Abstract
Estrogens play a crucial role in regulating the growth and differentiation of breast cancers, with approximately two-thirds of all breast tumours expressing the estrogen receptor alpha (ERα). Therefore, therapeutic strategies directed at inhibiting the action of ERα by using antiestrogens such as tamoxifen, or reducing estrogens levels by using aromatase inhibitors (AIs), such as letrozole, anastrozole, or exemestane, are the standard treatments offered to women with ERα-positive cancer. However, not all patients respond to endocrine therapies (termed de novo resistance), and a large number of patients who do respond will eventually develop disease progression or recurrence while on therapy (acquired resistance). Recently variant forms of the receptor due to alternative splicing or gene mutation have been identified. This article reviews these variant receptors and their clinical relevance in resistance to endocrine therapy, by addressing their molecular cross-talk with growth factor receptors and signaling components. Understanding the complexity of receptor-mediated signaling has promise for new combined therapeutic options which focus on more efficient blockade of receptor cross-talk.
Background
Human estrogen receptors (ERs) belong to a superfamily of nuclear hormone receptors that function as ligand-activated transcription factors. Two isoforms of ER have been described: ERα and ERβ, Each is encoded by unique genes, but share a common structural and functional organization. Classical ER (ERα or hERα-66) contains an amino-terminal region that harbors the ligand-independent activation function (AF-1), a central DNA binding domain (DBD), and a carboxy-terminal hormone binding domain (HBD) which contains the ligand-dependent activation function (AF-2) (Fig. 1). Binding of hormone to ERα facilitates “classical” genomic activities of the receptor (Fig. 2), and its’ binding to estrogen response elements (EREs) in target genes function to either activate or repress gene expression.
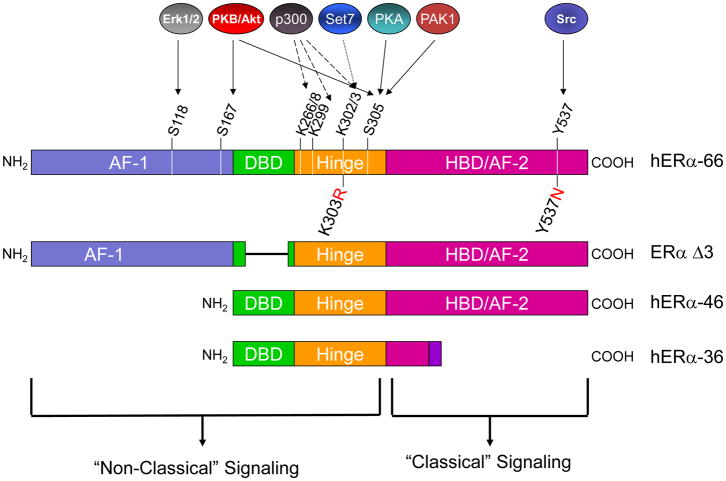
Figure 1.
A schematic representation of the structural domains, the sites of post-translational modifications and mutations within ERα. Growth factor signaling leads to numerous downstream phosphorylation events located within the AF-1, DBD, and hinge domains, thus affecting ERα signaling through the “non-classical” signaling pathways. Post-translational modifications located within the HBD/AF-2 domain affect the “classical” signaling pathway of ERα. In the AF-1 domain S118 and S167 are phosphorylated by Erk1/2 and Akt respectively, in the hinge domain, S305 by Akt, PKA and PAK-1. Phosphorylation of these three sites regulates both ERα’s sensitivity to tamoxifen as well as ligand independent activation of the receptor. Acetylation at residues K266/8, K299, and K302/3 by p300 also modulates ERα activation. A somatic mutation, K303R, allows ERα to be more highly phosphorylated by PKA and Akt, resulting in estrogen hypersensitivity and endocrine resistance. Methylation, occurring by Set7 interaction with ERα, causes increased receptor stability and a heightened recruitment of ERα to its target genes. In the AF-2 domain, the Y537 site is target of c-Src. The Y537N mutation eliminates this phosphorylation site, resulting in constitutive receptor activity. ERα can also be modified by alternative splicing at the DNA level, resulting in exon skipping and a truncated protein with a subsequently altered function. For example, in ERα Δ3, the third exon is alternatively spliced resulting in a truncated DBD. This isoform does not bind to DNA and is a dominant negative inhibitor to the WT ERα. Another truncated ERα isoform is ERα-46 which is missing the AF-1 domain and has been found to localize at the plasma membrane. It acts as a competitive inhibitor to full length ERα. A third alternatively spliced form of ERα is ERα-36, which lacks the AF-1 and AF-2 domains but has an additional 27 amino acid sequence added at the COOH terminus. This isoform also localizes to the plasma membrane as also acts as a suppressor of full length ERα.
