Abstract
Introduction
Diabetes mellitus (DM) has a bidirectional association with pancreatic cancer (PaC); however, its effect on clinical outcomes has not been thoroughly evaluated. We analyzed these data in a large sample of PaC subjects who had undergone surgical resection.
Methods
Subjects enrolled in the Mayo Clinic Pancreatic Cancer SPORE registry from 2000–2010 who had resection with curative intent were identified (n=488). Tumor size, cancer stage and postoperative median survival were evaluated. Median survivals were compared with Kaplan-Meier curves and Cox proportional hazards regression modelling.
Results
A total of 275 (56%) subjects had DM prior to surgery. DM subjects had larger tumors compared to those without DM (3.6 cm vs. 3.3, p=0.002), even after controlling for covariates including age, BMI, and tumor grade. Cancer stage at the time of surgery was not affected by DM status(p=0.575). Pre-operative DM was not associated with increased risk of death using a multivariable survival analysis (HR 1.06, 95% CI 0.81–1.38, p=0.676)). The median survival following cancer resection was similar between subjects with and without DM (24 vs. 26 months, p=0.610). Additionally, postoperative survival was similar on the basis of duration of DM (new-onset vs. long-standing) and prior use of anti-diabetic treatments in diabetic subjects.
Discussion
PaC subjects with DM have larger tumors than non-diabetic subjects. Despite this observation, preoperative DM does not negatively impact the cancer stage at the time of surgery or postoperative survival. Thus, the effect of DM on tumor size is either overshadowed by early metastatic spread of the cancer or is mitigated by the tumor resection.
Keywords: pancreatic cancer, diabetes mellitus, survival, insulin, metformin
INTRODUCTION
Pancreatic cancer (PaC) is the fourth most common cause of cancer death in United States (1). The 5-year survival rate remains a dismal 5%, reflecting the large proportion of patients with locally advanced or metastatic disease at initial presentation and poor treatment options. Progress in cancer screening and treatment options has been slow for multiple reasons. Further understanding of the complex relationship between PaC and diabetes mellitus (DM) may permit further advancements in these areas.
One unique clinical finding in subjects with PaC is a common association with DM. Although DM has been reported as a risk factor for other cancers, there is a disproportionately higher number of PaC patients with diabetes mellitus (DM) compared to other cancers(2). For PaC, long-standing DM has been identified as a risk factor in recent meta-analyses(3–5). Additionally, the risk for PaC is increasingly higher when the DM is new-onset (i.e., DM onset occurs within 36 months of cancer diagnosis)(6). New-onset DM represents almost 75% of DM that is associated with PaC(7). Although the DM can develop as the result of the tumor mass itself, it often occurs prior to the appearance of a radiographically visible mass, suggesting the potential role for a tumor-secreted humoral mediator of DM. The peculiarities of this relationship suggest DM plays an important role in the early stages of PaC, either as a tumor growth promoting factor or tumor by-product, so additional understanding has potential implications for both screening and potentially treatment(8).
Studies investigating the effect of DM on clinical outcomes in PaC are challenging to execute due to the relative rarity of the disease and need to control for a wide range of clinical variables. The ideal study population to examine for this association are subjects who undergo surgical resection because this provides complete pathologic staging (e.g., accurate tumor size, extension to surrounding tissues, lymph node status, and grade) which is often not obtained for subjects with apparently metastatic disease at diagnosis. Recently, Chu and colleagues examined over 200 patients who were treated with a “surgery first” approach and found that diabetic subjects had a larger tumor size and reduced postoperative survival(9). Interestingly, the effect sizes were larger in subjects with new-onset DM compared to long-standing DM.
Our institution also practices a “surgery first” approach whereby patients with an anatomically resectable cancer are offered surgery without neoadjuvant treatment (i.e., chemotherapy and/or radiation). Thus, in general, there is no confounding from the potential treatment effects of neoadjuvant therapy. It is important to confirm the previous observations and further understand the potential interaction between PaC and DM to increase our understanding of tumorigenesis and potentially identify a new pathway for targeted therapies. We examined a large cohort of PaC (ductal adenocarcinoma) patients who had undergone surgical resection to determine if DM status is associated with poorer clinical outcomes, including tumor size, stage of disease at the time of surgery, and postoperative survival.
METHODS
This study protocol was approved by the Mayo Clinic Institutional Review Board, and informed consent was obtained from all subjects.
