SUMMARY
OBJECTIVE
To assess the effectiveness of a peer-based intervention on adherence to and completion of latent tuberculous infection (LTBI) treatment.
METHODS
Patients prescribed self-administered LTBI treatment were enrolled in a randomized controlled trial of an experimental, peer-based adherence support intervention. Primary outcomes were treatment adherence and completion. Adherence was assessed through self-report, electronic monitoring devices and clinic visits.
RESULTS
Of 250 participants, 70% were male; 71% were Black and 20% Latino; the mean age was 40 years; 67% were foreign-born and 39% were married. No significant baseline differences were noted between the intervention groups. Treatment completion was 61% in the intervention group compared to 57% in the controls (P = 0.482). The corresponding completion rate for other clinic patients was 44%. Foreign birth, marriage and history of mental illness were associated with non-completion of treatment after controlling for the intervention group; increased completion rates were found among foreign-born married persons and older participants. A substantial difference in adherence rates was observed between the intervention groups. Adherence among non-completers decreased early, while adherence among completers remained constant.
CONCLUSIONS
The peer-based intervention was not significantly associated with LTBI treatment completion, but was associated with greater adherence. Findings suggest the importance of interventions to support adherence that target early non-adherence with LTBI treatment, particularly in the first 2 months, when there is a substantial risk of default.
Keywords: tuberculosis adherence, randomized controlled trial, peer-based intervention
AN ESTIMATED 9–14 million persons in the United States have latent tuberculous infection (LTBI) and are at risk for progression to active TB disease.1 Diagnosis and successful treatment of LTBI is a cornerstone of the US strategy to eliminate TB.2 An estimated 300 000 individuals are treated annually for LTBI in the United States;3 however, LTBI treatment completion rates fall well below established targets, ranging from 30% to 60% for a standard 9-month isoniazid regimen.4
Several US studies have evaluated interventions for improving adherence to LTBI treatment, including use of supervised therapy, financial incentives, counseling, peer education and case management.5 However, few studies have rigorously evaluated LTBI interventions through randomized controlled trials (RCTs),5 and most have focused on specific populations, such as jail/prison inmates,6 drug users,7–9 homeless persons10,11 and immigrants/refugees from TB-endemic countries,12 rather than the mix of populations eligible for LTBI treatment.
Peer workers are widely used to promote changes in health behavior and are thought to play a unique role in health care, in part due to shared ethnicity, sex, illness experience, sexual orientation, risk behaviors and/or socio-economic characteristics with the target population.13–15 Peer collaboration is recognized as a potentially powerful tool to influence the complex interaction of factors known to influence adherence.16,17
With TB rates greatly exceeding national averages and the concomitant human immunodeficiency virus epidemic, the Harlem community in New York is vulnerable to TB.18 For many community members, tuberculin skin testing and the determination of LTBI status is frequently required to access homeless shelters, substance use programs and community-based organizations.
The objective of this RCT was to assess the effectiveness of a peer-based intervention on adherence and completion of LTBI treatment in a general US clinic population.
STUDY POPULATION AND METHODS
Setting, sample and intervention
Between 2002 and 2005, patients offered LTBI treatment under prevailing Centers for Disease Control and Prevention (CDC)/American Thoracic Society guidelines2 at the Harlem Hospital Chest Clinic in New York, NY, USA, were recruited into the Tuberculosis Adherence Partnership Alliance Study. All patients recommended for LTBI treatment and aged ≥18 years were eligible, except for those receiving directly observed therapy for LTBI.
Participants signed informed consent documents approved by Columbia University’s Institutional Review Board at Harlem Hospital.
Following baseline interview, the participants were randomly assigned to intervention or control groups. All participants received self-administered 9-month isoniazid (INH) treatment and access to standard clinical services. The peer-based intervention utilized the health belief model,19,20 social learning theory,21 and the precaution adoption process model,22 enriched by social support concepts.23 Experimental subjects were paired with peer workers who had completed LTBI or anti-tuberculosis treatment at Harlem Hospital and had attended a 4-week training program that included role-playing exercises, informational sessions and observation, which was designed to enhance their ability to provide social support, information and instrumental support. Peers attempted to meet one-on-one with assigned subjects at least once a week. They provided health care and social service system navigation, liaised with patients and health workers to enhance patient-provider communication, educated and coached patients on adherence, and provided social and emotional support.
Measures
Data were gathered from interviews and abstracted clinic charts. Questionnaires were translated into French and Spanish; interviewers were experienced, trained research assistants.
Socio-demographic characteristics, substance use,24 social support (Dunckel-Schetter C, Feinstein L, Call J. UCLA Social Support Inventory [UCLA-SSI]. Los Angeles, CA, USA: UCLA, 1986. Unpublished psychometric instrument), life stressors,25 social desirability,26 quality of life, depression,27 perceived benefits/barriers,28 and TB knowledge and attitudes were obtained from baseline interviews. Adherence was assessed every month by self-reported missed doses, electronic monitoring devices (MEMS® caps; Aardex Pharmionic, Sion, Switzerland) and clinic attendance records; self-reported adherence was given priority. Prescription bottles with MEMS caps were distributed and collected at each monthly visit. Participants were followed until they completed treatment, stopped treatment without completing or were lost to follow-up. Participants returned monthly for medication refills, medical monitoring and interviews, and were compensated for their time and travel to the research site. Completion of LTBI treatment was determined according to CDC guidelines by the participants’ medical providers, who were blinded to study status and study group. As per CDC guidelines,2 subjects treated for 6 months were determined to be completers. Medical charts provided information on initiation and completion of treatment, treatment interruptions and clinic appointment adherence.
