Abstract
This review summarizes the data on the functional significance of ubiquitous (NKCC1) and renal-specific (NKCC2) isoforms of electroneutral sodium, potassium and chloride cotransporters. These carriers contribute to the pathogenesis of hypertension via regulation of intracellular chloride concentration in vascular smooth muscle and neuronal cells and via sensing chloride concentration in the renal tubular fluid, respectively. Both NKCC1 and NKCC2 are inhibited by furosemide and other high-ceiling diuretics widely used for attenuation of extracellular fluid volume. However, the chronic usage of these compounds for the treatment of hypertension and other volume-expanded disorders may have diverse side-effects due to suppression of myogenic response in microcirculatory beds.
Keywords: Hypertension, Intracellular chloride, Myogenic tone, Neuronal cell, NKCC cotransport, Smooth muscle
Abbreviations: CCC, ≿ation-chloride cotransporters; CNS, central nervous system; EFV, extracellular fluid volume; GABA, γ-aminobutiric acid; GFR, glomerular filtration rate; JGA, juxtaglomerular apparatus; KCC, K+,Cl− cotransport; MD, macula densa; NCC, Na+,Cl− cotransport; NKCC, Na+,K+,2Cl− cotransport; OSR1, oxidative stress response kinase; PVN, paraventricular nucleus; SMC, smooth muscle cells; SNS, sympathetic nervous system; SPAK, Ste20-related praline-alanine-rich kinase; TAHL, thick ascending limb of Henle's loop; TGF, tubuloglomerular feedback; WNK, with no K = lysine kinase
Na+ ,K+, 2Cl− cotransporters (NKCC) encoding by SLC12A2 (NKCC1) and SLC12A1 (NKCC2) belong to the subfamily of cation-chloride cotransporters (CCCs) that provide electroneutral transport of sodium, potassium and chloride across the plasma membrane and are inhibited by bumetanide, furosemide and several other structurally similar compounds (Table 1). Because the major target of these drugs is inhibition of ion transport in thick ascending limb of Henle's loop (TAHL), they were termed as high-ceiling diuretics. Gene structure, membrane architecture and pharmacology of СССs were subjected to detailed analysis in several comprehensive reviews.1, 2, 3 This review focuses on physiological significance of NKCCs and their involvement in the pathogenesis of hypertension via regulation of intracellular chloride concentration and sensing chloride concentration in the renal tubular fluid.
Table 1.
| Gene | Human chromosome localization | Protein | Alternative spicing | Tissue localization | Inhibitors, IC50 (μM) |
|---|---|---|---|---|---|
| SLC12A2 | 5 | NKCC1 | NA | Ubiquitous | Bumetanide, 0.05–0.60 Furosemide, 10–50 |
| SLC12A1 | 15 | NKCC2 | Isoforms A, B and F | Kidney | Bumetanide, 0.10–0.50 Furosemide 15–60 |
| SLC12A3 | 16 | NCC | NA | Kidney | Polythiazide, 0.5 |
| SLC12A4 | 16 | KCC1 | NA | Ubiquitous | Bumetanide, 60 |
| SLC12A5 | 20 | KCC2 | NA | Neurones | Bumetanide, 55 Furosemide, 10 |
| SLC12A6 | 15 | KCC3 | Isoforms A and A | Neurones | Bumetanide, 40 Furosemide, 25 |
| SLC12A7 | 3 | KCC4 | NA | Ubiquitous | Bumetanide, 900 Furosemide, 900 |
NA, information not available.
Functional implications of NKCCs
Cell volume regulation
Animal cells maintain their volume with an accuracy of 1%–2% by means of systems providing inwardly and outwardly directed fluxes of monovalent ions and organic osmolytes and termed as regulatory volume increase (RVI) and regulatory volume decrease (RVD), respectively.4, 5 In early studies with mammalian erythrocytes, we and others have shown that cell shrinkage and swelling result in activation of NКCC and K+, Cl− cotransport (KCC), respectively.6, 7, 8 We also demonstrated that in vascular smooth muscle cells (VSMC) subjected to hyperosmotic shrinkage, RVI is caused by activation of NКCC.9 It is noteworsy that under the baseline isosmotic conditions, inhibition of NKCC by bumetanide does not significantly affect the volume of human lung fibroblasts (Table 2), suggesting that in the absence of external stimuli, NКCC has a minor impact on the generation of net osmolite fluxes in these cells. However, under inhibition of the Na+, K+-ATPаse by ouabain, treatment with bumetanide resulted in ∼2-fold elevation in the volume of human lung fibroblasts (Table 2). This observation suggests that dissipation of transmembrane gradient of monovalent cations evoked by Na+, K+-ATPаse inhibition results in generation of inwardly-directed ion flux mediated by NКCС1, the only isoform of NКCС expressed in these cells. Functional consequences of the disturbances of cell volume regulatory machinery are discussed elsewhere.4, 10, 11
Table 2.
Effect of ouabain and bumetanide on the volume of human lung fibroblasts.
| Additions | Cell volume, pl per cell |
|---|---|
| None (control) | 0.23 ± 0.04 |
| Ouabain | 0.26 ± 0.04 |
| Bumetanide | 0.22 ± 0.01 |
| Ouabain + bumetanide | 0.43 ± 0.04* |
Cells were incubated in the presence of 0.1 μM ouabain and/or 10 μM bumetanide for 24 h. Cell volume was measured as 14C-urea available space.9 Means ± S.E. obtained in experiments performed in quadruplicate are shown. *p < 0.005 compared to control.
NKCC as a regulator of intracellular Cl− concentration
In all types of cells studied so far, СССs generate both inwardly and outwardly-directed ion movements and the direction of net flux depends on carrier's stoichiometry and transmembrane gradients of ≿ations created by Na+, K+-АТPase. Thus, the stoichiometry 1:1 predicts that the value of ion flux is in direct proportion to concentration of co-transporting ions. Because [Na+]o>>[Na+]i, [K+]i>>[K+]о аnd [Сl−]o>[Cl−]i, net fluxes generated by Na+, Cl−cotransport (NCC) and KCC exhibit inward and outward directions, respectively. In the majority of cells [Сl−]o2>>>[Cl−]i2, and net flux mediated by electroneutral NKCC functioning with the stoichiometry 1Na+:1K+:2Cl− has inward direction.
The above consideration suggests that СССs play a key role in the regulation of [Cl−]i whereas their contribution to the adjustment of intracellular concentration of monovalent cations is negligible because of the highly active Na+, K+-pump. Indeed, it was shown that inhibition of NCC and NКCC results in attenuation of [Cl−]i whereas suppression of КСС activity increases this parameter.12 Importantly, the alteration of CCC activity may lead to adjustment of [Cl−]i above or below the values corresponding to Nernst's equilibrium potential. This means that in cells abundant with anion channels, СССs contribute to maintenance of electrical potential thus having an impact on the whole spectrum of cellular functions controlled by potential-sensitive proteins localized within the plasma membrane. This conclusion is supported by data discussed in the next sections.