Patient recruitment and data collection
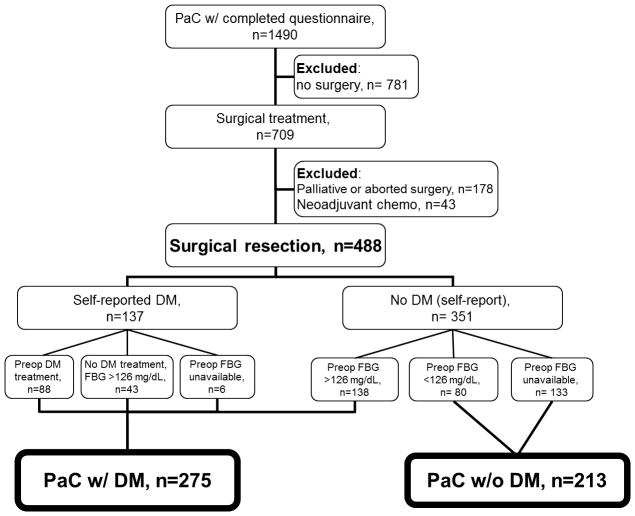
Pancreatic cancer patients (ductal adenocarcinoma) were rapidly and systematically identified and approached, using methodology reported previously at Mayo Clinic Rochester, Mayo Clinic Arizona, and Mayo Clinic Florida between January 1, 2000 and December 31, 2010(10). A total of 1490 enrolled patients completed the risk factor questionnaire (Figure 1). Subjects were also included if the questionnaire was completed after the date of surgery when the preoperative DM status could be confirmed. The questionnaire included medical and diabetes history, health habits, family history, and performance status.
Figure 1.
Study participant flow diagram.
Fasting blood glucose (FBG) levels were obtained from the electronic medical record for most subjects at study entry. DM was defined as those patients with a FBG level >126 mg/dL or, for those without FBGs, a self-report of DM on the risk factor questionnaire (Figure 1). In those with a self-report of DM, the status was confirmed initially by manual review of medical records to identify those receiving anti-diabetic treatment preoperatively, then by review of preoperative FBG status. New-onset DM was defined as the onset of DM up to 36 months prior to PaC diagnosis and long-standing DM if the duration was longer(11).
Additional data regarding preoperative laboratory data, and intraoperative and tumor data are prospectively collected and archived by an oncologist with an expertise in gastrointestinal cancers (PB). Intraoperative and tumor-related findings were independently reviewed by a second clinician (PH), and any discrepancies were resolved. Cancer staging was recorded according to AJCC 7th edition staging criteria. Vital status of subjects was collected using multiple sources as part of routine research followup; sources included periodic mailings, medical records, tumor registry, and death indices from online services.
The study group of interest was those undergoing surgical resection with intent to completely remove the cancer (i.e., a potentially “curative” surgery). Subjects with inoperable cancer were not included. Operative reports were reviewed if any surgery was performed to exclude subjects who had either a palliative or aborted (due to the presence of distant disease) procedure. In some instances surgery was performed elsewhere, so some operative details and tumor data were unavailable. Finally, 43 patients (6.1% of potentially eligible subjects undergoing surgery) who received neoadjuvant treatment (i.e., chemotherapy with or without radiation) were not included to minimize confounding from previous treatment effects on the tumor or glycemic status.
Statistical analyses
The date of cancer diagnosis was defined as the date of tissue diagnosis or surgical resection, whichever came first. Date of death or last known date alive was selected from the most current data among the multiple sources described above. Survival (the primary endpoint) was determined from the date of cancer diagnosis to the date of death or last known alive date. Three subgroups were identified at the time of study conception for comparison according to: i.) duration of DM prior to cancer diagnosis (new-onset vs. long-standing DM), ii.) DM treatment status (previous DM treatment vs. no DM treatment), and iii.) proximity to study center (limited to those residing within a 125 mile radius of Rochester, MN at study enrollment).
Clinical characteristics and operative findings were compared between DM and non-DM patients using Pearson chi-squared tests for categorical variables and Kruskal-Wallis tests for continuous variables. Kaplan-Meier curves with estimated median survival(logrank test for statistical significance) were used to explore survival. Cox proportional hazards regression analysis was used to further investigate the relationship between DM status and survival, multivariable models were considered to adjust for other clinically relevant covariates. These covariates were defined a priori as: age at PaC diagnosis, gender, body mass index (BMI), weight loss percentage, smoking status, family history of DM, DM status, DM treatment, maximum tumor size, tumor grade, number of positive lymph nodes, margin status, adjuvant chemotherapy, and stage at PaC diagnosis.
Tumor size was investigated as a secondary endpoint using multivariable regression modeling (test for significance from likelihood ratio tests). Clinically relevant covariates for this analysis were also defined a priori as: age at PaC diagnosis, gender, BMI, family history of DM, duration of DM, site of pancreatic mass, stage at diagnosis, tumor grade, number of positive nodes, angiolymphatic invasion, and perineural invasion. Statistical significance was P <0.05. All analyses were performed using SAS software, version 9.1.3 [SAS Institute, Cary, NC].
