Abstract
Human papillomavirus type 16 (HPV-16) infection is associated with a majority of cervical cancers and a significant proportion of head and neck cancers. Here, we describe a novel engineered DNA vaccine that encodes a HPV-16 consensus E6/E7 fusion gene (pConE6E7) with the goal of increasing its antitumor cellular immunity. Compared to an early stage HPV-16 E7 DNA vaccine (pE7), this construct was up to five times more potent in driving E7-specific cellular immune responses. Prophylactic administration of this vaccine resulted in 100% protection against HPV E6 and E7-expressing tumors. Therapeutic studies indicated that vaccination with pConE6E7 prevented or delayed the growth of tumors. Moreover, immunization with pConE6E7 could also partially overcome immune tolerance in E6/E7 transgenic mice. Such DNA immunogens are interesting candidates for further study to investigate mechanisms of tumor immune rejection in vivo.
Keywords: HPV-16, E6 and E7 protein, DNA vaccine
1. Introduction
Cancers associated with the human papillomavirus (HPV) infection pose a significant international health problem. Cervical cancer is the second most common cancer among women worldwide, but the most common cancer in developing countries [1]. Moreover, HPV-associated head and neck cancer affects as many as 115,000 people each year globally [2,3]. The development of these cancers is closely associated with infection by high-risk types of Human Papillomaviruses (HPV) [4]. Studies have shown that HPV-16 is the predominant subtype of both cancers, accounting for 46%–63% of cervical cancer cases and 90% of head and neck cancer cases [3,5].
There are two major targets for vaccines that can be considered in the fight against HPV-related cancer. Prophylactic vaccines are aimed at inducing immunity against HPV infection in naïve individuals. Anti L1 and L2-directed antigens are efficient in this role. Preventive vaccines developed to date [6] are constructed of VLPs or even pentameric L1 capsomerases. When used as vaccines, they generate strong virus-neutralizing antibodies in the serum. HPV prophylactic vaccines have shown significant clinical efficacy. They can prevent about 70–80% of all cervical cancers based on strains used in the vaccine [7]. However, prophylactic HPV vaccines would not be effective in controlling existing HPV infections or HPV-associated lesions. Therefore, an important need is to develop therapeutic HPV vaccines focused on patients with active disease and aimed at eliminating or controlling existing infection or disease progression through the specific induction of a strong cell-mediated response. Two HPV gene products, E6 and E7, are expressed in most and perhaps all HPV-related pre- and cancerous cells. These genes are responsible for the transformation of cells, and are required for the maintenance of HPV associated malignancies, thus making them ideal immunotherapeutic targets [8,9]. Therapeutic vaccines that target either HPV-16 E6 or HPV-16 E7 have been previously described [10,11]. However, few DNA vaccines that target both oncoproteins have been developed and demonstrated to have effective anti-tumor activity. Research suggests that the combination of E6 and E7 DNA vaccines may have better anti-tumor potential than either alone [12].
DNA vaccines have emerged as an increasingly important immunotherapeutic strategy for inducing anti-tumor cell-mediated immune responses. Their stability, safety, ease of production, lack of immune interference for boosting and cost efficiency makes them appealing to both medical professionals and manufacturers in the health care industry. However, initial data showed that DNA vaccines exhibited low potency. Accordingly, improving their immuno-potency is critical. Wu et al has been studying different intracellular targeting methods to enhance the potency of DNA vaccines delivered by the gene gun technique. Increased antigen presentation by APCs was increased by linking E7 to heat-shock protein 70 (HSP70) [13,14], a sorting signal and the lysosome-associated membrane protein I [15,16] or calreticulin (CRT) [17,18]. Moreover, several strategies aimed at improving the magnitude of the cellular immune responses induced by DNA vaccines have also been studied, such as codon optimization [19,20], RNA optimization [21,22] and the addition of immunoglobin leader sequences that have weak RNA secondary structure [23], as well as other approaches.
Recent studies have suggested that “centralized” immunogens such as consensus immunogens or ancestral immunogens may be useful to minimize the degree of sequence dissimilarity among different virus strains [24]. In the context of HPV where the initial phases of cell proliferation and tumor growth are slow probably resulting in peripheral tolerance to HPV antigens, the use of non-matched consensus immunogens may bypass this important immunological barrier. In our previous work, we have built novel engineered HIV and Influenza consensus optimized immunogens and the results suggested that these novel immunogens induced broader cellular immune responses than native DNA immunogens [25,26]. Hence, in this study, we proposed to utilize a multi-phase strategy to enhance anti-tumor cellular immune responses against HPV-16. First we generated an engineered HPV-16 optimized consensus E6 and E7 DNA vaccine (pConE6E7). We then tested the immunogenicity using quantitative T cell assays and found that ConE6E7 could induce stronger cellular immune responses compared to a primary E7 DNA immunogen. Both prophylactic and therapeutic studies indicated that ConE6E7 enhanced anti-tumor activities in the HPV-16 TC-1 tumor model system. Finally we observed that vaccination with pConE6E7 could partially overcome immune tolerance and induce anti-tumor effects in E6/E7 transgenic mice.
2. Materials and methods
2.1. HPV-16 E6 and E7 sequences
To generate HPV type 16 consensus E6 and E7 sequences, sixteen E6 and E7 gene sequences of the HPV-16 prevalent variants collected from different countries were selected from GenBank to avoid sampling bias.
2.2. Construction of HPV-16 E6 and E7 consensus sequences
The HPV type 16 E6 and E7 consensus nucleotide sequences were obtained after performing multiple alignment. The consensus amino acid sequences were obtained by translating the consensus nucleotide sequences.
2.3. Modifications of HPV-16 consensus E6 and E7 sequences
Several modifications were performed after obtaining HPV-16 consensus E6 and E7 sequences (Fig. 1A). Codon optimization and RNA optimization was performed by using GeneOptimizer™ (GENEART, Germany).
FIG. 1.