VSMC contraction
In skeletal and cardiac muscle, plasma membrane permeability for К+ (PK) plays a major role in the maintenance of electrical resistance and resting potential (Em). In contrast, the values of PK and PCl in smooth muscles are about the same,13 suggesting that in VSMC CCCs may be involved in regulation of the [Cl−]i/[Cl−]o ratio and therefore of Em and excitation-contraction coupling. Indeed, NKCC inhibition by furosemide or bumetanide resulted in a decreased [Cl−]i14, 15 and led to hyperpolarization of rat VSMC.14 These data suggest that decreased baseline tone seen in smooth muscles treated with Henle's loop diuretics,16, 17, 18 as well as attenuation by these compounds of contraction of smooth muscle strips evoked by modest increment of [K+]o,15 by electrical stimulation,19 by histamine,20 angiotensin II,21 thrombaxane A2,22, 23 oxitocyn,24, 25 α-adrenergic15, 26, 27, 28 or purinergic29 agonists is caused by Cl−i-dependent hyperpolarization and suppression of the activity of voltage-gated L-type Ca2+ channels.
Myogenic response
Myogenic tone (response) is a unique property of small (<100–200 μm) blood vessels to decrease rather than increase their inner diameter in response to elevated intraluminal pressure. Both kinetic and the amplitude of myogenic response are different in blood vessels of different origins. Importantly, myogenic response in blood vessels from the brain, skeletal muscle and renal afferent arteriole provides constant blood supply of these tissues independently of the changes in the systemic blood pressure.30, 31, 32
It was reported that bumetanide decreased the myogenic tone of mesenteric arteries33 and completely abolished myogenic response of renal afferent arteriole.23 We demonstrated that inhibitory action of bumetanide (but not of L-type Са2+ channel blocker nicardipine) on the myogenic response as well as on contraction triggered by α-adrenergic stimulation is absent in mesenteric arteries from NKCC1−/− mice.33 Because NKCC2 is not expressed in SMC, these data suffest that bumetanide and other loop's diuretics inhibit contraction and myogenic response of vascular SMC via their interaction with NKCC1, i.e., an ubiquitous isoform Na+, K+, 2Cl− cotransporter.
Synaptic transmission
Neuron–neuron interactions are controlled by neurotransmitters via regulation of transduction of electrical signals. Excitatory and inhibitory neurotransmitters lead to depolarization and hyperpolarization of postsynaptic membrane, respectively. For example, ionotropic glutamate receptor and acetylcholine receptors cause depolarization of postsynaptic membrane via increment of ion current mediated by ion channels permeable for Na+ and Ca2+. In contrast, hyperpolarization results from the increment of the permeability of K+ channels triggered by activation of metabotropic acetylcholine receptors. Unlike above-listed neurotransmitters, gamma-аminobutiric acid (GABA) increases permeability for Cl− and other low molecular weight anions via its interaction with ionotropic GABAA receptors. Direction of net flux mediated by these receptors is determined by transmembrane chloride gradient and electrical potential of postsynaptic membrane. In case RT/F × ln([Cl−]o/[Cl−]i)<Em, the net chloride flux will be directed into the cells which will lead to hyperpolarization and attenuation of neuronal activity. An elevation of [Cl−]i changes the direction of net chloride flux and in this case activation of GABAA receptors leads to elevation of neuronal activity.
Keeping this in mind, one may assume that the ratio of activity of NKCC1 and КСС2, providing inwardly and outwardly directed Cl− fluxes in neuronal cells, respectively, plays a key role in the function of GABAA receptors. Indeed, the attenuation of NKCC1 activity on the background of elevated КСС2 is the major mechanism of the alteration of the functional properties of GABAA receptors in central neuronal system (CNS) of mammals during ontogenesis: GABAA receptors function as activatory receptors in prenatal stage but became inhibitory ones few days after birth (for review see34, 35, 36).
Salt reabsorption by NKCC2
A major impact of NKCC in salt reabsorption and regulation of extracellular fluid volume (EFV) is well-established by the clinical use of furosemide and other inhibitors of these carriers as potent diuretics,37 as well as by salt-lasting characteristic of the loss-off-function mutations of NKCC2 that underpin type I Barter syndrome.38 Importantly, in addition to salt reabsorption in the TAHL, NKCC2 contributes to the regulation of salt excretion via tubuloglomerular feedback (TGF) in the juxtaglomerular apparatus (JGA) neighbouring to the distal segment of TAHL and consisting of epithelial cells of macula densa (MD), mesangial cells and VSMC. Such location is unique along the nephron and plays a key role in the JGA function. Indeed, [NaCl] in renal fluid delivered to the MD is in the range of 20–60 mM. This is in sharp contrast to the proximal tubule, where concentrations of most solutes deviate modestly from those in plasma (for more details, see 41, 40, 39). TGF is triggered immediately after elevation of salt concentration in the tubular fluid delivered to the JGA and results in the contraction of VSMC of afferent arterioles, thus causing increases in the exposure of proximal tubules to high-salt fluid via the attenuation of glomerular capillary pressure and the glomerular filtration rate (GFR). As a consequence of this negative feedback loop, salt delivery to the distal nephron is kept within a narrow range. This process facilitates the fine adjustment of salt handling in the distal tubules by corticosteroids and peptide hormones, such as aldosterone and arginine vasopressin (AVP).42, 43
Similar to TAHL, the apical membrane of the MD cells is abundant in NKCC2, which provides up to 80% of apical NaCl entry in these cells.44, 45, 46 Early studies of Wilcox and co-workers demonstrated that changes in the plasma [NaCl] affect renal blood flow in dogs mainly via modulation of plasma chloride concentration.47, 48 It was also shown that regulation of renal blood flow is mediated by activation of [Cl−]o-sensitive, osmolality-independent TGF.49, 50, 51 Because of its stoichiometry (1Na+:1K+:2Cl−), the [Cl−]o sensing by NKCC2 has an advantage compared to monovalent cations. Indeed, unlike Michael–Menten's pattern for Na+ and K+ dependencies, binding of Cl− to NKCC is a cooperative process with a Hill coefficient of 2,52 providing high-efficiency regulation of carrier activity in the range of [Cl−]o existing in the JGA and close to the EC50 value of the transporter.