Statistical methods
Data analysis followed the intent-to-treat principle. The intervention effect on treatment completion compares proportions of completers vs. non-completers between study groups, using Pearson’s χ2 or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. Potential confounding variables were examined using stratified analyses and Mantel-Haenszel summary measures. Variables significant at ≤0.10 were candidates for final models. Multivariate binomial regression was used to analyze the impact of the intervention on treatment completion after adjusting for covariates, including confounding variables reported in previous studies and interactions.
The impact of the intervention on treatment adherence was evaluated using mixed effects repeated measures models. Missing self-reported adherence data were imputed using chart abstractions, MEMS data (records of bottle openings used to approximate adherence) and hot-deck procedures (matched on treatment completion and adherence at adjacent visits). Heatmaps were used to illustrate adherence patterns.29
Knowledge score was constructed by calculating the mean number of correct answers to knowledge items. The distribution of each attitudinal item was examined and the category with most responses was designated as reference, or the middle categories (‘agree’/‘disagree’) were collapsed and used as reference. Factor analysis was used to develop scale scores for attitudes. Social support and perceived benefits/barriers scales were created by calculating the means of relevant individual items. The internal consistency reliability of the scales was tested with Cronbach’s alpha; values ≥0.6 were deemed reliable.
Statistical analyses used SAS (version 9.2, 2000; SAS Institute Inc, Cary, NC, USA), SPSS (version 17.0; SPSS Inc, Chicago, IL, USA), and R (R Development Core Team, Vienna, Austria).
RESULTS
Study population
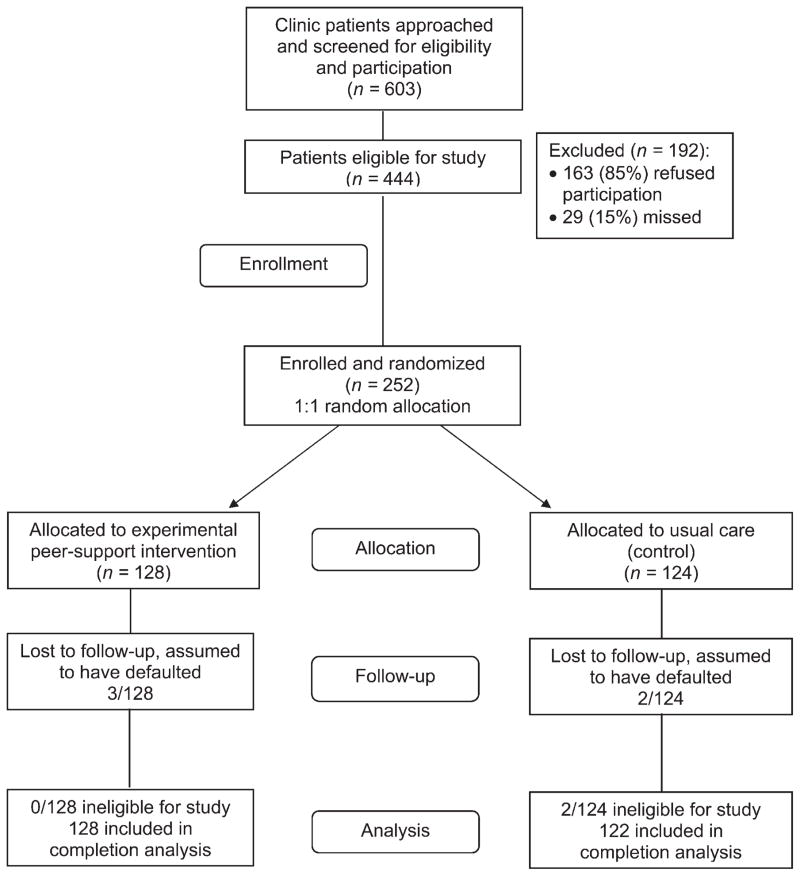
Figure 1 shows the participant flow from approach for participation, enrollment, and follow-up to outcome determination. Of the eligible participants, 163 refused to participate (44% too busy, 26% no interest, 21% other reasons, 8% no reason), and 29 were missed. A final 252 provided informed consent, completed baseline interviews and were randomized, 128 to the intervention group and 124 to the standard of care control group. After excluding two ineligible participants, the intervention (n = 128) and control groups (n = 122) had similar characteristics (Table 1). Participants were predominantly male (70%), with a mean age of 40 years; 35% were African-American, 20% Latino and 36% African. High school completion or equivalent was reported by 61% of the participants, 67% were foreign-born, and 39% reported marriage or common-law unions. A history of homelessness was reported by 33% and current homelessness by 16%; history of mental illness was reported by 8% of participants. Current alcohol use was reported by 32% and drug use by 16%. The mean Marlowe-Crowne scales for socially desirable responses did not differ significantly between the study groups.