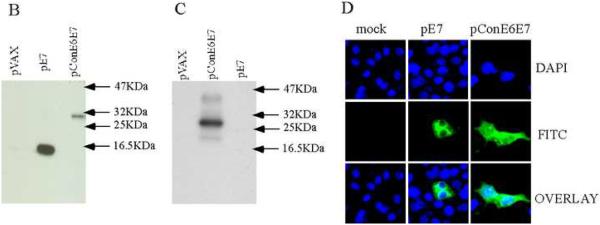
The nucleotide and amino acid sequences of ConE6E7 and expression of pConE6E7 and pE7. (A). The nucleotide and amino acid sequences of ConE6E7. The IgE leader sequence are underlined. The endoproteolytic cleavage site are boxed. The ▲ denotes the sites where deletion of domains required for p53 binding/degradation are performed. The mutation residues are italic. (B and C) In vitro translation to detect the expression of E6 and E7 fusion gene and E7 gene from the pConE6E7 and pE7 constructs, respectively. The gene products were immunoprecipitated using HPV-16 E7 (B) and E6-specific (C) monoclonal antibody, ran on SDS-PAGE gel, and detected by autoradiography. (D) Immunofluorescence assay of ConE6E7 and E7. The transfected RD cells expressing E7 protein showed typical FITC-fluorescence. HPV-16 E7-specific monoclonal antibody served as the source of primary antibody.
2.4. HPV-16 E6 and E7 immunogens
The fusion gene encoding modified HPV type 16 consensus E6/E7 fusion protein (ConE6E7, GenBank accession number: FJ229356) was synthesized and sequence verified by GENEART. The synthesized ConE6E7 was digested with EcoRI and NotI, cloned into the expression vector pVAX (Invitrogen) under the control of the cytomegalovirus immediate-early promoter and this construct was named as pConE6E7.
The primary HPV-16 E7 immunogen was generated from an E7-containing plasmid pE7/HisB. Sequence analysis indicated that this E7 gene was from a primary HPV-16 isolate (GenBank accession number: NC_001526). Briefly, the E7 gene was digested out by using BamHI and EcoRI from pE7/HisB and cloned into pVAX plasmid vector, which was also linearized with BamHI and NotI. This construct was named as pE7.
2.5. In vitro transcription and translation
Expression of pConE6E7 and pE7 was detected by utilizing TNT® Quick Coupled Transcription/Translation System (Promega). The gene product was immunoprecipitated using HPV-16 E7-specific monoclonal antibody (Invitrogen) and E6-specific monoclonal antibody (Invitrogen). After immunoprecipitation, the immunoprecipitated protein was run on the SDS-PAGE gel (12%). The gel was fixed, dried and the synthesized protein with incorporation of radioactive 35S was detected by autoradiography.
2.6. Indirect immunofluorescent assay
An indirect immunofluorescent assay for confirming the expression of pConE6E7 E6 and E7 fusion gene and pE7 E7 gene was performed. Human rhabdomyosarcoma (RD) cells were plated on two-well chamber slides (BD Biosciences), at a density to obtain 60–70% confluency the next day in complete DMEM medium with 10% FBS (GIBCO) and allowed to adhere overnight. The next day cells were transfected with pConE6E7 and pE7 and the control plasmid pVAX (1 ug/well) using DOTAP Transfection Reagent (Roche) according to the manufacturer's instructions. Thirty-six hours after transfection, the cells were washed gently three times with 1XPBS and fixed on slides using methanol for 15 min. Upon removal of the residual solvents from the slides, the cells were incubated with anti-mouse HPV-16 E7 monoclonal antibody (Abcam) at a 1:500 dilution for 60 min at 37°C. The slides were then incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary antibody (Amersham Pharmacia) for 45 min. The samples were viewed under microscope (Olympus, Tokyo, Japan) at an excitation wavelength of 480 nm for FITC. The images were acquired as TIFF files and further analyzed using Adobe Photoshop program.
2.7. Mice and cell line
Female 6-8-week-old C57BL/6 mice were purchased from Charles River Laboratory (Wilmington, MA). The E6/E7 transgenic mice were created on the C57BL/6 background that expresses the HPV-16 E6 and E7 under the control of the bovine thyroglobulin promoter. It is a useful model to determine the ability of HPV-16 E6/E7 vaccines to overcome immune tolerance [27]. The transgenic mice were bred at Penn Animal Facility. The female 4-6-week-old transgenic mice were used for study as described below. Their care was in accordance with the guidelines of the National Institutes of Health and the University of Pennsylvania Institutional Care and Use Committee (IACUC).
The TC-1 cell line is a well-characterized lung epithelial cell line immortalized with both HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene [28]. TC-1 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin, 100 μmol/L non-essential amino acids and 1% sodium pyruvate in a 37°C incubator with 5% CO2.
2.8. IFN-γ ELISpot assay
C57BL/6 or E6/E7 transgenic mice were separated into three groups of 5 mice each and immunized intramuscularly with pVAX (control group), pE7 and pConE6E7, respectively. Each mouse was immunized three times, each of 100ug of DNA at biweekly intervals. pVAX was used to keep the DNA vaccination amount consistent in each group. Mice were sacrificed one week after the third immunization and the spleens were removed aseptically. The spleen cells were collected and resuspended in RBC lysis buffer to remove erythrocytes. After lysis, the splenocytes from the same group were pooled and resuspended in RPMI 1640 medium with 10% FBS. Cells were counted and prepared for analysis. High-Protein Binding IP 96 well Multiscreen™ plates (Millipore, Bedford, MA, USA) were used. Plates were coated with the monoclonal antibody to mouse IFN-γ (R&D Systems, Minneapolis, MN) diluted in PBS, incubated overnight at 4°C. Plates were washed three times with PBS and then blocked for 2h at room temperature with PBS supplemented with 1% BSA and 5% sucrose. Mice splenocytes were added in triplicates at an input cell number of 2 × 105 cells per well resuspended in complete culture medium (RPMI 1640 supplemented with 10% FBS and antibiotics). A set of peptides each containing 15 amino acid residues overlapping by 8 amino acids representing the entire consensus E6/E7 fusion protein sequence of HPV-16 was synthesized from Invitrogen. This set of peptides was pooled at a concentration of 2 ug/ml/peptide into 2 pools, spanning the length of the E6 (pool 1) and E7 (pool 2) antigens, respectively. Concavalin A (Sigma–Aldrich, St. Louis, MO), at 5 ug/ml, and complete culture medium were used as positive and negative control, respectively. Plates were washed four times after a 24 h incubation at 37 °C, in a 5% CO2 atmosphere incubator. Then, a biotinilated anti-mouse IFN-γ detection antibody was added, and plates were incubated overnight at 4 °C. The plates were washed, and color development was followed according to the manufacturer's instructions (ELISPOT Blue Color Module, R&D Systems, Minneapolis, MN). Plates were air-dried and the spots were counted using an automated ELISPOT reader system (CTL Analyzers, Cleveland, OH) with the ImmnunoSpot® software. The average number of spot forming cells (SFC) was adjusted to 1 × 106 splenocytes for data display. The ELISpot assay was repeated three times in three separate experiments.