RESULTS
Study population characteristics
Surgical intervention was undertaken for 709/1490 (47.6%) of eligible participants, which reflects the referral bias of our center. Those who underwent surgery as a palliative bypass or had an aborted procedure due to locally unresectable or metastatic disease (n=178) or received neoadjuvant chemotherapy (n=43) were excluded from the analyses.
In 488 subjects undergoing surgical resection with curative intent, 382 (78.3%) had a pancreaticoduodenectomy, 84 (17.2%) had a distal pancreatectomy, and 22 (4.5%) had a total pancreatectomy. Portal or superior mesenteric vein reconstruction was performed for 73 (17%) subjects (data unavailable for 57 subjects). Complete gross resection (R0–R1) was achieved in 463/470 (99%) patients. Tumor characteristics for patients undergoing surgery with curative intent are shown (Table 1).
Table 1.
Tumor characteristics for 488 PaC patients who underwent surgical resection with curative intent.
| Variable | n (%) |
|---|---|
| Mean tumor size, cm (SD) | 3.5 (1.6) |
| Tumor Grade: | |
| Well-differentiated | 2 (0.4) |
| Moderately-differentiated | 83 (18.0) |
| Poorly-differentiated | 316 (68.7) |
| Undifferentiated | 59 (12.8) |
| Missing | 28 |
| Lymph node status: | |
| Positive | 258 (53.8) |
| Negative | 222 (46.3) |
| Missing | 8 |
| Resection status: | |
| R0 | 387 (82.3) |
| R1 | 76 (16.2) |
| R2 | 7 (1.5) |
| Missing | 18 |
All values are presented as n (% of available data) unless otherwise indicated.
Comparison based on diabetes mellitus status
DM status was determined for all patients as depicted by Figure 1. A total of 275 (56%) subjects with PaC had DM. The DM diagnosis was made on the basis of an elevated FBG for 138 subjects who were otherwise unaware of a history of DM. The clinical profiles of diabetic and non-diabetic patients were compared (Table 2). Those with DM had significantly higher usual adult BMI and were more likely to have a family history of DM, but the two groups otherwise had similar clinical profiles. There were no differences between the preoperative laboratory data including hemoglobin, leukocytes, absolute neutrophil count, Ca 19-9, albumin, AST, ALT, total/direct bilirubin, alkaline phosphatase, BUN, creatinine, and prothrombin time levels. Operative findings are compared in Table 3. Diabetic subjects had larger tumor sizes than non-diabetic subjects, but the distribution of AJCC stage at diagnosis was similar. Generally, subjects with DM were more likely to have a tumor with an advanced histologic grade (i.e., poorly or undifferentiated).
Table 2.
Clinical characteristics of pancreatic adenocarcinoma patients with and without DM who underwent curative surgical resection.
| Variable | DM (N=275) | Non-DM (N=213) | Total (N=488) | p-value* |
|---|---|---|---|---|
| Age at time of pancreatic cancer diagnosis | 0.401 | |||
| Mean (SD) | 65.3 (10.6) | 64.4 (10.8) | 64.9 (10.7) | |
| Gender | 0.096 | |||
| Male | 150 (54.5%) | 100 (46.9%) | 250 (51.2%) | |
| Race | 717 | |||
| White/Caucasian | 269 (97.8%) | 209 (98.1%) | 478 (98.0%) | |
| Black/African-American | 3 (1.1%) | 1 (0.5%) | 4 (0.8%) | |
| Other | 3 (1.1%) | 3 (1.4%) | 6 (1.2%) | |
| Usual Adult BMI | <0.001 | |||
| Mean (SD) | 29.0 (5.7) | 26.8 (4.6) | 28.0 (5.4) | |
| Percent of Total Weight Lost | 0.332 | |||
| 0–10 | 126 (46.3%) | 93 (44.1%) | 219 (45.3%) | |
| 10–20 | 110 (40.4%) | 90 (42.7%) | 200 (41.4%) | |
| >20 | 36 (13.2%) | 28 (13.3%) | 64 (13.3%) | |
| Ever Smoker | 0.906 | |||
| Yes | 168 (61.1%) | 129 (60.6%) | 297 (60.9%) | |
| Packyears | 0.443 | |||
| None | 112 (41.8%) | 87 (42.9%) | 199 (42.3%) | |
| <10 | 32 (11.9%) | 32 (15.8%) | 64 (13.6%) | |
| 10–19 | 30 (11.2%) | 25 (12.3%) | 55 (11.7%) | |
| 20+ | 94 (35.1%) | 59 (29.1%) | 153 (32.5%) | |
| Performance score (Karnofsky) | 0.315 | |||
| <50 | 5 (1.9%) | 5 (2.6%) | 10 (2.2%) | |
| 60 | 23 (8.8%) | 16 (8.2%) | 39 (8.6%) | |
| 70 | 79 (30.4%) | 44 (22.7%) | 123 (27.1%) | |
| 80 | 61 (23.5%) | 59 (30.4%) | 120 (26.4%) | |
| 90–100 | 92 (35.4%) | 70 (36.1%) | 162 (35.7%) | |
| Family History of Diabetes | 0.046 | |||
| Yes | 158 (57.5%) | 103 (48.4%) | 261 (53.5%) |
P-values for categorical variables were calculated using a Pearson chi-square test. A Kruskal-Wallis test was used for continuous variables.