Figure 1.
TAPAS study participant flow. TAPAS = Tuberculosis Adherence Partnership Alliance Study.
Table 1.
Baseline characteristics of study participants (n = 250)
| Total (N = 250) n (%) |
Intervention (n = 128) n (%) |
Control (n = 122) n (%) |
P value | |
|---|---|---|---|---|
| Age <40 years | 134 (53.6) | 71 (55.5) | 63 (51.6) | 0.544 |
| Male | 176 (70.4) | 88 (68.8) | 88 (72.1) | 0.558 |
| Race | 0.266 | |||
| African-American | 87 (34.8) | 50 (39.1) | 37 (30.3) | |
| Latino | 49 (19.6) | 25 (19.5) | 24 (19.7) | |
| African (foreign-born) | 91 (36.4) | 45 (35.2) | 46 (37.7) | |
| Other | 24 (9.2) | 8 (6.3) | 15 (12.3) | |
| Ever homeless | 83 (33.3) | 44 (34.7) | 39 (32.0) | 0.654 |
| Homeless in previous year | 40 (16.1) | 21 (16.5) | 19 (15.6) | 0.836 |
| Married/common-law | 97 (38.8) | 51 (39.8) | 46 (37.7) | 0.729 |
| Foreign-born | 167 (66.8) | 86 (67.2) | 81 (66.4) | 0.894 |
| Completed high school | 153 (61.2) | 78 (60.9) | 75 (61.5) | 0.931 |
| Unemployed | 147 (58.8) | 76 (59.4) | 71 (58.2) | 0.850 |
| Prior LTBI treatment | 15 (6.1) | 7 (5.5) | 8 (6.6) | 0.717 |
| History of mental illness | 21 (8.4) | 11 (8.6) | 10 (8.2) | 0.910 |
| Current smoker | 84 (33.6) | 46 (35.9) | 38 (31.2) | 0.423 |
| Ever alcohol use | 179 (71.6) | 94 (73.4) | 85 (69.7) | 0.509 |
| Current alcohol use | 80 (32.0) | 38 (29.7) | 42 (34.4) | 0.422 |
| Ever drug use | 129 (51.6) | 69 (53.9) | 60 (49.2) | 0.455 |
| Current drug user | 40 (16.0) | 20 (15.6) | 20 (16.4) | 0.868 |
| Depressed (CESD >16) | 86 (34.4) | 48 (37.5) | 38 (31.2) | 0.291 |
LTBI = latent tuberculosis infection; CESD = Center for Epidemiologic Studies Depression Scale.
LTBI treatment completion rates
Overall, 58.8% of the participants completed treatment: 60.9% in the intervention group vs. 56.6% in control group (risk ratio [RR] 1.096, 95% confidence interval [CI] 0.850–1.414). During study recruitment and follow-up, 1035 non-study patients in the clinic initiated LTBI treatment and 44.0% completed treatment.
Table 2 summarizes individual predictors of treatment completion, controlling for study group. Age ≥40 years (P = 0.002) was a significant predictor of completion, while being Latino (compared to African) was a predictor of non-completion (P = 0.042). History of mental illness (P = 0.055) showed a trend toward predicting non-completion.
Table 2.
Binomial regression analysis of predictors of completion of care, controlling for randomization group
| Regression coefficient | Standard error | Adjusted RR (95%CI) | P value | |
|---|---|---|---|---|
| Independent variables | ||||
| Demographics | ||||
| Age >40 years | 0.3247 | 0.1061 | 1.38 (1.12–1.70) | 0.002 |
| Male | −0.0512 | 0.1186 | 0.95 (0.75–1.20) | 0.666 |
| Race/ethnicity | 0.179 | |||
| African-American vs. African | −0.1468 | 0.1190 | 0.86 (0.68–1.09) | 0.217 |
| Latino vs. African | −0.3449 | 0.1692 | 0.71 (0.51–0.99) | 0.042 |
| Other vs. African | −0.0814 | 0.1826 | 0.92 (0.64–1.32) | 0.656 |
| Foreign-born | 0.0541 | 0.1149 | 1.06 (0.84–1.32) | 0.637 |
| Social characteristics | ||||
| Completed high school | −0.1249 | 0.1062 | 0.88 (0.72–1.09) | 0.239 |
| Employed | 0.0897 | 0.1059 | 1.09 (0.89–1.35) | 0.397 |
| Married | 0.0999 | 0.1088 | 1.11 (0.89–1.37) | 0.358 |
| Ever homeless | −0.0804 | 0.1168 | 0.92 (0.73–1.16) | 0.491 |
| Currently homeless | −0.1877 | 0.1676 | 0.83 (0.60–1.15) | 0.263 |
| Prior TB | −0.1042 | 0.2474 | 0.90 (0.55–1.46) | 0.674 |
| History of mental illness | −0.6013 | 0.3131 | 0.55 (0.30–1.01) | 0.055 |
| Depressed (CESD > 16) | 0.0478 | 0.1096 | 1.05 (0.85–1.30) | 0.663 |
| Substance abuse | ||||
| Current smoking | −0.0185 | 0.1127 | 0.98 (0.79–1.22) | 0.870 |
| Ever alcohol use | −0.1675 | 0.1075 | 0.85 (0.69–1.04) | 0.119 |
| Current alcohol use | −0.1559 | 0.1227 | 0.86 (0.67–1.09) | 0.204 |
| Ever drug use | 0.0003 | 0.1058 | 1.00 (0.81–1.23) | 0.997 |