Using isolated rabbit cortical TAHL with attached glomeruli, Laamerti and co-workers determined that NKCC in MD cells was activated by Na+o and Cl−o with the EC50 of 1.0 and 17 mM, respectively.45 Importantly, three alternatively-spliced NKCC2 isoforms (A, B and F) cloned from rabbit53 and mouse54 cDNA libraries. These splice variants, are differently distributed along the nephron: the NKCC2B and the NKCC2A are co-expressed in the MD, whereas the NKCC2F is prevalent in the medullary TAHL.55, 56, 56, 57, 57 In Xenopus oocytes, the EC50 values of mouse NKCC2B for K+o, Na+o and Cl−o are 0.8, 3.0 and 12 mM, respectively.58 Very similar EC50 values for Na+ and Cl− were obtained in a study of the rabbit NKCC2B.59 In Xenopus oocytes injected with NKCC2A, the EC50 values for Na+ are very close to the those for NKCC2B, whereas the affinity for Cl− is 2–5 folds lower.58, 59 It was also shown that the NKCC2F-transfected oocytes are more energy-efficient in Na+ uptake thus indicating a key role of this splice-variant in overall salt reabsorption in TAHL.60 Indeed, experiments performed in gene knock-out animals demonstrated that NKCC2B and NKCC2A contribute to salt absorption and MD function in the low and high NaCl concentration ranges, respectively.61, 62 Importantly, the range of NKCC activation by Cl−o was consistent with the range of modulation of TGF and renin production by the apical NaCl and was similar to the Cl− concentration in tubular fluid delivered to rat distal tubules.63 Downstream signaling events triggered by acute changes in apical [Cl−] were discussed in several comprehensive reviews.2, 44, 64
Salt secretion by NKCC1
Unlike apical location of NKCC2 in the absorptive epithelia, NKCC1 was found in the basolateral membrane of the secretory epithelia.65 Several laboratories including our team reported that activity of NKCC is decreased by 2–3 folds in erythrocytes from Blacks compared to Caucasians.66, 67, 68, 69, 70 Keeping in mind that NKCC1 is the only isoforms expressed in erythrocytes, we proposed that attenuated activity of this carrier evokes salt retention seen in African-Americans via its manifestation in the basolateral membrane of sweat glands.71 Several observations favour this hypothesis. First, both bumetanide-sensitive 86Rb uptake and chloride fluxes across secretory epithelium are suppressed in NKCC1−/− knock-out mice.72 Second, in humans, sweat glands contribute to up to 50% of total salt and water excretion during extensive exercise and psychoemotional activity.73 Third, in a hot environment, the function of sweat glands as a potent regulator of EFV is suppressed in Blacks compared to Caucasians.74 Fourth, salt retention by sweat glands results in augmented salt loading of renal distal tubule, which, in turn, leads to apical membrane depolarization and enhanced K+ secretion.75 The latter is consistent with more than a 5-fold prevalence of unprovoked hypokalemia detected in African-Americans compared to Caucasians.76
In contrast to human, sweat glands have a negligible impact on EFV regulation in rodents and other small mammals.77 Because of this, NKCC1−/− knockout mice possessing slightly attenuated blood pressure than wild-type animals72 cannot be used to examine the sweat gland-mediated mechanism of NKCC1 involvement in salt handling and hypertension. Keeping this comment in mind, the comparative analysis of Na+,K+,2Cl− cotransport and transcellular ion transport in secretory epithelial cells from Blacks and Whites is probably the best approach to examine the relative impact of NKCC1 in blood pressure regulation via its implication in the EFV adjustment.
Role of NKCC in the pathogenesis of hypertension
Elevation of systemic blood pressure has been documented in 25% of adults and is a major risk factor for stroke, heart failure, renal disease resulting in premature invalidization and death.78 A priori, elevation of the systemic blood pressure may be a consequence of the increase of peripheral resistance of blood flow, heart rate and EFV. In turn, above-listed parameters are under the control of dozen of hormones, neurotransmitters and sympathetic nerve system (SNS) affecting heart, blood vessels and renal function.79 In several forms of systematic hypertension, servomechanisms underlying long-term elevation of blood pressure are well-documented. This is the case of hypertension caused by adrenal tumors and renal insufficiency as well as by a set of single gene mutations in monogenous hypertension and hypotension. These forms of disease found in less than 5% of patients with elevated blood pressure are combined by common name of a secondary hypertension. In the rest of patients with primary or essential hypertension, the mechanisms of blood pressure elevation remain poorly understood.
Monogenous forms of secondary hypertension and hypotension
Monogenous forms of hypertension and hypotension identified so far are caused by mutations of genes involved in regulation of EFV by renal epithelial cells.80, 81 This is consistent with a key role of the kidney in long-term maintenance of elevated blood pressure as described by Artur Guyton.82 Among monogenous forms of hypertension and hypotension, three types of mutations result in altered function of СССs. The loss-off-function mutations of NKCC2 and NCC detected in patients with Barter type I and Gitelman syndrome, respectively, lead to attenuated salt reabsorption in thick ascending limb of Henle's loop and distal nephron, which, in turn, results in a decreased EFV and systemic blood pressure.38, 83 In both diseases inherited in accordance with classic Mendel's genetics, hypotension is accompanied by hypokalemia and alkaloidosis – universal markers of decreased reabsorption of salt in distal nephron. In contrast, in patients with pseudohypoaldosteronism type II (РНАII) also known as Gordon's syndrome, hypertension is caused by mutations in with-no-K(lysine) kinases WNK1 and WNK4 resulting in activation of NCC, increased sodium reabsorption and hyperkalemia.84
Primary hypertension
Unlike monogenous forms of secondary hypertension, elevation of blood pressure in primary hypertension is a consequence of a complex combination of inheriting traits and several environmental factors including limited physical activity, obesity, smoking, the augmented consumption of salt and alcohol. Inherited traits are probably caused by altered function of 4–5 genes whose combination may be different even within the same human population,85 underlining mosaic origin of the pathogenesis of essential hypertension as noted for the first time by Pickering.86 Up-to-now, there are no reports on mutations of NKCC2 and other renal-specific CCCs in patients with essential hypertension. Schiebl and co-workers found that in mice differential splicing of NKCC2 pre-mRNA is modulated by dietary salt intake,87 which may be triggered by recently discovered [Na+]i/[K+]i-mediated excitation-transcription coupling (for review, see88). Enhanced tubular reabsorption of salt and osmotically-obliged water is important determinant of obesity-related hypertension. Davies and co-workers reported that increased activity of NKCC2 in high-fat diet mice is caused by STE20/SPS1-related proline/alanine-rich kinase SPAK/OSR1-mediated phosphorylation of this carrier at serine-126.89 Additional studies should be performed to examine the role of this signaling pathway in the regulation of renal-specific CCCs in the pathogenesis of essential hypertension.