2.9. CD8+ T-cell depletion study
CD8 lymphocytes were depleted from splenocytes by using immune-magnetic beads coated with the antibody to CD8 (Dynal Biotech Inc., Lake Success, NY) following manufacturer's instructions. After depletion of CD8+ T-cells, IFN-γ ELISpot assay was performed as described above.
2.10. Epitope mapping study
In order to map the reactive epitopes, individual synthesized HPV-16 E6 and E7 peptide was used as a stimulator to perform IFN-γ ELISpot assay as described above. The epitope mapping assay was repeated twice in two separate experiments.
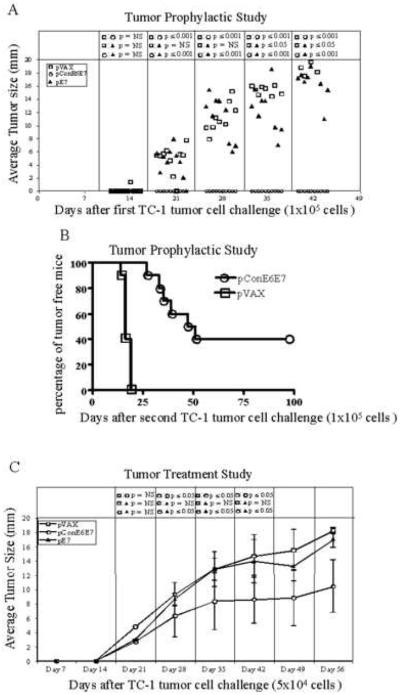
2.11. In vivo tumor prophylactic study
Female 6-8-week-old C57BL/6 or E6/E7 transgenic mice were separated into three groups of 10 mice each and vaccinated intramuscularly with pVAX, pE7 and pConE6E7, respectively. Each mouse was immunized three times, each of 100ug of DNA at biweekly intervals. pVAX was used to keep the DNA vaccination amount consistent in each group. One week after the last vaccination, each mouse was challenged with 1×105 TC-1 cells injected subcutaneously into the right flank. For the second tumor cell challenge, 1×105 TC-1 cells were injected subcutaneously into the right flank of the mice. At the same time, ten naïve mice were also challenged with 1×105 TC-1 tumor cells as a control group. Tumors were measured twice weekly thereafter with digital calipers spanning the shortest and longest surface diameters. The mean of these two measurements was plotted as the mean tumor diameter in millimeters against various time points. Mice were sacrificed when the tumor diameter reached 20 mm in compliance with our Institutional Animal Care and Use Committee protocols. Tumor measurements for each time point are shown only for surviving mice. For comparison of tumor diameters, statistical significance was calculated using the Student's test. P < 0.05 was considered significant.
2.12. In vivo tumor treatment studies
Female C57BL/6 or E6/E7 transgenic mice were separated into three groups of 10 mice each. On day 0, all mice were injected subcutaneously either with 5×104 (low dose) or 1×106 (high dose) TC-1 cells in the right flank. For the low dose challenge, on days 3, 10, and 17, each group of mice was injected intramuscularly with pVAX, pE7 and pConE6E7, respectively. For the high dose challenge, on days 7, 14, 21, 28 and 35, each mouse was immunized with 100ug of each DNA. pVAX was used to keep the DNA vaccination amount consistent in each group. Tumors measurement was performed as described above.
2.13. Adoptive transfer study
Female 6-8-week-old C57BL/6 were separated into two groups of 8 mice each and vaccinated intramuscularly with 100μg of pVAX and pConE6E7 respectively. One week after the third immunization, mice were sacrificed and splenocytes were isolated as described above. The CD8 and CD4 T cells were purified from splenocytes by using immune-magnetic beads coated with antibody to CD8 or CD4 (Dynal Biotech Inc., Lake Success, NY) following manufacturer's instructions. After purification, 1 × 107 CD8 or CD4 T cells were administered intravenously to each naïve mouse (5 mice/per group). Recipient naïve mice were challenged with 1×105 TC-1 cells injected subcutaneously into the right flank three days after CD8 or CD4 T cells injection. Tumors measurement was performed as described above.
2.14. Statistical analysis
Student t-test was used for comparison of the cellular immune responses and tumor diameters. In this study, p<0.05 has been considered statistically significant.
3. Results
3.1. Design and construction of a novel HPV type 16 E6 and E7 consensus-based fusion gene
The consensus sequences of HPV type 16 E6 and E7 proteins were generated from 16 sequences retrieved from GenBank. As summarized in Figure 1A, several modifications were carried out after generating the consensus E6/E7 fusion sequence. A highly efficient leader sequence (IgELS) was fused in frame upstream of the start codon to facilitate the expression [23]. Furthermore, in order to have a higher level of expression, the codon usage of this fusion gene was adapted to the codon bias of Homo Sapiens genes. In addition, RNA optimization [22] was also performed: regions of very high (>80%) or very low (<30%) GC content and the cis-acting sequence motifs such as internal TATA boxes, chi-sites and ribosomal entry sites were avoided. In addition, the domains of the E6 protein required for p53 binding/degradation were mutated or deleted [29,30]. The binding site of the E7 protein to the cellular retinoblastoma (Rb) protein was mutated [31]. An endoproteolytic cleavage site was introduced between E6 and E7 protein for proper protein folding and better CTL processing. The synthetic engineered ConE6E7 gene was 818 bp in length. The ConE6E7 gene was subcloned into the pVAX expression vector at the EcoRI and NotI sites for further study.