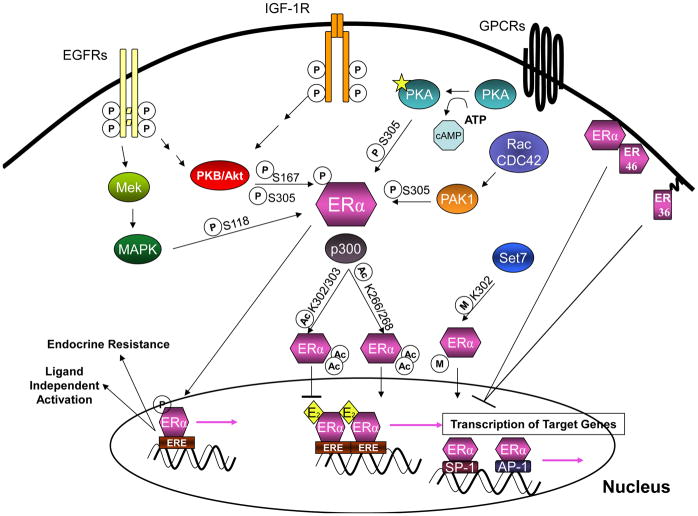
Figure 2.
A Schematic Representation of the “Classical” and “Non-Classical” Estrogen Receptor Signaling Pathways. Estrogen Receptor (ER) mediates transcription of its target genes using two types of mechanisms, these are known as “classical” and “non-classical”signaling. First, “classical” signaling initiates with the binding of estrogen to ER causing it to bind directly to regions of DNA called Estrogen Responsive Elements (EREs) located within transcriptional start sites of estrogen regulated genes and subsequently activate transcription of downstream genes. There are several mechanisms of “non-classical” signaling. The first of these mechanisms is mediated by the signaling of growth factors (such as IGFR and EGFR) and G-protein coupled receptors, through downstream signaling molecules to ER. These pathways mediate ERs state of post-transcriptional modification (by affecting its phosphorylaton, acetylation, methylation) and thus its activity, independent of estrogen binding. It is likely that crosstalk of these pathways not only results in estrogen independent activation of ER but also endocrine resistance. Signaling has also been shown to occur through truncated membrane bound forms of ER, this signaling is usually inhibitory of full length estrogen receptor activity. Finally, another mechanism of “non-classical” signaling requires the binding of estrogen receptor to other transcription factors (including SP-1 and AP-1) causing a recruitment of estrogen receptor to transcriptional start sites other than ERE’s and transcription of downstream genes.
Figure Key:
1.)
 Transcription
Transcription
2.)
 P Phosphorylation
P Phosphorylation
3.)
 M Methylation
M Methylation
4.)
 Ac Acetylation
Ac Acetylation
5.)