| Current drug use | −0.1958 | 0.1675 | 0.82 (0.59–1.14) | 0.242 |
| Benefits and barriers | ||||
| Benefits scale | 0.0386 | 0.1237 | 1.04 (0.82–1.32) | 0.755 |
| Barriers scale | −0.1704 | 0.1484 | 0.84 (0.63–1.13) | 0.251 |
| Quality of life, physical (mean) | −0.0034 | 0.0060 | 1.00 (0.99–1.01) | 0.570 |
| Quality of life, mental (mean) | 0.0056 | 0.0051 | 1.01 (1.00–1.02) | 0.273 |
| Social support scale | −0.0085 | 0.0560 | 0.99 (0.89–1.11) | 0.880 |
| Knowledge of TB transmission | ||||
| TB from crowded conditions | 0.0986 | 0.1894 | 1.10 (0.76–1.60) | 0.603 |
| TB from sharing dishes, etc. | 0.1014 | 0.1161 | 1.11 (0.88–1.39) | 0.383 |
| TB through kissing | 0.2526 | 0.1061 | 1.29 (1.05–1.58) | 0.017 |
| TB from stranger vs. family | 0.0526 | 0.1058 | 1.05 (0.86–1.30) | 0.619 |
| Knowledge of testing and treatment | ||||
| TST+ indicates active disease | −0.1315 | 0.1081 | 0.88 (0.71–1.08) | 0.224 |
| ‘Sleeping TB’ is contagious | 0.1485 | 0.1103 | 1.16 (0.93–1.44) | 0.178 |
| TST+ can mean need for drugs | 0.6988 | 0.4857 | 2.01 (0.78–5.21) | 0.150 |
| Most TB can be cured with drugs | 0.2275 | 0.2826 | 1.26 (0.72–2.18) | 0.421 |
| HIV+, more likely to get TB | −0.1380 | 0.1070 | 0.87 (0.71–1.07) | 0.197 |
| LTBI treatment can take 1 month | 0.2060 | 0.1210 | 1.23 (0.97–1.56) | 0.089 |
| Undocumented person may be deported for anti-tuberculosis treatment | 0.0185 | 0.1086 | 1.02 (0.82–1.26) | 0.865 |
| Knowledge of symptoms | ||||
| Weight loss | 0.1869 | 0.1713 | 1.21 (0.86–1.69) | 0.275 |
| Swollen feet | −0.1739 | 0.1159 | 0.84 (0.67–1.05) | 0.134 |
| Cough | 0.2686 | 0.3779 | 1.31 (0.62–2.74) | 0.477 |
| Vomiting | 0.0090 | 0.1142 | 1.01 (0.81–1.26) | 0.938 |
| Coughing up blood | 0.0567 | 0.1585 | 1.06 (0.78–1.44) | 0.721 |
| Knowledge score | 0.0451 | 0.0292 | 1.05 (0.99–1.11) | 0.123 |
| Attitudes | ||||
| BCG vaccine prevents TB disease | 0.0313 | 0.0541 | 1.03 (0.93–1.15) | 0.563 |
| No matter what, you could still get TB germ | 0.114 | |||
| Somewhat agree | −0.0127 | 0.1378 | 0.99 (0.75–1.29) | 0.927 |
| Somewhat disagree | 0.0809 | 0.1380 | 1.08 (0.83–1.42) | 0.558 |
| Strongly agree | −0.2876 | 0.1589 | 0.75 (0.55–1.02) | 0.070 |
| Strongly disagree | Reference | |||
| Taking TB medicines is important | 0.2409 | 0.1852 | 1.27 (0.89–1.83) | 0.193 |
| You know better than doctor when to stop medicines | −0.0174 | 0.0552 | 0.98 (0.88–1.10) | 0.753 |
| Clinic appointments are more trouble than they’re worth | −0.0158 | 0.0726 | 0.98 (0.85–1.13) | 0.828 |
| Do not trust doctor for best care | 0.336 | |||
| Strongly agree | −0.1216 | 0.3272 | 0.89 (0.47–1.68) | 0.710 |
| Strongly disagree | 0.1601 | 0.1444 | 1.17 (0.88–1.56) | 0.268 |
| Disagree/agree | Reference | |||
| Doing right thing can avoid getting TB | 0.176 | |||
| Strongly agree | 0.2654 | 0.1450 | 1.30 (0.98–1.73) | 0.067 |
| Strongly disagree | 0.1650 | 0.2211 | 1.18 (0.76–1.82) | 0.455 |
| Disagree/agree | Reference | |||
| Worry about passing TB germ to loved ones | 0.075 | |||
| Strongly agree | 0.0665 | 0.1289 | 1.07 (0.83–1.38) | 0.606 |
| Strongly disagree | 0.3124 | 0.1330 | 1.37 (1.05–1.77) | 0.019 |
| Disagree/agree | Reference | |||
| Embarrassed to say you have TB germ | 0.685 | |||
| Strongly agree | −0.0485 | 0.1408 | 0.95 (0.72–1.26) | 0.730 |
| Strongly disagree | −0.0904 | 0.1200 | 0.91 (0.72–1.16) | 0.451 |
| Disagree/agree | Reference | |||
| Believe that you have the TB germ | 0.073 | |||
| Somewhat agree | 0.3780 | 0.1768 | 1.46 (1.03–2.06) | 0.033 |
| Somewhat disagree | 0.0098 | 0.2576 | 1.01 (0.59–1.73) | 0.972 |
| Strongly agree | 0.1559 | 0.1791 | 1.17 (0.82–1.66) | 0.384 |
| Strongly disagree | Reference | |||
| Care about what family and friends think of anti-tuberculosis treatment | 0.967 | |||
| Strongly agree | 0.0401 | 0.1243 | 1.04 (0.82–1.33) | 0.747 |