In the early studies, it was shown that elevated permeability for monovalent cations of VSMC90 as well as erythrocytes from spontaneously hypertensive rats (SHR)91 and patients with essential hypertension92 is at least partially caused by augmented activity of NKCC (for review, see.93, 94, 95, 96, 97). Because NKCC1 is the only isoform of Na+, K+,2Cl− cotransporters identified in erythrocytes, these data indicate that at least in these experimental models of human primary hypertension, augmented activity of this carrier contributes to activation of servomechanisms for long-term elevation of systemic blood pressure. This conclusion can be supported by several observations. First, in erythrocytes of F1 hybrids obtained by crossing of Milan hypertensive strain (MHS) and Milan normotensive strain (MNS) rats and subjected to X-ray irradiation, NKCC activity was increased after transplantation of bone marrow from MHS but not from MNS.98 Second, in erythrocytes of F2 MHS × MNS hybrids as well as F2 hybrids obtained by crossing of SHR and normotensive Wistar-Kyoto (WKY) rats, NKCC activity positively correlated with blood pressure.98, 99 Third, several researchers demonstrated decreased blood pressure in NKCC1−/− knock out mice.72, 100, 101 Fourth, administration of bumetanide, a potent inhibitor of Na+, K+,2Cl− cotransport, decreased blood pressure in wild-type but not in NKCC1−/− mice.27
These findings raise a question on the mechanisms for the involvement of NKCC1 in the pathogenesis of hypertension. Data considered above suggest that these mechanisms may involve NKCC1-mediated regulation of [Cl−]i which, in turn, affects VSMC contraction and SNS activity. Indeed, it was shown that inhibitory action of bumetanide on the contraction of mesenteric arteries evoked by activation of α-adrenergic receptor is increased in SHR compared to normotensive controls.102, 103 Due to the methodological problems, data on the activity of NKCC in freshly isolated VSMC in primary hypertension are limited to few publications.94, 95 It was shown, however, that the content of NKCC1 mRNA and immunoreactive protein is increased in aorta and heart from SHR.102 Elevation of sympathetic tone may lead to elevation of systemic blood pressure via its impact on cardiovascular system and the kidney.104, 105, 106, 107 This hypothesis is consistent with numerous reports on activation of SNS in patients with essential hypertension104 and SHR.108
The major role of CNS in activation of paraventricular nucleus (PVN) in hypothalamus possessing hyperactivity in primary hypertension is well documented.109, 110, 111 It is also known that presynaptic neurons in PVN are activated by excitatory glutamatergic neurones and suppressed by inhibitory GABAergic neurons, respectively.112 Importantly, activity of GABAergic neurons is decreased in PVN of SHR.112, 113 As mentioned above, the relationship between the inhibitory and stimulatory actions of GABAА receptors is determined by intracellular chloride concentration that is under the control of the ratio of NKCC1 vs. KCC2 activities. Recent studies demonstrated that the value of the equilibrium potential for GABAА receptors (ЕGABA) is shifted to positive values in PVN of SHR by 15 mV, which corresponds to a 2-fold elevation of [Cl−]i compared to normotensive control.114 This difference as well as decreased inhibitory activity of GABAergic neurons in SHR was abolished by addition of low doses of bumetanide but not furosemide. These results allowed authors to propose that augmented [Cl−]i in SHR neurons is caused by activation of NKCC1 rather than inhibition of КСС2. This conclusion is in agreement with elevated of NKCC1 mRNA and immunoreactive protein levels with no changes in КСС2 content in PVN of SHR.114
Mechanisms of elevation of NKCC1 activity in primary hypertension remain poorly investigated, which probably reflects the polygenic and mosaic origin of this disease as well as diverse mechanisms of regulation of the activity and expression of CCCs. For example, elevation of [Ca2+]i activates whereas production of cAMP inhibits this carrier in VSMC.26, 115, 116 Numerous investigations documented abnormal activity of both signaling systems in primary hypertension.117, 118 Recent studies demonstrated a key role of WNK, SPAK and OSR1 kinases in regulation of several CCCs including NKCC1 and NKCC2 (for reviews, see119, 120, 121, 122). Bergaya and co-workers reported that both phosphorylation of NKCC1 and increment of blood pressure evoked by activation of α-adrenergic receptors is decreased in Wnk+/− mice.123 Bumetanide-sensitive component of vessel contraction was also attenuated in SPAK knock-out mice.124 To further examine the role of this signalling pathway, Rafiqi and co-workers generated knock-in mice in which SPAK cannot be activated by WNKs. These animals display reduced salt-dependent increment of blood pressure as well as decreased expression of NKCC2 and NCC proteins with no changes in mRNA level.125 It is noteworthy, however, that there is no evidence for mutations of genes encoding СССs or WNK/SPAK/ОSR1 regulatory pathway in primary hypertension in contrast to monogenic hypertension.
Epigenetic phenomena include DNA methylation, post-translation histone modification and expression of noncoding RNAs. Epigenetic mechanisms are under complex control of diverse environmental factors, which make them crucial for their involvement in the pathogenesis of hypertension and other complex and multifactorial disorders.126 Recent studies suggest that NKCC1-mediated abnormalities of ion transport have epigenetic origin.127 It has been shown that the contents of NKCC1 mRNA and protein are increased in aorta, heart and PVN neurones from rats with spontaneous hypertension.102, 114 In SHR aorta and heart, increased expression of NKCC1 is accompanied by attenuated methylation of its promoter.102 Importantly, methylation of NКСС1 promoter exhibited age-dependent increase in normotensive rats but not in SHR. It was also shown that the activity of DNA methyltransferase 3В (DNTB3B) is 3-fold higher in normotensive rats as compared to age-matched SHR.103 These results suggest that in this experimental model of primary hypertension, decreased activity of DNTB3B results in hypomethylation of NКСС1 promoter, which in turn leads to augmented NKCC1 expression, increment of [Cl−]i, depolarization and contraction of SMC, increased vascular resistance and elevation of blood pressure.
The role of epigenetic factors in augmented expression of NKCC1 in neurones of PVN of SHR, controlling activity of SNS, remains unknown. However, it was shown that in PVN from SHR, NKCC1 is highly glycosylated, which may contribute to increased content of membrane-bound protein, i.e., the fraction of the carrier providing Na+, K+,2Cl− cotransport.114 Thus, glycosylation of NKCC could represent another level of its regulation.
Complications caused by elevated blood pressure
The major cause of the premature death of patients with essential hypertension is a damage of the target organs such as brain vessels and the kidney due to chronic elevation of local blood pressure.128 Blood pressure elevation in the brain microcirculation increases the probability of nonreversible damage of the blood flow that results in stroke, whereas in the kidney high blood pressure leads to structural alterations in the nephron, changes of salt-water homeostasis and proteinuria.78
Keeping in mind that Rbf ∼ 1/d4 (where Rbf is the blood flow resistance and d is the inner vessel's diameter),129 myogenic response plays a key role in the protection of target organs from elevation of systemic arterial pressure. It was shown that chronic suppression of myogenic response in patients with essential hypertension as a consequence of hypertrophy of vessel's wall leads to attenuation of its sensitivity to the changes of intraluminal pressure. As a result of these changes, an increment of systemic blood pressure is transferred to microcirculation beds incorporated into the brain, heart, retina, kidney, which causes irreversible changes in the structure and function of these and other target-organs of hypertension.130, 131
To investigate the role of myogenic response in the kidney function, Loutzenhiser and co-workers employed isolated perfused kidney, which allowed them to study renal microcirculation in the absence of its modulation by JGA.132 Using this model, they have shown that bumetanide completely suppresses myogenic response of afferent arteriole in the rat kidney.23 Together with the absence of myogenic response in Nk≿≿1−/− mice33, these results may suggest that augmented activity of NKCC1 in SHR, MHS rats and in patients with essential hypertension protects kidney from the damage by prolonged elevation of the systemic blood pressure whereas chronic administration of furosemide and other NKCC inhibitors accelerates the renal insufficiency and proteinuria.71, 96, 97 In other words, high activity of NKCC1 in VSMC of the afferent arteriole maintains the constant renal blood flow even after elevation of systemic blood pressure caused by activation of this carrier in mesenteric arteries and other vessels contributing to regulation of peripheral resistance (Fig. 1). This hypothesis is consistent with 4-fold increase of renal complications in African-Americans with hypertension possessing up to 3-fold attenuation of NKCC activity in erythrocytes as compared to age-matched hypertensive Caucasians.70, 71 The relative contribution of Са2+ channels and NKCC1 in the myogenic response of coronary and SNS microcirculatory beds remains unexplored.