 E2 Estrogen
E2 Estrogen
Estrogen actions are also mediated by other “non-classical” mechanisms (Fig. 2): (a) ligand-independent ERα signaling, in which gene activation occurs through second-messengers downstream of growth factor signaling pathways (such as the epidermal growth factor receptor [EGFR], the insulin-like growth factor receptor [IGFR], and the G protein coupled receptor [GPCR] pathways) that alter intracellular kinase and phosphatase activity, resulting in altered phosphorylation of ERα (1); (b) rapid, “non-genomic” effects through a membrane-associated receptor; (c) and ERE-independent signaling, in which ERα regulates genes via protein-protein interactions with other transcription factors, such as c-Fos/c-Jun B (AP-1), Sp1 and NF-κB (2–4). These alternative mechanisms alter downstream target protein expression instrumental in cell division, angiogenesis and survival leading to sustained breast cancer growth and progression.
Several laboratories have evaluated the effects of phosphorylation by second-messengers on receptor action. Among the kinases that can phosphorylate ERα are important signaling molecules such as Akt, extracellular regulated kinase (Erk) 1/2 MAPK, p21-activated kinase 1 (PAK-1) and protein kinase A (PKA), resulting in diverse responses to ligands (Fig. 1) (5). For example, phosphorylation of ERα serine (S) 167 by Akt and S118 by Erk1/2 can result in acquired resistance to the antiestrogen tamoxifen, and ligand-independent activation of ERα (6–8). Phosphorylation of S305, which is mediated by both PKA and PAK-1 signaling, can impact estrogen hypersensitivity and tamoxifen responsiveness (9–11). These phosphorylation events are complex and interdependent. For instance, phosphorylation at ERα S305 can regulate the subsequent phosphorylation of S118 (12), and receptor acetylation (9).
The region between the DBD and the LBD, known as the hinge, has long been considered to simply serve as a flexible linker to orient the other two functional domains. However, it has now thought that this region is a multifunctional domain which binds a number of co-regulatory proteins and participates in the binding of ER to DNA (9, 13, 14). The lysine residues K266, K268, K299, K302, and K303 within this domain are conserved residues that can be acetylated by the histone acetylase protein p300 (Fig. 1) (14–17). Acetylation of K266 and K268 induced DNA-binding and ligand-dependent activation (14), while acetylation of K302 and K303 inhibited ERα activation (15). The phosphorylation status of ERα S305 coordinately regulates the acetylation of the K302/303 residues, sensitizing ERα to ligand stimulation (9). ERα K302 is also methylated by the SET7 methyltransferase (Fig. 1); this methylation stabilizes the receptor and is necessary for the efficient recruitment of ERα to its target genes and subsequent transactivation (18). Acetylation of K303 attenuates ERα-driven transcription, not just from antagonism via acetylation, but also by inhibition of K302 methylation and subsequent destabilization of ERα. Other modifications, such as ubiquitination at K302 (19) and sumoylation at K266 and K268 (20), have also been shown to affect ERα stability and activity. Thus residues in the hinge domain are frequent targets for post-translational modifications that affect hormone sensitivity through alteration of receptor stability or regulation of estrogen-dependent gene transcription. It is tempting to speculate that immunohistochemical quantitation of these post-translational modifications could provide important prognostic or predictive information in clinical samples.
Variant ERα Protein Isoforms
Several groups have identified ERα splice variants in a number of different normal tissues such as human breast epithelium, endometrium, and pituitary, as well as various tumor types including breast cancer, endometrial carcinoma, and meningiomas; these mRNA variants are usually coexpressed along with the wild-type receptor [reviewed in (21)]. These splice variants can confer either dominant-positive or dominant-negative effects on cancer cells, and are hypothesized to contribute to the hormone-independent phenotype of some breast tumors. Among these variants is ERα exon Δ3, that is missing part of the DBD (22), which showed the most significant increase in levels in breast cancer tissue (Fig. 1) (23). The ERα Δ3 isoform functions as a dominant-negative receptor, able to suppress estrogen-induced transcriptional activity (24), reduce anchorage-dependent growth, soft-agar colony forming ability, and in vitro invasion when transfected in breast cancer cells (25). It is hypothesized that this reduction in ER signaling may lead to unchecked estrogen stimulation, establishing permissive conditions for further carcinogenetic events.