Table 3.
Operative findings of subjects who underwent curative surgical resection of pancreatic cancer based on DM status.
| Variable | DM (N=275) | Non-DM (N=213) | Total (N=488) | p-value* |
|---|---|---|---|---|
| Site of Pancreatic Mass | 0.063 | |||
| Head/uncinate | 222 (81.6%) | 146 (71.2%) | 368 (77.1%) | |
| head and body | 12 (4.4%) | 9 (4.4%) | 21 (4.4%) | |
| body | 12 (4.4%) | 14 (6.8%) | 26 (5.5%) | |
| body and tail | 5 (1.8%) | 9 (4.4%) | 14 (2.9%) | |
| tail | 21 (7.7%) | 27 (13.2%) | 48 (10.1%) | |
| Operation Type | 0.005 | |||
| Pancreaticoduodenectomy | 222 (80.7%) | 160 (75.1%) | 382 (78.3%) | |
| Distal pancreatectomy | 36 (13.1%) | 48 (22.5%) | 84 (17.2%) | |
| Total pancreatectomy | 17 (6.2%) | 5 (2.3%) | 22 (4.5%) | |
| Portal or Superior Mesenteric Vein Reconstruction | 0.208 | |||
| Yes | 49 (18.8%) | 24 (14.1%) | 73 (16.9%) | |
| Resection Status | 0.829 | |||
| R0 (negative margins) | 221 (81.0%) | 166 (80.2%) | 387 (80.6%) | |
| R1 (microscopically positive margins) | 42 (15.4%) | 34 (16.4%) | 76 (15.8%) | |
| R2 (grossly positive margins) | 5 (1.8%) | 2 (1.0%) | 7 (1.5%) | |
| Maximum tumor dimension (cm) | 0.002 | |||
| Mean (SD) | 3.59 (1.42) | 3.27 (1.69) | 3.45 (1.55) | |
| Tumor Grade | 0.012 | |||
| Well differentiated | 0 (0.0%) | 2 (1.0%) | 2 (0.4%) | |
| Moderately differentiated | 36 (13.7%) | 47 (23.9%) | 83 (18.0%) | |
| Poorly differentiated | 190 (72.2%) | 126 (64.0%) | 316 (68.7%) | |
| Undifferentiated | 37 (14.1%) | 22 (11.2%) | 59 (12.8%) | |
| Number of Positive Nodes | 0.317 | |||
| Mean (SD) | 2.15 (3.85) | 2.06 (3.96) | 2.11 (3.90) | |
| Ratio of Positive Nodes/Total Number | 0.306 | |||
| Mean (SD) | 0.15 (0.21) | 0.18 (0.48) | 0.16 (0.35) | |
| Stage at Diagnosis | 0.907 | |||
| I | 48 (17.6%) | 36 (17.2%) | 84 (17.4%) | |
| II | 220 (80.6%) | 168 (80.4%) | 388 (80.5%) | |
| III | 5 (1.8%) | 5 (2.4%) | 10 (2.1%) | |
| Adjuvant Chemotherapy | 0.752 | |||
| Yes | 230 (85.2%) | 169 (86.2%) | 399 (85.6%) | |
| Adjuvant Radiotherapy | 0.122 | |||
| Yes | 189 (69.2%) | 130 (62.5%) | 319 (66.3%) |
P-values for categorical variables were calculated using a Pearson chi-square test. A Kruskal-Wallis test was used for continuous variables.
Comparisons in population-based subgroup
Our clinical practice is predominantly referral-based. To evaluate referral bias, particularly in regards to cancer stage, univariable comparisons were repeated between diabetic and non-diabetic groups for subjects (n=151) living within 125 miles of Rochester, MN (Table 4). Diabetic subjects remained more likely to have a higher adult BMI, family history of DM, and have a pancreaticoduodenectomy. Additionally, there was a greater proportion of weight loss and higher median CA 19-9 levels in diabetics at the time of cancer diagnosis. Tumor size and histologic tumor grade were no longer statistically significant, but the direction of effect was the same as in the total study population. The AJCC stage at diagnosis was similar between those with and without DM for this non-referral based subgroup.