| Strongly disagree | 0.0160 | 0.1368 | 1.02 (0.78–1.33) | 0.907 |
| Disagree/agree | Reference | |||
| Try hard, will still miss some medicines | 0.824 | |||
| Somewhat agree | 0.0887 | 0.1202 | 1.09 (0.86–1.38) | 0.460 |
| Somewhat disagree | −0.0315 | 0.1564 | 0.97 (0.71–1.32) | 0.841 |
| Strongly agree | −0.0804 | 0.2421 | 0.92 (0.57–1.48) | 0.740 |
| Strongly disagree | Reference | |||
| Taking TB medicines is a hassle | 0.202 | |||
| Strongly agree | 0.4126 | 0.1645 | 1.51 (1.09–2.09) | 0.012 |
| Strongly disagree | 0.1033 | 0.1247 | 1.11 (0.87–1.42) | 0.407 |
| Disagree/agree | Reference | |||
| Only something really serious would prevent me from taking TB medicines | 0.946 | |||
| Strongly agree | 0.0483 | 0.1302 | 1.05 (0.81–1.35) | 0.711 |
| Strongly disagree | 0.0518 | 0.1341 | 1.05 (0.81–1.37) | 0.699 |
| Disagree/agree | Reference | |||
RR = risk ratio; CI = confidence interval; TB = tuberculosis; CESD = Center for Epidemiologic Studies Depression Scale; TST = tuberculin skin test; + = positive; HIV = human immunodeficiency virus; LTBI = latent tuberculous infection.
The constructed scales—social support, perceived benefits and perceived barriers—were found to have good reliability, but none were significant predictors. Of 16 knowledge items, correct answers on two statements were found to be significant predictors of treatment completion or showed a trend: ‘you can get TB by kissing’ (P = 0.017) and ‘treatment of LTBI can take 1 month’ (P = 0.089).
Factor analysis of attitudinal items yielded five factors; scales were created but none were found to have adequate reliability. Two individual attitudinal items were found to be significant: strong disagreement with the statement ‘you worry about passing the TB germ to loved ones’, was significantly associated with treatment completion compared to neutral (agree/disagree) responses (P = 0.0189). Similarly, agreement with the statement ‘you believe you have the TB germ’, was significantly associated with treatment completion compared to strong disagreement (P = 0.0325).
While not significantly associated with treatment completion, foreign birth and being married were further considered in multivariate models because of suspected interactions. As noted in Table 3, while study group was not significantly associated with LTBI treatment completion, being married (adjusted risk ratio [aRR] 0.508, 95%CI 0.258–0.998) was significantly associated with non-completion; however, marriage and foreign-birth were modified by interaction terms. Unmarried foreign-born participants were less likely than US-born participants to complete treatment, while married foreign-born participants were substantially more likely than US-born participants to complete treatment (aRR 2.379, 95%CI 1.148–4.930). Age ≥40 years was a predictor of completion of treatment (aRR 1.303, 95%CI 1.054–1.612), while history of mental illness (aRR 0.561, 95%CI 0.307–1.023) showed a trend for non-completion of treatment. No knowledge and attitudinal items remained in the multivariate model. Diagnostic statistics confirmed that the final model conforms to statistical assumptions for binomial regression.
Table 3.
Multivariate binomial regression analysis of effect of the intervention on LTBI treatment completion
| Independent variable | Regression coefficient | Standard error | Adjusted RR (95%CI) | P value |
|---|---|---|---|---|
| Study group | 0.0380 | 0.1000 | 1.04 (0.85–1.26) | 0.704 |
| Married | −0.6778 | 0.3447 | 0.51 (0.26–1.00) | 0.049 |
| Foreign-born | −0.1581 | 0.1399 | 0.85 (0.65–1.12) | 0.258 |
| Married and foreign-born | 0.8666 | 0.3718 | 2.38 (1.15–4.93) | 0.100 |
| Age >40 years | 0.2649 | 0.1085 | 1.30 (1.05–1.61) | 0.015 |
| History of mental illness | −0.5788 | 0.3068 | 0.56 (0.31–1.02) | 0.059 |
LTBI = latent tuberculous infection; RR = risk ratio; CI = confidence interval.