Despite the different ER splice variants described thus far, relatively few variant ERα protein isoforms have been characterized, in part due to the practical limitations of their detection (26–28). Previous reports have shown the presence of three predominant bands of 35–39, 46, and 66 kDa in immunoblots probed with an anti-ERα antibody raised against the LBD (26–28). There is accumulating evidence that these isoforms could play significant roles in ER signaling events.
For instance, the hERα–46 isoform has been biochemically isolated from MCF-7 breast cancer cells (29), vascular endothelial cells (30), and osteoblasts (26). This isoform lacks the first 173 amino acids in the amino-terminal the AF-1 domain due to alternative splicing of exon 1, and it co-purifies with plasma membrane markers (Fig. 1) (31). This altered location allows cells to mediate rapid estrogen signaling events, such as stimulation of nitrogen oxide synthesis (27). hERα–46 forms heterodimers with full-length ERα acting as a strong competitive inhibitor in ERα-positive cells, and it can promote activation of genomic activities in ERα-negative tissues (26, 29). Further studies are needed to identify the exact function of this isoform in estrogen target cell proliferation, and to understand its potential prognostic role in clinical samples.
hERα–36 is a naturally-occurring isoform that is expressed in both ERα-positive and negative breast cancer cells, and is generated from a promoter located in the first intron of the hERα–66 gene (32, 33). It lacks both AF-1 and AF-2, but retains the DBD and portions of the HBD (Fig. 1). It possesses an extra, unique 27 amino acid domain that replaces the last 138 amino acids encoded by exons 7 and 8 of the hERα–66 gene (28). The hERα–36 isoform also contains three potential myristoylation sites located near the amino-terminal region which are postulated to direct it to the plasma membrane. This isoform lacks intrinsic transcriptional activity, but it efficiently suppresses the transactivation activities mediated by full-length ERα, suggesting that it is a potent inhibitor of genomic estrogen signaling. Interestingly, the hERα–36 isoform primarily localizes to the plasma membrane, where it transduces “nongenomic” signaling cascades initiated by both estrogens and antiestrogens, such as activation of the MAPK/ERK signaling pathway thus stimulating cell proliferation (34). In clinical samples, overexpression of hERα-36 was associated with poorer disease free-survival in patients, identifying a subset of patients that are less likely to benefit from tamoxifen treatment (33). In summary, ERα protein isoforms capable of modulating ERα-mediated signaling have been identified, and their integration into the accurate classification of ERα status may be warranted.
ERα Mutations in Tumors
The number of naturally-occurring mutations identified in breast cancers to date is relatively low (35), surprising since mutation of the clinical target is a common resistance mechanism in tumors. The Y537N (Tyr537Asn) mutation was discovered in a metastatic breast tumor (36). This mutation eliminates a carboxy-terminal tyrosine residue that is considered to be an important c-Src phosphorylation site with potential roles in regulating ligand binding, homodimerization, and transactivation of ERα. It was demonstrated that the Y537N ERα mutant exhibits constitutive transactivation activity, and that this activity was only slightly affected by estradiol, tamoxifen, or the steroidal antiestrogen ICI 164,384 (36). A mutation at this site may allow ERα to escape phosphorylation-mediated controls, providing cells with a potential selective advantage, but unfortunately only a few metastatic breast tumors have yet been examined for mutations at this site.
A somatic mutation at nucleotide 908 of ERα (A908G) has been identified in about a third of premalignant breast hyperplasias and one-half of invasive breast tumors from untreated patients (37, 38). The A908G mutation introduces a lysine to arginine transition at residue 303 (termed K303R) within the hinge domain. Molecular analyses of the K303R ERα mutation have shown that the mutated arginine at the 303 position allows ERα to be more highly phosphorylated by protein kinase A (PKA) (9) and Akt kinase signaling (39), and alters the dynamic recruitment of coactivators and corepressors, such as BRCA-1 or calmodulin (40, 41).