Table 4.
Univariable comparisons for the primary variables of interest and those achieving statistical significance between diabetic and non-diabetic subjects in the population-based (in proximity to Rochester, MN) group.
| Variable | DM (N=101) | Non-DM (N=50) | Total (N=151) | p-value* |
|---|---|---|---|---|
| Usual Adult BMI | 0.004 | |||
| Mean (SD) | 29.1 (6.1) | 26.2 (4.7) | 28.1 (5.8) | |
| Percent of Total Weight Lost | 0.008 | |||
| 0–10 | 40 (39.6%) | 28 (56.0%) | 68 (45.0%) | |
| 10–20 | 41 (40.6%) | 17 (34.0%) | 58 (38.4%) | |
| >20 | 20 (19.8%) | 5 (10.0%) | 25 (16.6%) | |
| Family History of Diabetes | 0.002 | |||
| Yes | 57 (56.4%) | 15 (30.0%) | 72 (47.7%) | |
| Median Ca 19-9 (IQR) | 0.027 | |||
| Median | 341.0 (82–726) | 79.0 (34–367) | 189.5 | |
| Operation Type | 0.011 | |||
| Pancreaticoduodenectomy | 87 (86.1%) | 33 (66.0%) | 120 (79.5%) | |
| Distal pancreatectomy | 12 (11.9%) | 16 (32.0%) | 28 (18.5%) | |
| Total pancreatectomy | 2 (2.0%) | 1 (2.0%) | 3 (2.0%) | |
| Maximum tumor dimension (cm) | 0.083 | |||
| Mean (SD) | 3.6 (1.4) | 3.2 (1.6) | 3.5 (1.5) | |
| Tumor Grade | 0.063 | |||
| Well differentiated | -- | -- | -- | |
| Moderately differentiated | 11 (11.1%) | 13 (26.0%) | 24 (16.1%) | |
| Poorly differentiated | 72 (72.7%) | 31 (62.0%) | 103 (69.1%) | |
| Undifferentiated | 16 (16.2%) | 6 (12.0%) | 22 (14.8%) | |
| Stage at Diagnosis | 0.330 | |||
| I | 17 (17.0%) | 13 (26.5%) | 30 (20.1%) | |
| II | 82 (82.0%) | 35 (71.4%) | 117 (78.5%) | |
| III | 1 (1.0%) | 1 (2.0%) | 2 (1.3%) |
P-values for categorical variables were calculated using a Pearson chi-square test. A Kruskal-Wallis test was used for continuous variables.
Comparisons based on duration of diabetes mellitus
To determine if the duration of DM influenced the clinical outcomes, 204 DM patients were further evaluated on the basis of the duration of DM (duration of DM could not be determined for 71 subjects). DM was of new-onset for the majority of diabetic subjects (164, 80%). The clinical characteristics presented in Table 2 were compared between those with new-onset DM and long-standing DM. Usual adult BMI was higher in those with long-standing DM compared to new-onset DM (30.8 kg/m2 vs. 28.5, respectively, p=0.012). Additionally, subjects with new-onset DM were less likely to be smokers (60.4% vs. 80.0%, p=0.020 or to have a family history of DM (56.1% vs. 82.5%, p=0.002). The groups were similar in regards to preoperative laboratory and operative findings with the exception of mean tumor size (cm), which was larger in new-onset DM (3.7 vs. 3.2, p = 0.022). Findings in the new-onset DM group were similar when compared to non-diabetic subjects, but the difference in family history of DM was no longer present.
Comparisons based on diabetes mellitus treatment
DM subjects were also compared on the basis of use of antidiabetic medications. A total of 88 DM subjects were using either an oral medication or insulin at the time of cancer diagnosis. Those receiving treatment had a higher mean adult BMI (30.0 kg/m2 (SD 5.3) vs. 28.5 kg/m2 (5.9), p=0.014) and higher proportion of family history for DM (69.3% vs. 51.9%, p=0.006).
Tumor size
Several factors were significantly associated with increased tumor size in addition to DM on univariable analysis, including increasing usual adult BMI (p=0.033), site of mass (body/tail vs. head) (p<0.001), advanced tumor grade (i.e., poorly differentiated or undifferentiated) (p<0.001), >3 positive lymph nodes (p=0.023), positive family history of DM (p=0.004), and recent onset of DM (vs. long-standing) (p=0.025). Multivariable analysis controlling for these variables and others with p<0.20 on univariable analysis demonstrated an independent relationship between tumor size and age, adult BMI, site of mass, stage at diagnosis, tumor grade, and duration of DM (Table 5).