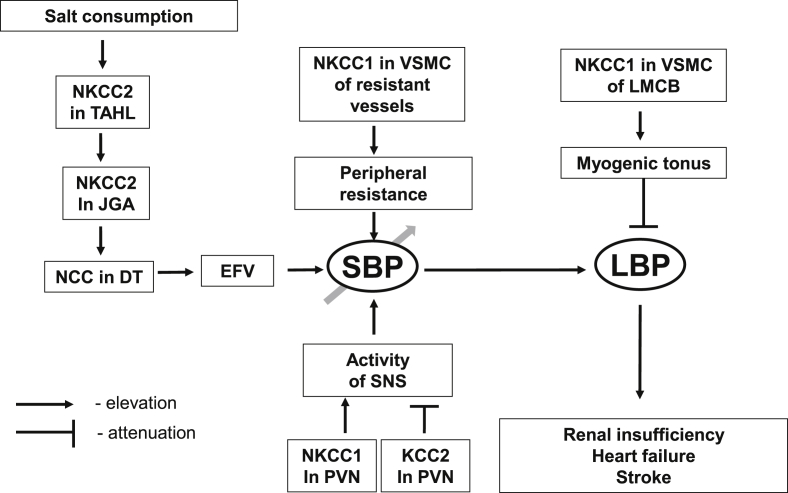
Figure 1.
The scheme showing implication of cation-chloride cotransporters in the pathogenesis of essential hypertension and its cardiovascular and renal complications. Augmented salt consumption leads to elevation of extracellular fluid volume (EFV) via salt reabsorption by NKCC2 in the thick ascending limb of Henle's loop (TAHL), NCC in distal tubule (DT) and sensinig of Cl− conceration in tubular fluid delivered to juxtaglomerular apparatus (JGA) by NKCC2. Augmented activity of NKCC1 in vascular smooth muscle cells (VSMC) resulted in elevation of peripheral resistance and systemic blood presure (SBP). Augmemted activity of NKCC1 and attenuated of KCC2 in neuronal cells of paraventricular nucleus (PVN) also contributes to SBP elevation via activation of sympathetic nervous system (SNS). On the other hand, augmented NKCC1 activity in VSMC of local microcirculatory bed (LMCB) resulted in suppression of myogenic tonus, elevation of local blood pressure (LBP) that, in turn, contributes to the pathogenesis of renal insufficiency, heart failure and stroke.
Ischemia is the major consequence of even a brief disturbance of blood circulation in the brain vessels that leads to irreversible damage of the neuronal function. It was shown that attenuation of the oxygen partial pressure results in astrocyte swelling, which in turn leads to a release of glutamate and other neurotransmitters triggering massive Ca2+ influx in neurones and their death.133, 134 It was shown that bumetanide suppresses astrocyte swelling, neurotransmitter's release135 and the death of neurones in the hippocampus subjected to hypoxia and hypoglycemia.136 Moreover, both [K+]o-induced swelling and release of neurotransmitters evoked by ischemia are sharply decreased in astrocytes from NKCC1−/− mice,137 thus indicating that activation of NKCC1 is a mechanism for astrocyte swelling. We have reported that elevation of [HCO3–]o decreases NKCC activity in VSMC from rat aorta.138 Thus, activation of NKCC1 under hypoxic conditions might be caused by acidosis-mediated attenuation of [HCO3−] in cerebrospinal fluid. Hypoxia is also accompanied by sharp attenuation of intracellular ATP content and inhibition of the Na+,K+-ATPase.139 As shown in Table 2, inhibition of the Na+,K+-ATPase changes the direction of NKCC1-mediated net ion fluxes and evokes cell swelling. Additional experiments should be performed for evaluation of the relative impact of Na+,K+-pump and NKCC1 in regulation of astrocyte volume in ischemic conditions.
Conclusion
The data discussed in this review leads to several conclusions.
First, the transport of monovalent ions across the cells as well as the regulation of intracellular chloride concentration and of cell volume are major functions of NKCCs. In renal epithelial cells, reabsorption of salt and osmotically-obliged water is provided by NKCC2, whereas NKCC1 has a major impact on [Cl−]i, in VSMC and neuronal cells.
Second, in primary hypertension, NKCC1 activity is increased in VSMC and neurons of PVN leading to elevation of peripheral resistance in systemic circulation and activation of SNS, respectively. In both cases, these abnormalities are mediated by elevation of [Cl−]i and plasma membrane depolarization.
Third, furosemide and other loop's diuretics decrease systemic blood pressure via inhibition of NKCC2 in TAHL and of NKCC1 in smooth muscles of resistant vessels. However, the same compounds suppress the myogenic response in microcirculatory beds of the kidney and the brain thus increasing the risk of renal and cerebral complications.
In spite of the progress in the understanding the role of NKCC in the regulation of cellular functions at physiological and pathophysiological conditions, several questions remain unanswered. In cultured VSMC as well as in isolated blood vessels, NKCC1 is activated by phenylephrine, angiotensin II and other vasoconstrictors and is inhibited by vasodilators whose action is mediated by cAMP.26, 115 Recently, Danielsson and co-workers have reported that the combination of Cl-channel blockers and bumetanide potentiates relaxation of airway smooth muscle rings by β-adrenergic agonists.140 Hence, the question is whether NKCC1 contributes to the regulation of vascular tone by these compounds. A key role of NKCC1 in regulation of myogenic response of renal afferent arteriole is well-documented. The question is what is the relative contribution of NKCC1 to the regulation of myogenic response of microcirculatory beds of the brain and other target-organs of hypertension.
In vitro experiments have demonstrated that loop's diuretics might be used as a pharmacological tools augmenting inhibitory function of GABAA receptors. It was shown that at least furosemide fluently penetrates human blood–brain barrier.141 This finding together with the data discussed in the previous sections suggests that high-ceiling diuretics may be used for the regulation of sympathetic tone via inhibition of NKCC1 in neuronal cells. Indeed, it was shown that orally administrated furosemide at doses commonly used in clinic reduced spinal inhibitory interneuronal activity.142 It is important to point out that currently used high-ceiling diuretics exhibit the same affinity for NKCC1 and NKCC2. Because an apparent affinity for furosemide and bumetanide is proportional to carrier's activity,143 inhibition of highly active NKCC2 and diuretic action of these compounds is much greater than their vasodilatory and neuronal effects. It is also important to mention another side effect of these drugs, i.e., their prolonged administration may result in the development of deafness due to inhibition of NKCC1 in epithelial cells of the inner ear.144, 145 These issues become important for the development of novel antihypertensive drugs lacking side-effects caused by NKCC inhibition in epithelial cells and myogenic tonus in microcirculatory beds.