Overexpression of the K303R ERα mutation in ERα-positive MCF-7 breast cancer cells confers estrogen hypersensitivity (37), and decreased sensitivity to tamoxifen treatment when engaged in cross-talk with growth factor receptor signaling pathway (42). Enhanced growth factor receptor cross-talk with ERα is a known mechanism of hormone resistance in breast cancer (43). Expression of the K303R ERα mutation also conferred resistance to the non-steroidal aromatase inhibitor anastrozole in ERα-positive cells, via a dynamic interaction between the K303R ERα mutation, S305 phosphorylation, and the IGF-1R signaling pathway (39, 44). Signaling components both upstream and downstream of the IGF-1R were altered in mutant-overexpressing cells. The frequency of the mutation is still contentious (45–49), however the sequencing method used in some of these studies might not have been sensitive enough for straight-forward detection of this specific mutation (38). The presence of the K303R ERα mutation was associated with poor outcomes in univariate analyses of tumors from untreated breast cancer patients, and its presence was correlated with older age, larger tumor size, and lymph node-positive disease, all clinical factors associated with worse outcomes (38). Collectively these data suggest that this mutation could play an important role as a predictive marker in breast cancer and strategies to accurately measure it in clinical samples are currently underway.
Clinical Translational Advances
Combination Therapies: How Do ERα Variants Come Into Play?
Since a number of different signaling pathways can be simultaneously active in ERα-positive breast tumors, and a variety of ER activities, such as receptor turnover, cellular localization, and hormone responsiveness can all be influenced by the presence of ERα variants, then strategies to prevent signaling to these variants may present a unique opportunity for complete blockade of ER. Future directions include peptide mimetics capable of blocking altered post-translational modifications on the receptor (39, 42). We speculate that the ERα hinge region may be a particularly attractive target to block the multiple posttranslational modifications, including phosphorylation, acetylation, methylation, sumoylation, and ubiquitination, occuring in this important regulatory region.
Major breakthroughs in understanding the molecular dynamics of cell signaling networks operative in tumors are rapidly being translated into the clinic, with a number of potent drugs selectively targeting the MAPK, and PI3K/Akt pathways entering into clinical trials (Table 1). There is also evidence of potential cross-talk between these signaling pathways, necessitating horizonal blockade, or combined use of multiple signaling inhibitors. The complex bidirectional cross-talk that exists between growth factor receptors, these second-messenger signaling pathways, and ERα, along with the variant ERα forms suggests that simultaneous blockade will be required to bypass resistance mechanisms or restore hormone sensitivity in some breast cancer patients. The potential utility of these new therapeutics to signaling components, along with combined ERα-directed therapy is predicted to improve clinical care and reduce mortality from breast cancer.
Table 1.
Clinical trials using drugs which target pathways known to interact with the ERα pathway in patients with ERα-positive breast disease*.
| Drug/Combination | Pathway Target(s) | Patient Disease Info | Phase | Status |
|---|---|---|---|---|
| PD-3259011 | MEK (MAPK Pathway) | Advanced Breast Cancer, Colon Cancer, and Melanoma | I–II | Terminated |
| Bevacizumab + sorafenib tosylate2 | RAF (MAPK Pathway) | Refractory, Metastatic, or Unresectable Solid Tumors | I | Ongoing |
| Paclitaxel and RAD001 Followed by FEC (chemotherapy)3,4 | mTOR | Triple Negative Breast Tumors | II | Recruiting |
| Ritonavir (Pre-Operative)5 | Akt | Newly Diagnosed Breast Cancer Patients | I–II | Not yet open |
| GSK21417956 | Akt | Solid tumors/lymphomas not responsive to other therapies | I | Recruiting |
| GDC-0941+ bevacizumab+ paclitaxel7 | PI3K | Locally recurrent or metastatic breast cancer | Ib | Recruiting |
| BGT2264 | PI3K | Advanced solid malignancies including breast cancer | I–II | Recruiting |
| BEZ2354 | PI3K | Advanced solid malignancies including breast cancer | I | Recruiting |
| Temsirolimus2,8 | mTOR | Locally recurrent or metastatic breast cancer | II | Ongoing |
| XL147+ XL6479 | PI3K | Solid tumors including breast cancer | I | Suspended |
Study sponsors:
Pfizer,
NCI,
MD Anderson, Houston TX,
Novartis,
Masonic Cancer Center, University on Minnesota,
GlaxoSmithKline,
Genentech,
University of Chicago,
Exelixis
Information from ClinicalTrials.gov
Conclusions
A significant challenge for effective blockade of ERα signaling is the inherent cellular heterogeneity present in breast tumors. Not only can variant forms of ERα be expressed along with wild-type receptor in tumors, but ERα-positive patients can present with alterations in the growth factor receptors themselves, as well as critical signaling molecules such as PI3K and Akt (50). Accurate determination of this molecular heterogenity is requisite for accurate and effective treatment decisions. The development of new biomarkers to detect this heterogenity is key.