LTBI treatment adherence
MEMS were used always or often by most participants (86%). Participants also reported that it was easy to understand how to use the electronic cap (94%) and found using it to be easy (98%). When self-reported adherence was compared with data from the MEMS cap for 3 days prior to self-report, good agreement (κ = 0.687) was found. Where information did not match between the two methods, MEMS adherence was generally lower than self-reported adherence. This suggests that when MEMS data were used for imputation, adherence may have been imputed at lower values than the self-report.
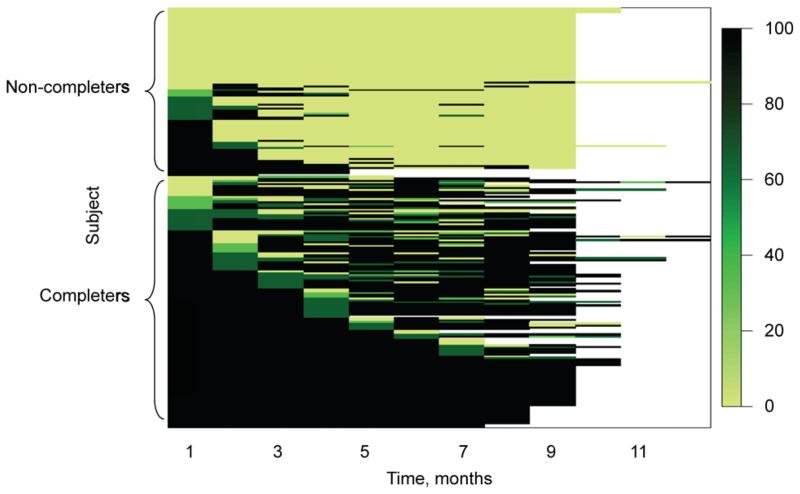
Models were run on 232 participants with 2043 records of monthly adherence: 1257 (62%) were reported via interview, 589 (29%) were determined from chart and MEMS data and 188 (9%) were imputed via a stochastic adherence algorithm; 18 participants had missing data. Figure 2 plots adherence over time for individuals using heatmaps. As shown in the plot, while adherence generally decreased over the course of treatment, non-completers’ adherence decreased dramatically and more rapidly early in the course of treatment.
Figure 2.
TAPAS adherence over time using heatmaps. Adherence over time for individuals was mapped using heatmaps, where each participant is represented by a layer and intensity of the color is utilized to show the level of adherence. TAPAS = Tuberculosis Adherence Partnership Alliance Study. This image can be viewed online in color at http://www.ingentaconnect.com/content/iuatld/ijtld/2013/00000017/00000009/art00009
A final multivariate model is presented in Table 4, with repeated measures analysis used to compare adherence as a continuous variable over treatment duration. A substantial difference in adherence rates was observed between study groups (9.7%, P = 0.043). Being married and foreign-born, age ≥40 years, not homeless, not using alcohol and knowing that ‘TST+ can mean need for medications’ were important predictors. The most common reasons reported for not adhering to treatment were ‘forgot’, ‘ran out of medications’ and ‘other priorities.’
Table 4.
Predictors of treatment adherence over time by repeated measures analysis
| Estimate | Standard error | t-value | P value | |
|---|---|---|---|---|
| Base adherence level | 15.8189 | 14.9026 | 1.06 | 0.290 |
| Study group | 9.7063 | 4.7684 | 2.04 | 0.043 |
| Married | −15.1533 | 9.8339 | −1.54 | 0.643 |
| Foreign-born | −6.9232 | 6.5853 | −1.05 | 0.342 |
| Married and foreign-born | 24.9865 | 11.6543 | 2.14 | 0.033 |
| Age >40 years | 16.8777 | 5.0227 | 3.36 | 0.001 |
| Currently homeless | −15.2774 | 6.8059 | −2.24 | 0.026 |
| Current alcohol use | −10.4141 | 5.1117 | −2.04 | 0.043 |
| Knowing that TST+ can indicate need for medication | 30.0988 | 13.0337 | 2.31 | 0.022 |
TST+ = tuberculin skin test positive.
DISCUSSION
Adherence and completion of LTBI treatment are crucial factors in eliminating TB in the United States and are thus of major public health relevance. This study assessed an innovative, peer-based adherence support intervention for LTBI treatment among individuals with multiple barriers to adherence in an urban US setting. The intervention was multifaceted and addressed recognized barriers to adherence using an approach that could be replicated in other settings. An intervention effect for LTBI treatment completion was observed but was not statistically significant; however, the intervention was found to be associated with statistically significantly higher rates of adherence. Interestingly, the study also showed that non-completers’ adherence decreased early in the course of treatment, while completers had fairly steady levels of adherence throughout treatment, an observation that could inform future interventions.