Conflicts of interest
All authors have none to declare.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research (MOP-81392) (S.N.O.), Russian Foundation for Fundamental Research ##14-04-31705 (S.V.K.), 15-04-00101 (S.N.O.), the Russian Scientific Foundation #14-15-00006 (S.N.O.) and the USA National Institutes of Health Award R01-GM85058 (N.O.D.).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 2.Orlov S.N., Mongin A.A. Salt sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H2039–H2053. doi: 10.1152/ajpheart.00325.2007. [DOI] [PubMed] [Google Scholar]
- 3.Markadieu N., Delpire E. Physiology and pathophysiology of SLC12A1/2 transporters. Pfluger Arch - Eur J Physiol. 2014;466:91–105. doi: 10.1007/s00424-013-1370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang F., Busch G., Ritter M. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 5.Mongin A.A., Orlov S.N. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology. 2001;8:77–88. doi: 10.1016/s0928-4680(01)00074-8. [DOI] [PubMed] [Google Scholar]
- 6.Orlov S.N., Pokudin N.I., Kotelevtsev YuV., Gulak P.V. Volume-dependent regulation of ion transport and membrane phosphorylation in human and rat erythrocytes. J Membr Biol. 1989;107:105–117. doi: 10.1007/BF01871716. [DOI] [PubMed] [Google Scholar]
- 7.Adragna N., Di Fulvio M., Lauf P.K. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. doi: 10.1007/s00232-004-0695-6. [DOI] [PubMed] [Google Scholar]
- 8.Orlov S.N. Ion transport across erythrocyte membrane: mechanisms and volume-dependent regulation. Sov Sci Rev F Physiol Gen Biol. 1994;8:1–48. [Google Scholar]
- 9.Orlov S.N., Tremblay J., Hamet P. Cell volume in vascular smooth muscle is regulated by bumetanide-sensitive ion transport. Am J Physiol. 1996;270:C1388–C1397. doi: 10.1152/ajpcell.1996.270.5.C1388. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann E.K., Lambert I.H., Pedersen S.F. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 11.Orlov S.N., Platonova A.A., Hamet P., Grygorczyk R. Cell volume and monovalent ion transporters: their role in the triggereing and progression of the cell death machinery. Am J Physiol Cell Physiol. 2013;305:C361–C372. doi: 10.1152/ajpcell.00040.2013. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Leefmans F.J. Intracellular chloride regulation. In: Sperelakis N., editor. Cell Physiology Source Book. A Molecular Approach. Academic; San Diego, CA: 2001. pp. 301–318. [Google Scholar]
- 13.Chipperfield A.R., Harper A.A. Chloride in smooth muscle. Prog Biophys Mol Biol. 2001;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 14.Davis J.P.L., Chipperfield A.R., Harper A.A. Accumulation of intracellular chloride by (Na-K-Cl) cotransport in rat arterial smooth muscle is enhanced in deoxycorticosterone acetate (DOCA)/salt hypertension. J Mol Cell Cardiol. 1993;25:233–237. doi: 10.1006/jmcc.1993.1029. [DOI] [PubMed] [Google Scholar]
- 15.Anfinogenova Y.J., Baskakov M.B., Kovalev I.V., Kilin A.A., Dulin N.O., Orlov S.N. Cell-volume-dependent vascular smooth muscle contraction: role of Na+,K+,2Cl- cotransport, intracellular Cl- and L-type Ca2+ channels. Pflьgers Arch. 2004;449:42–55. doi: 10.1007/s00424-004-1316-z. [DOI] [PubMed] [Google Scholar]
- 16.Barthelmebs M., Stephan D., Fontaine C., Grima M., Imbs J.L. Vascular effects of loop diuretics: an in vivo and in vitro study in the rat. Schmiedeb Arch Pharmacol. 1994;349:209–216. doi: 10.1007/BF00169839. [DOI] [PubMed] [Google Scholar]
- 17.Lavallee S.L., Iwamoto L.M., Claybaugh J.R., Dressel M.V., Sato A.K., Nakamura K.T. Furosemide-induced airway relaxation in guinea pigs: relation to Na-K-2Cl cotransporet function. Am J Physiol. 1997;273:L211–L216. doi: 10.1152/ajplung.1997.273.1.L211. [DOI] [PubMed] [Google Scholar]
- 18.Tian R., Aalkjaer C., Andreasen F. Mechanisms behind the relaxing effect of furosemide on the isolated rabbit ear artery. Pharmacol Toxicol. 1990;67:406–410. doi: 10.1111/j.1600-0773.1990.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 19.Kovalev I.V., Baskakov M.B., Anfinogenova Y.J. Effect of Na+,K+,2Cl- cotransport inhibitor bumetanide on electrical and contractile activity of smooth muscle cells in guinea pig ureter. Bull Exp Biol Med. 2003;136:145–149. doi: 10.1023/a:1026310722105. [DOI] [PubMed] [Google Scholar]
- 20.Kovalev I.V., Baskakov M.B., Medvedev M.A. Na+,K+,2Cl- cotransport and chloride permeability of the cell membrane in mezaton and histamine regulation of electrical and contractile activity in smooth muscle cells from the guinea pig ureter. Russ Physiol J. 2008;93:306–317. [PubMed] [Google Scholar]
- 21.Stanke F., Devillier P., Breant D. Furosemide inhibits angiotensin II-induced contraction on human vascular smooth muscle. Br J Clin Pharmacol. 1998;46:571–575. doi: 10.1046/j.1365-2125.1998.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanke-Labesque F., Craciwski J.L., Bedouch P. Furosemide inhibits thrombaxane A2-induced contraction in isolated human internal artery and saphenous vein. J Cardiovasc Pharmacol. 2000;35:531–537. doi: 10.1097/00005344-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Breaks J., Loutzenhiser K., Loutzenhiser R. Effects of inhibition of the Na+/K+/2Cl- cotransporter on myogenic and angiotensin II responses of the rat afferent arteriole. Am J Physiol Ren Physiol. 2007;292:F999–F1006. doi: 10.1152/ajprenal.00343.2006. [DOI] [PubMed] [Google Scholar]
- 24.Mozhayeva M.G., Bagrov Y.Y. The inhibitory effects of furosemide on Ca2+ influx pathways associated with oxytocin-induced contractions of rat myometrium. Gen Physiol Biophys. 1995;14:427–436. [PubMed] [Google Scholar]
- 25.Mozhayeva M.G., Bagrov Y.Y., Ostretsova I.B., Gillespie J.I. The effect of furosemide on oxytocin-induced contractions of the rat myometrium. Exp Physiol. 1994;79:661–667. doi: 10.1113/expphysiol.1994.sp003798. [DOI] [PubMed] [Google Scholar]
- 26.Akar F., Skinner E., Klein J.D., Jena M., Paul R.J., O'Neill W.C. Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+-K+-2Cl- cotransporter in rat aorta. Am J Physiol. 1999;276:C1383–C1390. doi: 10.1152/ajpcell.1999.276.6.C1383. [DOI] [PubMed] [Google Scholar]