Table 5.
Factors independently associated with increased tumor size with multivariable regression modeling.
| Covariate* | Estimate** (95% CI) | p-value |
|---|---|---|
| Age at PaC Diagnosis | 0.001 | |
| Ordinal Effect | 0.02 (0.01, 0.02) | |
| Usual Adult BMI | 0.004 | |
| Ordinal Effect | 0.04 (0.01, 0.06) | |
| Site of Pancreatic Mass | <.001 | |
| Head/Head and Body | 0.00 (ref) | |
| Body/Tail | 1.13 (0.75, 1.50) | |
| NOS | −0.22 (−0.92, 0.49) | |
| Stage at Diagnosis | 0.026 | |
| I | 0.00 (ref) | |
| II | 0.56 (0.13, 1.00) | |
| III | 0.00 (−1.10, 1.10) | |
| Tumor grade | 0.008 | |
| Moderately/Well differentiated | 0.00 (ref) | |
| Poorly differentiated | 0.25 (−0.13, 0.63) | |
| Undifferentiated | 0.86 (0.31, 1.41) | |
| Duration of Diabetes | <.001 | |
| Non-DM | 0.00 (ref) | |
| Long-standing | −0.50 (−1.03, 0.04) | |
| New-onset | 0.41 (0.09, 0.73) |
Also included gender, number of positive lymph nodes, angiolymphatic invasion, family history of diabetes and perinuerual invasion. Only significant covariates are shown.
The estimate reflects the increase in tumor size (cm) associated with each covariate.
Survival analysis
The postoperative median survival for all subjects undergoing cancer resection with curative intent was 24.4 months. Median survival was similar in those with and without DM (23.7 months vs. 26.4 months, respectively, p=0.610) (Figure 2). On multivariable survival analysis the presence of diabetes mellitus was not associated with an increased risk for death (HR 1.06, 95% CI 0.81–1.38, p=0.676). Although the median survival tended to be shorter in those with new-onset compared to long-standing DM, the difference was not statistically different (23.5 months vs. 27.1 months, respectively, p=0.715) (Figure 3). In the DM group, median survival was not influenced by preoperative diabetic treatment status (23.5 months for treated vs. 23.8 months for untreated, p=0.743). Specifically, the median post-operative survival in those who used metformin (27.1 months) or insulin (25.3 months) was similar to that of diabetics not on treatment (23.8 months, p=0.367 and 0.912, for the respective comparisons).
Figure 2.
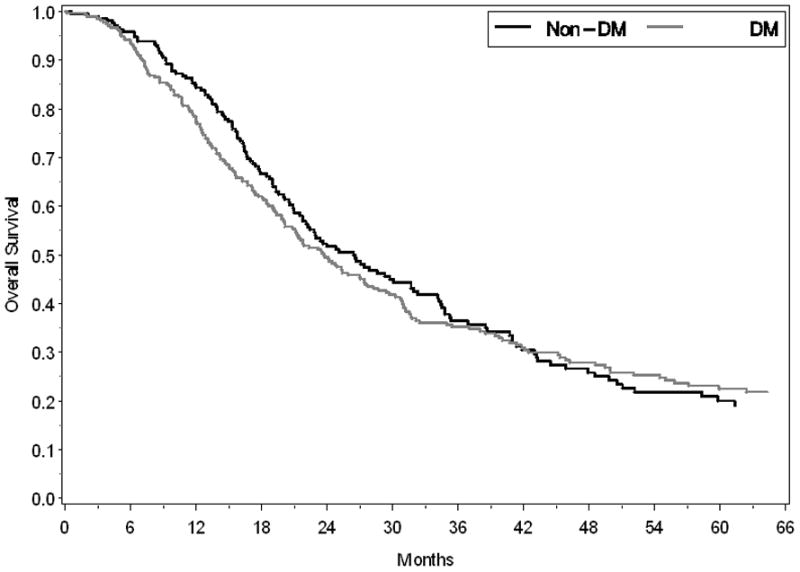
Kaplan-Meier survival curves demonstrate a similar median survival (p=0.610) following pancreatic cancer resection for subjects with DM (gray line) and without DM (black line).
Figure 3.