Foreign birth, marriage and history of mental illness were associated with non-completion of LTBI treatment; increased completion rates were found among foreign-born married persons and participants aged ≥40 years. Similar results were found in the adherence analysis regarding foreign birth, marriage and older age. Homelessness and current alcohol use were strongly associated with non-adherence to LTBI treatment. Furthermore, understanding that a positive TST may indicate need for LTBI treatment was associated with improved adherence.
As adherence tapered off early during the treatment course for non-completers, it may be beneficial to intervene early for patients whose poor adherence may indicate increased likelihood of discontinuing treatment. Prior studies found adherence in the first month of LTBI treatment to be predictive of completion.30–33 In an urban TB clinic, Parsyan et al. found that among those who failed to complete LTBI treatment, 54% defaulted during the first month.31 Sebastian et al. found that the failure to attend the first appointment identified all defaulters,32 and in an RCT comparing 4 months of rifampin with 9 months of INH, the percentage of doses taken and variability of the interval between doses in the first month was found to be highly predictive of LTBI treatment completion.30 This is similar to findings by Trajman et al., which showed that the regularity of treatment and percentage of doses taken were predictive of successful treatment completion.33 Identifying reasons for missing medication doses can suggest possible foci for interventions in the early months, such as weekly reminders to take medications and ensuring that prescriptions are refilled on schedule.
A unique aspect of our study is that it offers a systematic, in-depth examination of TB knowledge and attitudes. Three knowledge and two attitudinal items were found to be possible predictors of treatment completion, but these relationships were not evident in multivariate modeling. This suggests that knowledge and attitudes may be less important than social factors in determining treatment completion.
Few prior studies have evaluated interventions to promote LTBI treatment completion using RCT designs.5 Furthermore, existing RCTs conducted involved specific high-risk groups such as the homeless,10,11 drug users7–9 and jail inmates,6 rather than a general clinic population. Using a clinic population enables the generalizability of study findings to similar settings, which is important in considering how the study can inform public health practice. The inclusion of multiple measures of adherence and detailed assessment of knowledge and attitudes are additional strengths of our study.
The study has some limitations. Information on treatment completion was abstracted from clinic medical charts and not ascertained through participant interviews. However, chart information was completed by physicians blinded to study group assignment; such information is used for TB surveillance reports to the municipal health department. Another limitation is that participants in both study groups had considerably higher treatment completion rates than non-study participants who received LTBI treatment in the same clinic during the study period, which may have reduced the power to detect an intervention effect. While MEMS were utilized in this study as a measurement tool to monitor adherence, there might have been an unintended intervention effect, an issue that has been acknowledged by other researchers.34 Self-reporting may have been subject to social desirability bias in face-to-face interviews; however, it is reassuring that most risk factors reported were balanced between the groups, as were Marlowe-Crowne scores measuring the tendency to present oneself in a socially desirable way.
CONCLUSIONS
This RCT, which evaluated the effectiveness of a peer-based intervention, demonstrated improved adherence in the intervention group, although it did not show significant improvement in treatment completion. Another key finding is the importance of providing interventions to promote adherence to LTBI treatment during the first 2 months of treatment, when patients are at higher risk of defaulting. Focusing support on this ‘danger period’ could enhance the impact of interventions to improve LTBI treatment outcomes.
Acknowledgments
The authors thank all the patients who participated in this study, the staff at the Harlem Hospital Center and the Tuberculosis Adherence Partnership Alliance Study staff. This study was supported by the National Heart, Lung, & Blood Institute, National Institutes of Health (Tuberculosis Adherence Partnership Alliance Study, R01 HL66782, Wafaa El-Sadr, PI).
Footnotes
Conflict of interest: none declared.
References
- 1.Bennett DE, Courval JM, Onorato I, et al. Prevalence of tuberculosis infection in the United States population: the National Health and Nutrition Examination Survey, 1999–2000. Am J Respir Crit Care Med. 2008;177:348–355. doi: 10.1164/rccm.200701-057OC. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;49:1–51. [PubMed] [Google Scholar]
- 3.Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173:927–931. doi: 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 4.Horsburgh CR, Jr, Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401–409. doi: 10.1378/chest.09-0394. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch-Moverman Y, Daftary A, Franks J, Colson PW. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis. 2008;12:1235–1254. [PubMed] [Google Scholar]