- 27.Garg P., Martin C., Elms S.C. Effect of the Na-K-2Cl cotransporter NKCC1 on systematic blood pressure and smooth muscle tone. Am J Physiol Heart Circ Physiol. 2007;292:H2100–H2105. doi: 10.1152/ajpheart.01402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios J., Espinoza F., Munita C., Cifuentes F., Michea L. Na+-K+-2Cl- cotransporter is implicated in gender differences in the response of the rat aorta to phenylephrine. Br J Pharmacol. 2006;148:964–972. doi: 10.1038/sj.bjp.0706818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koltsova S.V., Maximov G.V., Kotelevtsev S.V. Myogenic tome in mouse mesenteric arteries: evidence for P2Y receptor-mediated, Na+,K+,2Cl- cotransport-dependent signaling. Purinergic Signal. 2009;5:343–349. doi: 10.1007/s11302-009-9160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis M.J., Hill M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 31.Hill M.A., Davis M.J., Meininger G.A., Potocnik S.J., Murphy T.V. Arteriolar myogenic signaling mechanisms: implications for local vascular functions. Clin Hemorheol Microcirc. 2006;34:67–79. [PubMed] [Google Scholar]
- 32.Schubert R., Mulvany M.J. The myogenic response: established facts and attractive hypothesis. Clin Sci. 1999;96:313–326. [PubMed] [Google Scholar]
- 33.Koltsova S.V., Kotelevtsev S.V., Tremblay J., Hamet P., Orlov S.N. Excitation-contraction coupling in resistant mesenteric arteries: evidence for NKCC1-mediated pathway. Biochem Biophys Res Commun. 2009;379:1080–1083. doi: 10.1016/j.bbrc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Delpire E., Austin T.M. Kinase regulation of Na+-K+-2Cl- cotransport in primary neurons. J Physiol. 2010;588:3365–3373. doi: 10.1113/jphysiol.2010.190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahle K.T., Staley K.J., Nahed B.V. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 36.Loscher W., Puskarjov M., Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 37.Wright F.S., Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974;53:1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon D.B., Karet F.E., Hamdan J.M., Di Pietro A., Sanjad S.A., Lifton R.P. Bartter's syndrome, hypokalemic alkalosis with hypercalciuria, is caused by mutation of Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 39.Briggs J.P., Schnermann J. Control of renin release and glomerular vascular tone by the juxtaglomerular apparatus. In: Laragh J.H., Brenner B.M., editors. Hypertension: Pathophysiology, Diagnosis, and Management. 2nd ed. Raven Press Ltd.; New York: 1995. pp. 1359–1384. [Google Scholar]
- 40.Schermann J., Briggs J.P. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int. 2008;74:418–426. doi: 10.1038/ki.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh P., Thomson S.C. Renal homeostasis and tubuloglomerular feedback. Curr Opin Nephrol Hypert. 2010;19:59–64. doi: 10.1097/MNH.0b013e3283331ffd. [DOI] [PubMed] [Google Scholar]
- 42.Knepper M.A., Inoue T. Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr Opin Cell Biol. 1997;9:560–564. doi: 10.1016/s0955-0674(97)80034-8. [DOI] [PubMed] [Google Scholar]
- 43.Borgnia M., Nielsen S., Engel A., Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 44.Bell P.D., Lapointe J.-Y., Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003;65:481–500. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- 45.Laamarti M.A., Bell P.D., Lapointe J.-Y. Transport and regulatory properties of the apical Na-K-2Cl cotransporter of macula densa cells. Am J Physiol. 1998;275:F703–F709. doi: 10.1152/ajprenal.1998.275.5.F703. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen S., Maunsbach A.B., Ecelbarger C.A., Knepper M.A. Ultrasructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol. 1998;275:F885–F893. doi: 10.1152/ajprenal.1998.275.6.F885. [DOI] [PubMed] [Google Scholar]
- 47.Nashat F.S., Tappin J.W., Wilcox C.S. The renal blood flow and the glomerylar filtration rate of anaesthetized dogs during acute changes in plasma sodium concentration. J Physiol. 1976;256:731–745. doi: 10.1113/jphysiol.1976.sp011348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox C.S. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briggs J.P., Schermann J., Wright F.S. Failure of tubule fluid osmolarity to affect feedback regulation of glomerular filtration. Am J Physiol. 1980;239:F427–F432. doi: 10.1152/ajprenal.1980.239.5.F427. [DOI] [PubMed] [Google Scholar]
- 50.Schnermann J., Briggs J., Wright F.S. Feedback-mediated reduction of glomerular filtration rate during infusion of hypertonic saline. Kidney Int. 1981;20:462–468. doi: 10.1038/ki.1981.162. [DOI] [PubMed] [Google Scholar]
- 51.Schnermann J., Ploth D.W., Hermle M. Activation of tubulo-glomerular feedback by chloride transport. Pflugers Arch. 1976;362:229–240. doi: 10.1007/BF00581175. [DOI] [PubMed] [Google Scholar]
- 52.Russell J.M. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:212–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 53.Payne J.A., Forbush B. Alternatively spliced isoforms of the putative renal Na-K-Cl cotransporter are differently distributed within the rabbit kidney. Proc Natl Acad Sci U. S. A. 1994;91:4544–4548. doi: 10.1073/pnas.91.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igarashi P., Vandel Heuvel G.B., Payne J.A., Forbush B.I. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am J Physiol. 1995;269:F405–F418. doi: 10.1152/ajprenal.1995.269.3.F405. [DOI] [PubMed] [Google Scholar]
- 55.Castrop H., Schnermann J. Isofroms of renal Na-K-2Cl cotransporter NKCC2: expression and functional significance. Am J Physiol Ren Physiol. 2008;295:F859–F866. doi: 10.1152/ajprenal.00106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang T., Huang Y.C., Singh I., Schnermann J., Briggs J.P. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol. 1998;271:F931–F939. doi: 10.1152/ajprenal.1996.271.4.F931. [DOI] [PubMed] [Google Scholar]
- 57.Brunet G.M., Gagnon E., Simard C.F. Novel insights regarding the operational characteristics and theological purpose of the renal Na+-K+-Cl- cotransporter (NKCC2s) splice variants. J Gen Physiol. 2005;126:325–337. doi: 10.1085/jgp.200509334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plata C., Meade P., Vazquez N., Hebert S.C., Gamba G. Functional properties of the apical Na+,K+,2Cl- cotransporter isoforms. J Biol Chem. 2002;277:11004–11012. doi: 10.1074/jbc.M110442200. [DOI] [PubMed] [Google Scholar]
- 59.Gimenez I., Isenring P., Forbush B.I. Spatially distributed alternatively spliced variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem. 2002;277:8767–8770. doi: 10.1074/jbc.C200021200. [DOI] [PubMed] [Google Scholar]