3.2. In Vitro and in Vivo Expression of ConE6E7
To verify the expression of pConE6E7 and pE7, in vitro translation was carried out by T7 coupled transcription and translation reaction. After immunoprecipitation with E6 or E7-specific monoclonal antibodies, the expression of E6 and E7 genes was assessed by SDS-PAGE gel (15%). The E6/E7 fusion protein migrated corresponding to a molecular weight of approximately 30 kDa when immunoprecipitated with either E6- or E7-specific antibodies. The primary E7 protein with a molecular weight of about 11 kDa was detected only when immunoprecipitated with E7-specific antibody. As a negative control, no protein band could be detected in the pVAX group (Figure 1B and 1C).
To further confirm the expression of this fusion construct, an indirect immunofluorescent assay was performed using transfected RD cells. High specific expression was observed under fluorescent microscope in the pConE6E7 transfected cells (Fig. 1D). As indicated in Fig. 1D, the transfected cells expressing E6/E7 fusion protein showed a typical FITC-fluorescence, supporting the synthetic fusion protein was expressed and exhibited a relatively native conformation. As a control, expression was not detected in pVAX transfected RD cells.
3.3. Vaccination with pConE6E7 generates strong E6- and E7-specific CD8+ T-cell immune responses in C57BL/6 mice
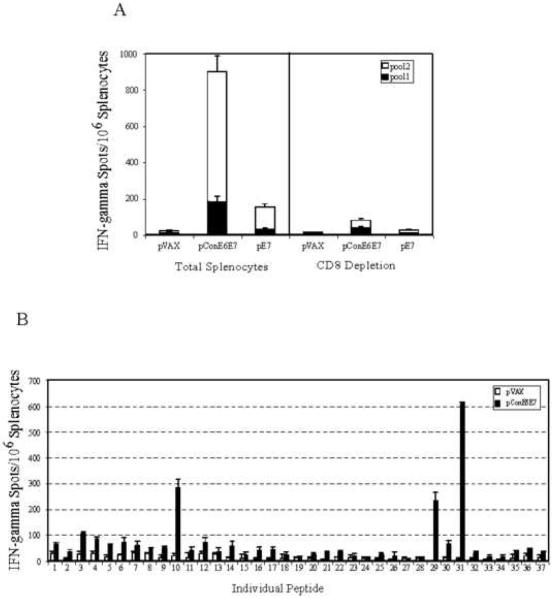
In order to determine whether mice immunized with pConE6E7 or pE7 mount E6 or E7-specific cellular immune responses, ELISpot analysis was performed to determine the number of antigen-specific IFN-γ secreting cells in response to stimulation with the E6 (pool 1) and E7 (pool 2) peptide pools. As shown in Fig 2A, immunization with pConE6E7 was able to induce strong both E6 and E7-specific cellular immune responses. When compared to the pE7 vaccine, pConE6E7 was about five times more potent (720 ± 82.7 vs 142 ± 17.6) in driving the E7-specific immune responses, suggesting that utilization of the multi-phase strategy was able to significantly increase the magnitude of cellular immune responses induced. We also determined whether CD8+ lymphocytes were responsible for the IFN-γ secretion detected in the C57BL/6 mice immunized with pConE6E7. The number of SFU/per million cells were reduced to 80.57 ± 17.56 after CD8+ depletion, indicating that there was more than a 90% decrease in the frequencies of IFN-γ producing cells observed in CD8+ T-cell depleted ELISpot assays. The data supports that the IFN-γ production induced by pConE6E7 is mediated mainly by CD8+ T-cells.
FIG. 2.
pConE6E7 induced strong cell-mediated immune responses in C57BL/6 mice. Frequencies of HPV-16 consensus E6 and E7-specific IFN-γ spot forming units (SFU) per million total splenocytes and CD8 depleted splenocytes after DNA vaccination with pConE6E7 and pE7 were determined by ELISpot assay for C57BL/6 mice (A). The splenocytes were isolated from individual immunized mice (five mice per group) and stimulated in vitro with the overlapping consensus HPV-16 E6 (pool 1) and E7 (pool 2) peptides pools. pVAX immunized mice were included as a negative control. (B) Characterization of HPV-16 E6- and E7-specific dominant epitopes. The splenocytes collected from pConE6E7 vaccinated C57BL/6 mice were cultured with individual HPV-16 E6 and E7 peptide for 24 hours. IFN-γ secreting cells were determined by ELISpot assay as described above. Backbone pVAX immunized mice were included as a negative control.
In addition, we were interested in further detailing the cellular immune responses induced by pConE6E7. Accordingly, ELISpot assay was performed against individual peptides spanning consensus E6/E7 fusion protein (Fig. 2B). IFN-γ ELISpot analysis of splenocytes derived from pConE6E7-vaccinated C57BL/6 mice recognized LCIVYRDGNPYAVCD (peptide 10) and AEPDRAHYNIVTFCC (peptide 31) as dominant epitopes for E6 and E7 immunogens, respectively. These peptides overlap with the well-characterized Db-restricted E6 epitope YRDGNPYAV [32] and Db-restricted E7 epitope RAHYNIVTF [33], supporting the normal effective processing of these two antigens during vaccination. Another E7 peptide (peptide 29), SSEEEDEIDGPAGQA, was recognized as a subdominant peptide for the E7 immungen. This epitope is interesting for further study.
3.4. Vaccination with pConE6E7 completely prevents growth of E6/E7 expressing tumors and elicits anti-tumor memory responses in C57BL/6 mice
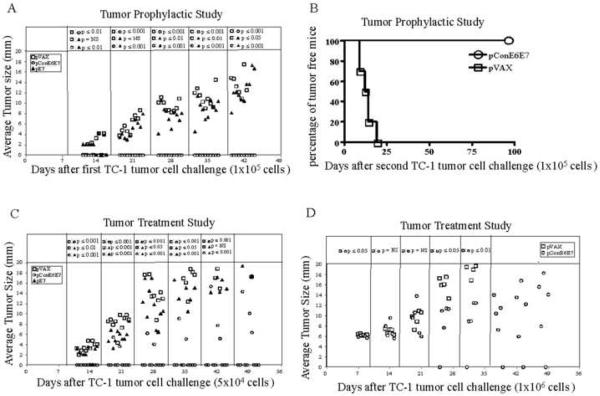
After confirmation of immunogenicity, an in vivo tumor prophylactic study was performed to assess the anti-tumor immunity induced by pConE6E7 or pE7. Prior to subcutaneous injection of 1×105 TC-1 cells on day 0, C57BL/6 mice were immunized three times biweekly with pVAX, pE7 and pConE6E7, respectively. As shown in Fig. 3A, on day 42, all ten mice immunized with pConE6E7 were tumor free. In contrast, the mice immunized with pE7 exhibited tumor growth beginning two weeks after TC-1 injection continuing until the mice required euthanization. However, as expected, the pE7-immunized group exhibited smaller tumors when compared to the pVAX-immunized group (p<0.05). This suggests that vaccination with pConE6E7 is capable of generating stronger antitumor effects to completely prevent growth of tumors in C57BL/mice after prophylactic challenge.