- 6.White MC, Tulsky JP, Goldenson J, Portillo CJ, Kawamura M, Menendez E. Randomized controlled trial of interventions to improve follow-up for latent tuberculosis infection after release from jail. Arch Intern Med. 2002;162:1044–1050. doi: 10.1001/archinte.162.9.1044. [DOI] [PubMed] [Google Scholar]
- 7.Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug Alcohol Depend. 2002;66:283–293. doi: 10.1016/s0376-8716(01)00208-3. [DOI] [PubMed] [Google Scholar]
- 8.Chaisson RE, Barnes GL, Hackman J, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. 2001;110:610–615. doi: 10.1016/s0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- 9.Malotte CK, Hollingshead JR, Larro M. Incentives vs. outreach workers for latent tuberculosis treatment in drug users. Am J Prev Med. 2001;20:103–107. doi: 10.1016/s0749-3797(00)00283-x. [DOI] [PubMed] [Google Scholar]
- 10.Tulsky JP, Hahn JA, Long HL, et al. Can the poor adhere? Incentives for adherence to TB prevention in homeless adults. Int J Tuberc Lung Dis. 2004;8:83–91. [PubMed] [Google Scholar]
- 11.Tulsky JP, Pilote L, Hahn JA, et al. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Arch Intern Med. 2000;160:697–702. doi: 10.1001/archinte.160.5.697. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg SV, Wallace J, Jackson JC, Chaulk CP, Nolan CM. Cultural case management of latent tuberculosis infection. Int J Tuberc Lung Dis. 2004;8:76–82. [PubMed] [Google Scholar]
- 13.Baker EA, Bouldin N, Durham M, et al. The Latino Health Advocacy Program: a collaborative lay health advisor approach. Health Educ Behav. 1997;24:495–509. doi: 10.1177/109019819702400408. [DOI] [PubMed] [Google Scholar]
- 14.Pilote L, Tulsky JP, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless. A trial to improve adherence to referral. Arch Intern Med. 1996;156:161–165. [PubMed] [Google Scholar]
- 15.Colson PW, Francis LE. Consumer staff and the role of personal experience in mental health services. Social Work Mental Health. 2009;7:385–401. [Google Scholar]
- 16.Witmer A, Seifer SD, Finocchio L, Leslie J, O’Neil EH. Community health workers: integral members of the health care work force. Am J Public Health. 1995;85:1055–1058. doi: 10.2105/ajph.85.8_pt_1.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoni JM, Nelson KM, Franks JC, Yard SS, Lehavot K. Are peer interventions for HIV efficacious? A systematic review. AIDS Behav. 2011;15:1589–1595. doi: 10.1007/s10461-011-9963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.New York City Department of Health and Mental Hygiene. Tuberculosis in New York City, 2011: information summary. New York, NY, USA: NYC DOHMH; 2012. [Google Scholar]
- 19.Becker M. The health belief model and personal behavior. Health Educ Monogr. 1974;1:324–508. [Google Scholar]
- 20.Becker M, Maiman L, Kirscht J, Haefner D, Drachman R, Taylor D. Patient perceptions and compliance: recent studies of the Health Belief Model. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance in health care. Baltimore, MD, USA: Johns Hopkins University Press; 1979. pp. 78–109. [Google Scholar]
- 21.Bandura A, editor. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ, USA: Prentice-Hall; 1986. [Google Scholar]
- 22.Weinstein ND, Rothman AJ, Sutton SR. Stage theories of health behavior: conceptual and methodological issues. Health Psychol. 1998;17:290–299. doi: 10.1037//0278-6133.17.3.290. [DOI] [PubMed] [Google Scholar]
- 23.Berkman L, Glass T. Social integration, social networks, social support and health. In: Berkman L, Kawachi I, editors. Social epidemiology. Oxford, UK: Oxford University Press; 2000. pp. 137–173. [Google Scholar]
- 24.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ford J, Findley S, Richardson M, Meyer I. REACH Baseline Study. New York, NY, USA: Columbia University Press; 1995. Life events questionnaire. Comprehensive asthma interview. [Google Scholar]
- 26.Reynolds W. Development of reliable and valid short forms of the Marlowe-Crowne Social Desirability Scale. J Clin Psychol. 1982;38:119–125. [Google Scholar]
- 27.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 28.Strecher VJ, Rosenstock IM. The Health Belief Model. In: Glanz K, Lewis ML, Rimer B, editors. Health behavior and health education theory, research and practice. San Francisco, CA, USA: Jossey-Bass; 1997. pp. 42–59. [Google Scholar]
- 29.Swihart BJ, Caffo B, James BD, Strand M, Schwartz BS, Punjabi NM. Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology. 2010;21:621–625. doi: 10.1097/EDE.0b013e3181e5b06a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menzies D, Dion M-J, Francis D, et al. In closely monitored patients, adherence in the first month predicts completion of therapy for latent tuberculosis infection. Int J Tuberc Lung Dis. 2005;9:1343–1348. [PubMed] [Google Scholar]
- 31.Parsyan AE, Saukkonen J, Barry MA, Sharnprapai S, Horsburgh CR., Jr Predictors of failure to complete treatment for latent tuberculosis infection. J Infect. 2007;54:262–266. doi: 10.1016/j.jinf.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Sebastian MS, Bothamley GH. Tuberculosis preventive therapy: perspective from a multi-ethnic community. Respir Med. 2000;94:648–653. doi: 10.1053/rmed.1999.0877. [DOI] [PubMed] [Google Scholar]
- 33.Trajman A, Long R, Zylberberg D, Dion MJ, Al-Otaibi B, Menzies D. Factors associated with treatment adherence in a randomised trial of latent tuberculosis infection treatment. Int J Tuberc Lung Dis. 2010;14:551–559. [PubMed] [Google Scholar]
- 34.Nieuwkerk PT. Does electronic monitoring of adherence reduce social desirability on patients’ self-reports of adherence? J Acquir Immune Defic Syndr. 2004;36:880. doi: 10.1097/00126334-200407010-00018. [DOI] [PubMed] [Google Scholar]