Acknowledgments
Grant support: NCI RO1 CA72038
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have not received commercial research grants.
References
- 1.Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med. 1998;76:469–79. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- 2.Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990;63:1267–76. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- 3.Ray A, Prefontaine KE, Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269:12940–6. [PubMed] [Google Scholar]
- 4.Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–56. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 5.Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol Endocrinol. 2006;20:3120–32. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Jiang F, Wang Q, et al. MEKKI activation of human estrogen receptor alpha and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol Endocrinol. 2000;14:1882–96. doi: 10.1210/mend.14.11.0554. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64:9199–208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 10.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. Embo J. 2002;21:5437–47. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalides R, Griekspoor A, Balkenende A, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Rayala SK, Talukder AH, Balasenthil S, et al. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006;66:1694–701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 13.Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007;67:4514–23. doi: 10.1158/0008-5472.CAN-06-1701. [DOI] [PubMed] [Google Scholar]
- 14.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20:1479–93. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Fu M, Angeletti RH, et al. Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem. 2001;276:18375–83. doi: 10.1074/jbc.M100800200. [DOI] [PubMed] [Google Scholar]
- 16.Fu M, Wang C, Zhang X, Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol. 2004;68:1199–208. doi: 10.1016/j.bcp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Fu M, Wang C, Zhang X, Pestell R. Nuclear receptor modifications and endocrine cell proliferation. J Steroid Biochem Mol Biol. 2003;85:133–8. doi: 10.1016/s0960-0760(03)00223-1. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian K, Jia D, Kapoor-Vazirani P, et al. Regulation of estrogen receptor alpha by the SET7 lysine methyltransferase. Mol Cell. 2008;30:336–47. doi: 10.1016/j.molcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry NB, Fan M, Nephew KP. Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol. 2008;22:1535–51. doi: 10.1210/me.2007-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol Endocrinol. 2005;19:2671–84. doi: 10.1210/me.2005-0042. [DOI] [PubMed] [Google Scholar]
- 21.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–98. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Miksicek RJ. Identification of a dominant negative form of the human estrogen receptor. Mol Endocrinol. 1991;5:1707–15. doi: 10.1210/mend-5-11-1707. [DOI] [PubMed] [Google Scholar]
- 23.Poola I, Speirs V. Expression of alternatively spliced estrogen receptor alpha mRNAs is increased in breast cancer tissues. J Steroid Biochem Mol Biol. 2001;78:459–69. doi: 10.1016/s0960-0760(01)00118-2. [DOI] [PubMed] [Google Scholar]
- 24.Iwase H, Omoto Y, Iwata H, Hara Y, Ando Y, Kobayashi S. Genetic and epigenetic alterations of the estrogen receptor gene and hormone independence in human breast cancer. Oncology. 1998;55 (Suppl 1):11–6. doi: 10.1159/000055254. [DOI] [PubMed] [Google Scholar]
- 25.Erenburg I, Schachter B, Mira y Lopez R, Ossowski L. Loss of an estrogen receptor isoform (ER alpha delta 3) in breast cancer and the consequences of its reexpression: interference with estrogen-stimulated properties of malignant transformation. Mol Endocrinol. 1997;11:2004–15. doi: 10.1210/mend.11.13.0031. [DOI] [PubMed] [Google Scholar]