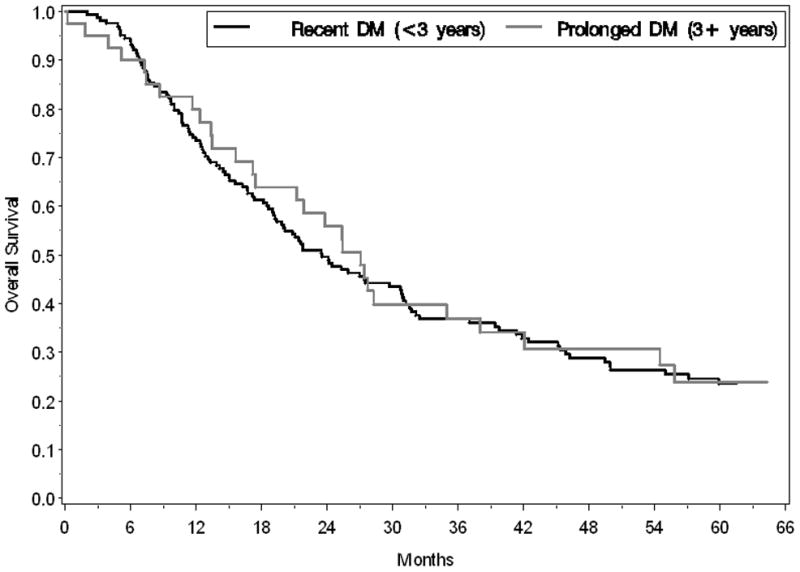
Kaplan-Meier survival curves demonstrate a similar median survival (p=0.715) following pancreatic cancer resection for subjects with new-onset DM (black line) and longstanding DM (gray line).
DISCUSSION
In this cohort study of PaC subjects who had undergone surgical resection, we demonstrated that, although subjects with DM have a greater tumor size, this is not associated with worse survival following cancer resection. Despite the larger tumor size, DM and non-DM subjects have a similar cancer stage at the time of surgery. The tumor size is independently associated with preoperative DM and is more prominent in diabetics with new-onset compared to long-standing DM. The duration of DM (new-onset vs. long-standing) and the use of anti-diabetic medications prior to diagnosis did not affect the postoperative survival. In brief, preoperative DM status does not influence clinical outcomes, with the exception of tumor size, in those undergoing surgical resection for PaC.
Previous large epidemiologic studies have repeatedly shown that those with DM are more likely to develop and die from PaC compared to subjects without DM. These predominantly database-derived analyses lacked the necessary information to control for important confounders, including age at diagnosis, tumor grade, and cancer stage. A previous, well-designed case-control study evaluating 209 subjects who had undergone surgical resection provided results from a single center study(9). The investigators noted a similar increase in tumor size in subjects with DM compared to those without DM (3.8 vs. 3.2 cm, p<0.01). They found the postoperative median survival was reduced in DM compared to non-DM subjects (15 vs. 17 months, p=0.02). Although the absolute decrease in median survival for DM subjects in our study was similar, the increased overall median survival in our series (24 months) compared to their series (16 months) may partially explain our inability to conclude a statistically significant difference. However, their multivariable survival analysis did not account for age and margin status (the subjects in our study were younger and less likely to have positive margins or nodal involvement). In summary, when important confounders were considered in the analysis of our large cohort (nearly 500 subjects), we were unable to confirm the previous observation that preoperative DM negatively influences postoperative survival.
The dramatic effect on survival of early metastases and lymphatic spread in PaC cannot be overlooked. As a consequence of the large effect of cancer stage on survival, the potential influence of a single comorbidity or disease modifier must have a large effect to be clinically apparent. Therefore, even if DM affects prognosis, this effect is likely overshadowed by the powerful influence of early metastases. Nonetheless, the increased tumor size may still be mechanistically relevant. Since tumors are 3-dimensional objects, an increase in diameter results in an exponential increase in tumor volume (volume of a sphere = 4/3·π·r3). By this measure, the observed increase in tumor size between DM and non-DM subjects, although <5 mm in diameter, results in an approximate 30% increase in tumor volume. Since it remains unclear whether DM incites tumor growth and/or is a consequence of tumor growth (likely via humoral mechanisms), the true importance of the increased size is not fully realized at this time. An alternative explanation is that the negative impact on survival by the diabetic state is neutralized by cancer resection. In a previous study, cancer resection has been shown to ameliorate the hyperglycemic changes of PaC-associated DM(7). This effect was even more apparent in those with new-onset DM, in which the DM resolved in more than half of patients following surgical resection. According to this hypothesis, neutralization of the diabetic state by surgical resection could potentially mitigate any potential influence of DM, making postoperative survival comparable to those who were not diabetic preoperatively.
It has not been directly demonstrated that systemic lowering of serum glucose (i.e., by providing anti-diabetic treatments) in subjects with PaC influences clinical outcomes or even decreases the amount of glucose available in the tumor microenvironment. In our study, data regarding hemoglobin A1c values were not consistently available for enough subjects to make any meaningful comparison in this regard. Future studies presenting data on the degree of glycemic control prior to, at the time of diagnosis, and following diagnosis may provide further insights. There is increasing attention to the potential for anti-diabetic medications to alter the risk of developing PaC; however, results have been mixed(12). A recent meta-analysis demonstrated increased risk for developing PaC in subjects on sulfonylureas, but there was no significant association with metformin, thiazolidinediones, and insulin(13). In our subgroup analysis of diabetic subjects who received the various anti-diabetic medications (e.g., metformin, insulin, and others) prior to cancer diagnosis, we were unable to demonstrate a significant difference in postoperative survival compared to diabetics who did not receive these treatments.