FIG. 3.
Vaccination with pConE6E7 prevents tumor growth upon TC-1 implantation (A), provides long-lasting memory (B) and reduces tumor growth kinetics (C and D) in C57BL/6 mice. For the prophylactic study, C57BL/6 mice (10 mice/per group) were immunized with pVAX, pE7 and pConE6E7, and challenged as described in the Materials and Methods to assess the antitumor effects. Mice were re-challenged with 1×105 TC-1 cells 7 weeks post first challenge. For the therapeutic study, on day 0, 5×104 (C) or 1×106 (D) TC-1 cells were implanted subcutaneously into C57BL/6 mice. Starting on day 3 or 7, each group of mice was immunized with pVAX, pE7 and pConE6E7 at weekly intervals either three times or five times. Tumors were measured twice weekly in two dimensions, and the average of those values was plotted against days post tumor implantations for each mouse. Tumor measurements for each time point are shown only for surviving mice. pVAX immunized mice served as negative control.
In order to determine whether immunization with pConE6E7 could also induce anti-tumor memory responses, we re-challenged these tumor-free C57BL/6 mice in pConE6E7 immunized group with 1×105 TC-1 cells and measured the tumor growth thereafter. The results showed that vaccination with pConE6E7 could still provide complete protection 96 days after second tumor implantation (Figure 3B), suggesting that pConE6E7 may also induce clear anti-tumor memory responses.
3.5. Vaccination with pConE6E7 prevents/delays the growth of tumors in C57BL/6 mice
Given the promising results obtained from the prophylactic tumor studies, in vivo tumor therapy studies were performed to determine the therapeutic effect of vaccination with pConE6E7. We initiated a tumor treatment study by challenging mice with TC-1 tumor cells at low dose (5×104) on day 0. Three days after TC-1 cells implantation, 10 mice/per group were immunized with pVAX, pE7 or pConE6E7 and boosted on day 10 and 17. As shown in Fig. 3C, seven out ten mice in the pConE6E7 vaccinated group were tumor free 49 days after initial tumor implantation. Only three mice in the pConE6E7-vaccinated group developed tumors, but the tumors were smaller than those in the pE7 immunized group (p<0.001). In contrast, all mice in the pE7 group developed tumors 21 days after initial tumor implantation although the tumors in this group were smaller than those in the control group (p<0.05) until day 35. P values were not determined on day 49 since most of the mice either in the control or pE7 group were dead/sacrificed. In order to further assess the therapeutic potential of pConE6E7, we performed another tumor treatment study by increasing the challenge dose of TC-1 cells to 1×106. Moreover, in this study, the mice didn't receive the vaccination until the average tumor size was about 6 mm (on day 7). The growth of the well-established tumors after treatment with pConE6E7 is depicted in Fig. 3D. One out of five mice showed complete tumor regression after vaccination with pConE6E7, and the tumors in the rest of four mice grew much slower compared to those in the control group (p<0.05). Again, p values were not determined after day 35 since all mice in the control group were dead/sacrificed. These data indicated that the DNA vaccine pConE6E7 could prevent or delay the growth of tumors in C57BL/6 mice, even when the mice had a very high tumor burden in a difficult immune therapy model.
3.6. CD8 T cells play the dominant role in control of tumor growth
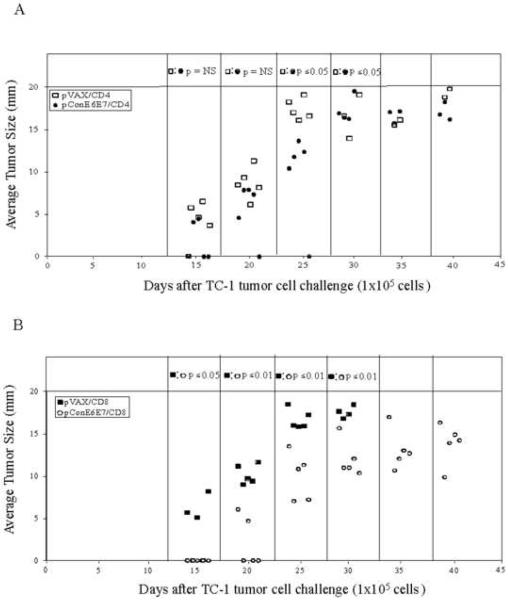
To explore differential antitumor function of anti-E6/E7 CD8 or CD4 cells in vivo, we chose to deliver the T cells systemically and monitor their ability to control tumor growth. As indicated in Fig. 4A, mice adoptively transferred with anti-E6/E7 CD4 cells when challenged showed smaller tumors compared to the mice in the control group until 30 days post TC-1 tumor cell challenge (p<0.05). Importantly, the tumors in mice adoptively transferred with anti-E6/E7 CD8 cells were better controlled compared to these mice adoptively transferred with CD8 cells from the control group (p<0.01) (Fig. 4B). In summary, the data suggest that anti-E6/E7 CD8 cells play a dominant role in control of tumor growth driven by DNA vaccination in this model.
FIG. 4.