- 26.Denger S, Reid G, Kos M, et al. ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol. 2001;15:2064–77. doi: 10.1210/mend.15.12.0741. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–12. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–7. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 29.Flouriot G, Brand H, Denger S, et al. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19:4688–700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci U S A. 2000;97:5930–5. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez DC, Pietras RJ. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene. 2001;20:5420–30. doi: 10.1038/sj.onc.1204729. [DOI] [PubMed] [Google Scholar]
- 32.Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-alpha36, a novel variant of ER-alpha, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–83. [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Dong B, Li Z, et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27:3423–9. doi: 10.1200/JCO.2008.17.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–8. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roodi N, Bailey LR, Kao WY, et al. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. J Natl Cancer Inst. 1995;87:446–51. doi: 10.1093/jnci/87.6.446. [DOI] [PubMed] [Google Scholar]
- 36.Zhang QX, Borg A, Wolf DM, Oesterreich S, Fuqua SA. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997;57:1244–9. [PubMed] [Google Scholar]
- 37.Fuqua SA, Wiltschke C, Zhang QX, et al. A hypersensitive estrogen receptor-alpha mutation in premalignant breast lesions. Cancer Res. 2000;60:4026–9. [PubMed] [Google Scholar]
- 38.Herynk MH, Parra I, Cui Y, et al. Association between the estrogen receptor alpha A908G mutation and outcomes in invasive breast cancer. Clin Cancer Res. 2007;13:3235–43. doi: 10.1158/1078-0432.CCR-06-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barone I, Iacopetta D, Covington KR, et al. Phosphorylation of the mutant K303R estrogen receptor α at serine 305 affects aromatase inhibitor sensitivity. Oncogene. 2010 doi: 10.1038/onc.2009.520. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Fan S, Hu C, et al. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Mol Endocrinol. 24:76–90. doi: 10.1210/me.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herynk MH, Hopp T, Cui Y, Niu A, Corona-Rodriguez A, Fuqua SA. A hypersensitive estrogen receptor alpha mutation that alters dynamic protein interactions. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giordano C, Cui Y, Barone I, et al. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;119:71–85. doi: 10.1007/s10549-009-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 44.Barone I, Cui Y, Herynk MH, et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res. 2009;69:4724–32. doi: 10.1158/0008-5472.CAN-08-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies MP, O’Neill PA, Innes H, Sibson DR. Hypersensitive K303R oestrogen receptor-alpha variant not found in invasive carcinomas. Breast Cancer Res. 2005;7:R113–8. doi: 10.1186/bcr965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tebbit CL, Bentley RC, Olson JA, Jr, Marks JR. Estrogen receptor alpha (ESR1) mutant A908G is not a common feature in benign and malignant proliferations of the breast. Genes Chromosomes Cancer. 2004;40:51–4. doi: 10.1002/gcc.20017. [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga E, Kimura Y, Maehara Y. No hypersensitive estrogen receptor-alpha mutation (K303R) in Japanese breast carcinomas. Breast Cancer Res Treat. 2004;84:289–92. doi: 10.1023/B:BREA.0000019963.67754.93. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Yamashita H, Toyama T, et al. Estrogen receptor alpha mutation (A-toG transition at nucleotide 908) is not found in different types of breast lesions from Japanese women. Breast Cancer. 2003;10:70–3. doi: 10.1007/BF02967628. [DOI] [PubMed] [Google Scholar]
- 49.Conway K, Parrish E, Edmiston SN, et al. The estrogen receptor-alpha A908G (K303R) mutation occurs at a low frequency in invasive breast tumors: results from a population-based study. Breast Cancer Res. 2005;7:R871–80. doi: 10.1186/bcr1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–8. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]