This was a retrospective cohort study of patients who had completed an extensive risk factor questionnaire. Availability of extensive historical and pre- and postoperative clinical details allowed us to simultaneously assess a large number of potential confounding variables within the same study population. The high availability of preoperative FBG values also allowed us to identify many subjects with new-onset DM. Unfortunately many subjects with self-reported absence of DM did not have an available preoperative blood glucose collected in the fasting state. A sensitivity analysis was conducted (data not shown) excluding these subjects, and the results remained unchanged. The current study is the largest evaluation of DM status in subjects who underwent surgical resection of PaC. This is a reflection of the existing high-volume, referral-based medical and surgical Pancreas clinic and incorporation of the three Mayo Clinic practice sites. To ensure the findings were not altered by the referral nature of our practice, we performed subgroup analyses for subjects living in proximity to the primary study site; similar results for tumor size, cancer stage at diagnosis, and postoperative survival were seen.
In conclusion, although the presence of DM at the time of PaC diagnosis is associated with increased tumor size, there is no negative impact on the cancer stage at time of surgery or survival following cancer resection. Even though the absolute difference in tumor size is relatively small (approximately 3 mm), it reflects a noteworthy increase in relative tumor volume. If DM negatively affects these outcomes, the effect is either mitigated by tumor resection or simply overshadowed by the profound influence of early metastases. Further studies exploring the influence of DM on tumor size may help understand factors involved in tumor growth but may not yield meaningful treatment options.
STUDY HIGHLIGHTS.
1. WHAT IS CURRENT KNOWLEDGE?
Long-standing diabetes mellitus (DM) is an established risk factor for pancreatic cancer.
New-onset DM is a stronger risk factor and likely and early manifestation of pancreatic cancer.
The influence of DM status on clinical outcomes in pancreatic cancer (PaC) is poorly defined.
2. WHAT IS NEW HERE?
Patients with DM have a larger tumor size at the time of initial cancer diagnosis, particularly those with new-onset DM. The increased tumor size in diabetics is likely a clue to the underlying tumor pathogenesis, and warrants further investigations
Despite the increase in tumor size, the AJCC cancer stage at presentation and postoperative survival are similar between diabetics and non-diabetics.
Anti-diabetic medications did not alter the survival in diabetics compared to those who did not receive these medications.
Acknowledgments
Grant Support: Dr Chari’s research was funded by a grant from the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701).
Abbreviations
- CA 19-9
carbohydrate antigen 19-9
- DM
diabetes mellitus
- FBG
fasting blood glucose
- PaC
pancreatic ductal adenocarcinoma
Footnotes
Conflicts of interest/disclosures: No conflicts of interest exist.
- Phil A. Hart, MD – study concept and design, acquisition of data, analysis and interpretation of data, drafting of initial manuscript, critical revision of the final manuscript, and final approval of the version to be published
- Ryan J. Law, DO – acquisition of data, analysis and interpretation of data, critical revision of the final manuscript and final approval of the version to be published
- Ryan D. Frank, BA - acquisition of data, analysis and interpretation of data, critical revision of the final manuscript and final approval of the version to be published
- William R. Bamlet, MS - analysis and interpretation of data, critical revision of the final manuscript and final approval of the version to be published
- Patrick A. Burch, MD – acquisition of data, analysis and interpretation of data, critical revision of the final manuscript and final approval of the version to be published
- Gloria M. Petersen, PhD - analysis and interpretation of data, critical revision of the final manuscript and final approval of the version to be published
- Kari G. Rabe, MS - analysis and interpretation of data, critical revision of the final manuscript and final approval of the version to be published
- Suresh T. Chari, MD - study concept and design, acquisition of data, analysis and interpretation of data, drafting of initial manuscript, critical revision of the final manuscript, final approval of the version to be published, and study guarantor
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42:198–201. doi: 10.1097/MPA.0b013e3182592c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. European Journal of Cancer. 2011;47:1928–37. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 5.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British Journal of Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sah RP, Nagpal SJ, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423–33. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–7. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sah RP, Nagpal SJ, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013 doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Annals of Surgical Oncology. 2010;17:502–13. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–62. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:510–9. doi: 10.1038/ajg.2013.7. quiz 520. [DOI] [PubMed] [Google Scholar]