CD8 T cells play the dominant role in prevention of tumor growth and mortality. CD4 and CD8 T cells were purified from mice immunized with pConE6E7 and adoptively transferred to naïve mice (5 mice/per group). Each mouse received 1×107 purified CD4 (A) or CD8 (B) cells intravenously and challenged with 1×105 TC-1 cells 3 days post adoptive transfer. Tumor growth was monitored by measuring tumor size. (A) The average tumor size (mm) of each mouse adoptively transferred with CD4 cells over time after tumor implantation. (B) The average tumor size (mm) of each mouse adoptively transferred with CD8 cells over time after tumor implantation. (C) The percentage of surviving mice over time after tumor implantation in each group adoptively transferred with CD4 or CD8 cells. Backbone pVAX immunized mice were included as a negative control.
3.7. Vaccination with pConE6E7 is capable of partially overcome immune tolerance in E6/E7 transgenic mice
Once we demonstrated that pConE6E7 was capable of preventing tumor growth and slowing the growth of well-established tumors significantly in C57BL/6 mice, we applied a more rigorous challenge model to test the efficacy of the DNA immunogen pConE6E7. E6/E7 transgenic mice were used since these mice are more difficult to treat due to the immune tolerance they exhibit to these proteins [27]. Accordingly, they represent a more stringent challenge model system. In this regard the consensus immunogens may have an advantage as it is not entirely homologous with the host HPV antigens and in theory this miss-match could facilitate cross-reactive immune responses, which would bypass peripheral tolerance.
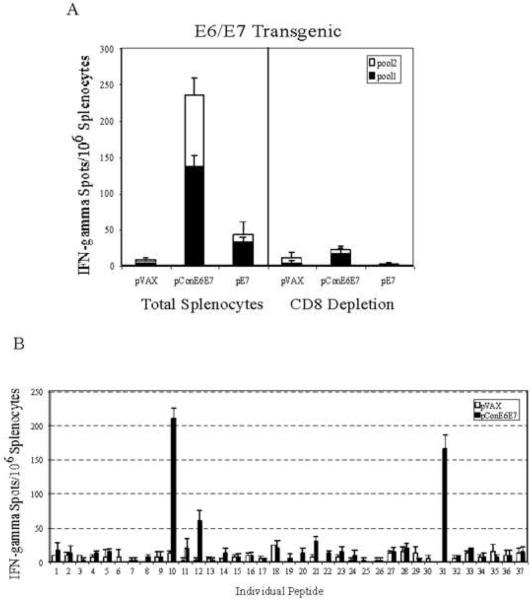
We next examined the immunogenicity of pConE6E7 in these E6/E7 transgenic mice. We observed that although the immune responses induced by pConE6E7 were lower compared to those generated in C57BL/6 mice, similar immunogenicity patterns were still obtained in E6/E7 transgenic mice (Fig. 5A). The number of E7-specific IFN-γ secreting cells in the pConE6E7-immunized mice was 108 ± 17.3, while there was hardly any E7-specific immune response detected in the pE7 immunized transgenic mice (23.6 ± 20). Again, following depletion studies the CD8+ lymphocytes were responsible for the IFN-γ secretion detected in E6/E7 transgenic mice immunized with pConE6E7. Epitope mapping results indicated that the E6/E7 transgenic mice immunized with pConE6E7 recognized the same dominant epitopes both for E6 and E7 immunogens (Fig. 5B). The results suggest that at least potentially the pConE6E7 can overcome immune tolerance in this model.
FIG. 5.
pConE6E7 induced strong cell-mediated immune responses in E6/E7 transgenic mice. Frequencies of HPV-16 consensus E6 and E7-specific IFN-γ spot forming units (SFU) per million total splenocytes and CD8 depleted splenocytes after DNA vaccination with pConE6E7 and pE7 were determined by ELISpot assay for E6/E7 transgenic mice (A). (B) Characterization of HPV-16 E6- and E7-specific dominant epitopes. The splenocytes collected from pConE6E7 vaccinated E6/E7 transgenic mice were cultured with individual HPV-16 E6 and E7 peptide for 24 hours. IFN-γ secreting cells were determined by ELISpot assay as described above. Backbone pVAX immunized mice were included as a negative control.
In order to study whether vaccination with pConE6E7 could elicit antitumor immunity in E6/E7 transgenic mice, a similar prophylactic challenge experiment was performed as was performed in C57BL/6 mice. All ten E6/E7 transgenic mice immunized with pConE6E7 were tumor free 42 days after tumor implantation (Fig. 6A). All mice immunized with pE7 exhibited tumor growth, however the tumors were smaller than those in the control pVAX-immunized group (p<0.05). Therefore, pConE6E7 was also able to generate antitumor immunity and completely prevent growth of implanted tumors in these E6/E7 transgenic mice. When the tumor-free transgenic mice in the pConE6E7 immunized group were re-challenged with 1×105 TC-1 cells to detect E6- and E7-specific memory responses, all rechallenged mice exhibited some resistance to this rechallenge and four out of ten mice vaccinated with pConE6E7 remained tumor free 96 days after TC-1 second challenge (Fig. 6B). These data indicated that the E6 and E7-specific memory responses were induced in these tolerant mice that represent a much more stringent challenge model.
FIG. 6.
Vaccination with pConE6E7 is capable of partially overcome immune tolerance in E6/E7 transgenic mice. (A) Vaccination with pConE6E7 in E6/E7 transgenic mice prevents tumor growth upon TC-1 implantation (B) Vaccination with pConE6E7 in E6/E7 transgenic mice provides long-lasting memory. (C and D) Vaccination with pConE6E7 in E6/E7 transgenic mice reduces tumor growth kinetics. For the prophylactic study, E6/E7 transgenic mice (10 mice/per group) were immunized with pVAX, pE7 and pConE6E7, and challenged as described in the Materials and Methods to assess the antitumor effects. Mice were re-challenged with 1×105 TC-1 cells 7 weeks post first challenge. For the therapeutic study, on day 0, 5×104 TC-1 cells were implanted subcutaneously into E6/E7 transgenic mice (10 mice/per group). Starting on day 3, each group of mice was immunized with pVAX, pE7 and pConE6E7 at weekly intervals three times. Tumors were measured twice weekly in two dimensions, and the average of those values was plotted against days post tumor implantations for each mouse. Tumor measurements for each time point are shown only for surviving mice.
Next, a similar therapeutic study was performed in these transgenic mice as we performed in C57BL/6 mice. We observed that mice immunized with pConE6E7 exhibited smaller tumors compared to those immunized with pE7 or pVAX (p<0.05) (Fig. 6C). Thus, the vaccine pConE6E7 was competent to partially overcome the immune tolerance and slow the growth of the tumors in E6/E7 transgenic mice. Again, these results contrasted with the results in the non-transgenic mice suggest the clear rigorous of this model.
4. Discussion
DNA vaccines have become an attractive approach for antigen-specific immunotherapy. However, increasing the potency of DNA vaccines is still among the most important challenges for DNA vaccine development. In this study, we constructed a novel HPV-16 E6 and E7 fusion antigen using several features of immunogens that have previously been studied to increase the immunogenicity of HIV or influenza DNA vaccines [25,26]. This is the first study to use these collective strategies in a single HPV construct. Our data demonstrate that the combination of these strategies is likely beneficial and that further combined strategies should be examined to improve immune potency.
DNA vaccines have significant potential, compared to traditional protein vaccines, in terms of priming CTL responses and generating memory CD8 T-cell responses [34,35]. This study tested this hypothesis in two models, a C57BL/6 syngeneic challenge model and a novel E6/E7 transgenic mouse model. Vaccination with pConE6E7 provided complete protection of tumor growth even 96 days post the second tumor implantation in C57BL/6 mice, supporting that the strong cellular immune responses were elicited and long-lasting E6- and E7-specific memory T-cell responses were induced. Furthermore, CD4+ T-cell help has been shown to be required for the generation of CD8+ T-cells capable of efficient recall responses to antigen [36]. Vaccination with pConE6E7 could also generate antigen-specific CD4 helper T cells, suggesting a positive effect on MHC Class II presentation. Another major theoretical advantage of DNA vaccination is that these vaccines can be used for repeated administration as the efficacy of plasmid vectors are not influenced by pre-existing neutralizing antibodies [37]. In present study, apparently smaller tumors were observed by vaccination with pConE6E7 five times to treat the well-established tumors. Preexisting immunity to HPV in patients ultimately should not be a substantial issue for boosting in such pattern.
CD8+ T-cells have been shown to be the primary effectors of immune surveillance against intracellular microbes and cancers and are thus prime targets for achieving effective tumor immunity by therapeutic and preventative treatments. It has been suggested that activated CD4 T cells can also have a direct role on tumor growth by recruiting and activating macrophages and driving effector CD8 T-cell help [38]. Prior studies have indicated that a protein-based vaccine (h) hspE7 could prime E7-specific IFN-γ release by CD4+ T cells and regress neoplastic lesions in a mouse model of cervical cancer [39]. In support of these prior findings, in the adoptive transfer study presented in this manuscript, we observed that CD4 T cell-mediated antigen-specific responses induced by pConE6E7 slowed tumor growth. Combination studies with these different approaches might further improve on these results.
Previous studies have suggested that consensus or ancestral immunogens could enhance the breadth of cellular immune responses [25,40,41]. Chan et al performed a phylogenetic analysis of E6 gene sequences collected from 57 HPV types and found that most of these high-risk types were phylogenetically related to either HPV16 (31, 33, 35, 52 and 58) or HPV18 (39, 45, 59 and 68) [42]. Therefore, generation of a HPV 16 type-specific consensus immunogen may be important to maximize potential immune responses, especially at the T-cell level. Importantly, the slow growth and low antigen exposure during the HPV life cycle is hypothetically to drive peripheral tolerance. The consensus immunogens cannot match entirely the endogenous HPV isolate and may therefore provide beneficial in this regard.
Most animal models for HPV vaccine studies are based on analysis of fast-growing transplantable tumors, however, malignancies in cervical and head and neck cancer patients in general develop much more slowly, which as described may lead to immune suppression or immune tolerance [43]. Therefore, whether HPV-specific vaccines are potent enough to overcome the tolerance becomes an important concern. In this regard, research has demonstrated that vaccination of HPV16 E7 protein-based vaccine reduced the growth of pre-established TC-1 tumors although there was no measurable CTL response detected [44]. Previous studies have also indicated that recombinant Semliki Forest virus (SFV) expressing a fusion E6/E7 protein had the capacity to induce HPV16 E7-specific cytotoxic T cells in HPV-transgenic mice [43]. Furthermore, Souders et al found that Listeria-based vaccines can partially break tolerance by expanding low avidity CD8+ T cells capable of slowing the growth of tumors in the same E6/E7 transgenic mouse model used in our study [27]. In contrast, several recent studies showed that immunization with HPV16 E6/E7 DNA vaccines was not able to induce HPV-specific CTL activity in E6/E7 transgenic mice and therefore could not overcome E6 or E7-specific tolerance [43,45]. Therefore, this manuscript provides the first evidence that a HPV E6/E7 DNA vaccine could induce HPV-specific CTL activity and partially overcome immune tolerance in the E6/E7 transgenic mouse model system. However, since peripheral tolerance mechanisms are most likely at work in cervical cancer patients, more studies focusing on determining the contributions of peripheral tolerance to the immune responses induced by pConE6E7 in this transgenic model will be important. The experiment to determine whether immunization with pConE6E7 can reduce the incidence of E6/E7-associated thyroid tumors in these transgenic mice will be also interesting.
In conclusion, we have studied a novel HPV16 DNA vaccine encoding an E6/E7 fusion consensus protein. We observe that vaccination with pConE6E7 can generate prophylactic and therapeutic antitumor effects as well as partially break immune tolerance in E6/E7 transgenic mice. Further examination of these approaches is in progress.
Acknowledgments
This work was supported in part by NIH grants awarded to Dr. David B. Weiner, NCI grant K08 CA097218 awarded to Dr. Duane Sewell. The laboratory notes possible commercial conflicts associated with this work, which may include: Wyeth, VGX, BMS, Virxsys, Ichor, Merck, Althea, and Aldeveron.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Franceschi S, Rajkumar T, Vaccarella S, Gajalakshmi V, Sharmila A, Snijders PJ, et al. Human papillomavirus and risk factors for cervical cancer in Chennai, India: a case-control study. Int J Cancer. 2003;107:127–133. doi: 10.1002/ijc.11350. [DOI] [PubMed] [Google Scholar]
- [2].Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- [3].Gillison M, Koch WM, Capone RB. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- [4].Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- [5].Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- [6].Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines. 2003;2:495–516. doi: 10.1586/14760584.2.4.495. [DOI] [PubMed] [Google Scholar]
- [7].Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat. Rev. Cancer. 2006;6:753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- [9].Eiben GL, Velders MP, Kast WM. The cell-mediated immune response to human papillomavirus-induced cervical cancer: implications for immunotherapy. Adv Cancer Res. 2002;86:113–148. doi: 10.1016/s0065-230x(02)86004-3. [DOI] [PubMed] [Google Scholar]
- [10].Peng S, Trimble C, He L, Tsai YC, Lin CT, Boyd DA, et al. Characterization of HLA-A2-restricted HPV-16 E7-specific CD8(+) T-cell immune responses induced by DNA vaccines in HLA-A2 transgenic mice. Gene Ther. 2006;13:67–77. doi: 10.1038/sj.gt.3302607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lin CT, Tsai YC, He L, Calizo R, Chou HH, Chang TC, et al. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J Biomed Sci. 2006;13:481–488. doi: 10.1007/s11373-006-9086-6. [DOI] [PubMed] [Google Scholar]
- [12].Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006;13:257–265. doi: 10.1038/sj.gt.3302646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll D, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- [14].Hsu KF, Hung CF, Cheng WF, He L, Slater LA, Ling M, et al. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther. 2001;8:376–383. doi: 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- [15].Wu TC, Guarnieri FG, Staveley-O'Carroll KF, Viscidi RP, Levitsky H, Hedrick L. Engineering an intracellular pathway for MHC class II presentation of HPV-16 E7. Proc Natl Acad Sci U S A. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ji H, Wang TL, Chen CH, Pai SI, Hung CF, Lin KY, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–2740. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- [17].Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz M, Boyd DA, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22:3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- [19].Andre S, Seed B, Eberle J, Schraut W, Bultmann A, Haas J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol 1998. 72:1497–1503. doi: 10.1128/jvi.72.2.1497-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R, et al. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 gag protein. J. Virol. 2001;75:10991–11001. doi: 10.1128/JVI.75.22.10991-11001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY, et al. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo. Virology. 2003;314:134–146. doi: 10.1016/s0042-6822(03)00459-8. [DOI] [PubMed] [Google Scholar]
- [22].Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang JS, Kim JJ, Hwang DS, Choo AY, Dang K, Maguire H, et al. Induction of potent Th1-Type immune responses from a novel DNA vaccine for West Nile Virus New York Isolate (WNV-NY1999) J. Infect Diseases. 2001;184:809–816. doi: 10.1086/323395. [DOI] [PubMed] [Google Scholar]
- [24].Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296 doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- [25].Yan J, Yoon H, Kumar K, Ramanathan MP, Corbitt N, Kutzler M, et al. Enhanced diversity and magnitude of cellular immune responses elicited by a novel engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Molecular Therapy. 2007:411–421. doi: 10.1038/sj.mt.6300036. [DOI] [PubMed] [Google Scholar]
- [26].Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner D. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Souders NC, Sewell D, Pan ZK, Hussain SF, Rodriguez A, Wallecha A, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immunity. 2007;7 [PMC free article] [PubMed] [Google Scholar]
- [28].Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky H, August T, Pardoll D, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–16. [PubMed] [Google Scholar]
- [29].Pim D, Storey A, Thomas M, Massimi P, Banks L. Mutational analysis of HPV-18 E6 identifies domains required for p53 degradation in vitro, abolition of p53 transactivation in vivo and immortalisation of primary BMK cells. Oncogene. 1994;9:1869–1876. [PubMed] [Google Scholar]
- [30].Crook T, Tidy JA, Vousden K. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- [31].Boursnell ME, Rutherford E, Hickling JK, Rollinson EA, Munro AJ, Rolley N, et al. Construction and characterisation of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996;14:1485–1494. doi: 10.1016/S0264-410X(96)00117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78:8468–8476. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de JB, Drijfhout JW. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- [34].Van Herck K, Van Damme P, Lievens M, Stoffel M. Hepatitis A vaccine: indirect evidence of immune memory 12 years after the primary course. J Med Virol. 2004;72:194–196. doi: 10.1002/jmv.10574. [DOI] [PubMed] [Google Scholar]
- [35].Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- [36].Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr Opin in Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [37].Kuck D, Leder C, Kern A, Muller M, Piuko K, Gissmann L, et al. Efficiency of HPV 16 L1/E7 DNA immunization: Influence of cellular localization and capsid assembly. Vaccine. 2006;24:2952–2965. doi: 10.1016/j.vaccine.2005.12.023. [DOI] [PubMed] [Google Scholar]
- [38].Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4+ T cells in the antitumor immune responses. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Daniel D, Chiu C, Giraudo E, Inoue M, Mizzen LA, Chu NR, et al. CD4+ T cell-mediated antigen-specific immunotherapy in a mouse model of cervical cancer. Cancer Res. 2005;65:2018–2025. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- [40].Gao F, Weaver EA, Lu Z, Li Y, Liao HX, Ma BJ, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group M consensus envelope glycoprotein. J. Virol. 2005;79:1154–1163. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rolland M, Jensen MA, Nickle DC, Yan J, Learn GH, Heath L, et al. Reconstruction and function of ancestral central-of-tree (COT) Human Immunodeficiency Virus Type 1 proteins. J. Virol. 2007;81:8507–8514. doi: 10.1128/JVI.02683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chan SY, Delius H, Halpern AL, Bernard HU. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J Virol. 1995;69:3074–3083. doi: 10.1128/jvi.69.5.3074-3083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12:1410–1414. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- [44].Gerard CM, Baudson N, Kraemer K, Ledent C, Pardoll D, Bruck C. Recombinant Human Papillomavirus Type 16 E7 protein as a model antigen to study the vaccine potential in control and E7 transgenic mice. Clin Cancer Res. 2001;7:838s–847s. [PubMed] [Google Scholar]
- [45].Michel N, Osen W, Gissmann L, Schumacher TN, Zentgraf H, Muller M. Enhanced immunogenicity of HPV 16 E7 fusion proteins in DNA vaccination. Virology. 2002;294:47–59. doi: 10.1006/viro.2001.1321. [DOI] [PubMed] [Google Scholar]