- 60.Lu L., Fraser J.A. Functional consequences of NKCC2 splice isoforms: insight from a Xenopus oocyte model. Am J Physiol Ren Physiol. 2014;306:F710–F720. doi: 10.1152/ajprenal.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oppermann M., Mizel D., Huang G. Macula densa control of renin secretion and proglomerular resistance in mice with selective deletion of the B isoform of Na, K,2Cl co-transporter. J Am Soc Nephrol. 2006;17:2143–2152. doi: 10.1681/ASN.2006040384. [DOI] [PubMed] [Google Scholar]
- 62.Oppermann M., Mizel D., Kim S.M. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl- co-transporter. J Am Soc Nephrol. 2007;18:440–448. doi: 10.1681/ASN.2006091070. [DOI] [PubMed] [Google Scholar]
- 63.Lorenz J.N., Kotchen T.A., Ott C.E. Effect of Na and Cl infusion on loop function and plasma renin activity. Am J Physiol. 1990;258:F1328–F1335. doi: 10.1152/ajprenal.1990.258.5.F1328. [DOI] [PubMed] [Google Scholar]
- 64.Peti-Peterdi J., Bebok Z., Lapointe J.-Y., Bell P.D. Cytosolic [Na] regulation in macula densa cells: novel role for an apical H: K-ATPase. Am J Physiol Ren Physiol. 2002;282:F329. doi: 10.1152/ajprenal.00251.2001. [DOI] [PubMed] [Google Scholar]
- 65.O'Grady S.M., Palfrey H.C., Field M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am J Physiol. 1987;253:C177–C192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- 66.Tuck M.L., Gross C., Maxwell M.H., Brickman A.S., Krasnoshtein G., Mayes D. Erythrocyte Na+,K+ cotransport and Na+,K+ pump in black and caucasian hypertensive patients. Hypertension. 1984;6:536–544. doi: 10.1161/01.hyp.6.4.536. [DOI] [PubMed] [Google Scholar]
- 67.Weder A.B., Torretti B.A., Julius S. Racial differences in erythrocyte cation transport. Hypertension. 1984;6:115–123. doi: 10.1161/01.hyp.6.1.115. [DOI] [PubMed] [Google Scholar]
- 68.Canessa M. The Na-K-Cl cotransport in essential hypertension: cellular functions and genetic environment interactions. Int J Cardiol. 1989;25:S37–S45. doi: 10.1016/0167-5273(89)90091-0. [DOI] [PubMed] [Google Scholar]
- 69.Orlov S.N., Pausova Z., Gossard F. Na+,K+,Cl- cotransport is decreased in African American vs French-Canadian hypertensives: lack of impact of gender and plasma lipids (Abstract) J Hypertens. 2001;19(suppl. 2):S46. [Google Scholar]
- 70.Orlov S.N., Gossard F., Pausova Z. Decreased NKCC1 activity in erythrocytes from African-Americans with hypertension and dyslipidemia. Am J Hypertens. 2010;23:321–326. doi: 10.1038/ajh.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orlov S.N. Decreased Na+,K+,Cl- cotransport and salt retention in Blacks: a provocative hypothesis. J Hypertens. 2005;23:1929–1930. doi: 10.1097/01.hjh.0000181324.51786.29. [DOI] [PubMed] [Google Scholar]
- 72.Flagella M., Clarke L.L., Miller M.L. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 73.Saito T., Hartell N.A., Muguruma H., Hotta S., Sasaki S., Ito M. Light dose and time dependency of photodynamic cell membrane damage. Photochem Photobiol. 1998;68:745–748. [PubMed] [Google Scholar]
- 74.Dill D.B., Yousef M.K., Goldman A., Hillyard S.D., Davis T.P. Volume and composition of hand sweat of white and black men and women in desert walk. Am J Antropol. 1983;61:67–73. doi: 10.1002/ajpa.1330610107. [DOI] [PubMed] [Google Scholar]
- 75.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 76.Andrew P.M., Jones D.W., Wofford M.R. Ethnicity and unprovoked hypokalemia in the atherosclerosis risk in communities study. Am J Hypertens. 2002;15:594–599. doi: 10.1016/s0895-7061(02)02270-7. [DOI] [PubMed] [Google Scholar]
- 77.Sato K., Kang W.H., Saga K., Sato K.T. Bilogy of sweet glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20:537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- 78.O'Shaughnessy K.M., Karet F.E. Salt handling in hypertension. Annu Rev Nutr. 2006;26:343–365. doi: 10.1146/annurev.nutr.26.061505.111316. [DOI] [PubMed] [Google Scholar]
- 79.Guyton A.C. WB Saunders Co; Philadelphia: 1980. Arterial Pressure and Hypertension. [Google Scholar]
- 80.Lifton R.P., Gharavi A.G., Geller D.S. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 81.Lifton R.P. Genetic dissection of human blood pressure variation: common pathways from rare phenotypes. Harvey Lect. 2005;100:71–101. [PubMed] [Google Scholar]
- 82.Guyton A.C. Blood pressure control – special role of the kidney and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 83.Simon D.B., Nelson-Williams C., Bia J. Gitelman's varaint of Bartter's syndrome, inhereted hypokalemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 84.Wilson F.H., Disse-Nicodeme S., Choate K.A. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 85.Hamet P., Pausova Z., Adarichev V., Adaricheva K., Tremblay J. Hypertension: genes and environment. J Hypertens. 1998;16:397–418. doi: 10.1097/00004872-199816040-00001. [DOI] [PubMed] [Google Scholar]
- 86.Pickering G.W. Systematic arterial pressure. In: Fishman A.P., Richards D.W., editors. Circulation of the Blood. Men and Ideas. 1964. pp. 487–541. London. [Google Scholar]
- 87.Schiebl I.M., Rosenauer A., Kattler V., Minuth W.W., Oppermann M., Castrop H. Dietary salt intake modulates differential splicong of the Na-K-2Cl cotransporter NKCC2. Am J Physiol Ren Physiol. 2013;305:F1139–F1148. doi: 10.1152/ajprenal.00259.2013. [DOI] [PubMed] [Google Scholar]
- 88.Orlov S.N., Hamet P. Salt and gene expression: evidence for Na+i,K+i-mediated signaling pathways. Pflugers Arch. 2015;467(3):489–498. doi: 10.1007/s00424-014-1650-8. [DOI] [PubMed] [Google Scholar]
- 89.Davies M., Fraser S.A., Galic S. Novel mechanisms of Na+ retention in obesity: phosphorylation of NKCC2 and regulation of SPAK/OSR1 by AMPK. Am J Physiol Ren Physiol. 2014;307:F96–F106. doi: 10.1152/ajprenal.00524.2013. [DOI] [PubMed] [Google Scholar]
- 90.Jones A.W. Altered ion transport in vascular smooth muscle from spontaneously hypertensive rats. Influence of aldosterone, norepinephrine and angiotensin. Circ Res. 1973;33:563–572. doi: 10.1161/01.res.33.5.563. [DOI] [PubMed] [Google Scholar]
- 91.Postnov YuV., Orlov S.N., Gulak P.V., Shevchenko A.S. Altered permeability of the erythrocyte membrane for sodium and potassium in spontaneously hypertensive rats. Pflugers Arch. 1976;365:257–263. doi: 10.1007/BF01067026. [DOI] [PubMed] [Google Scholar]
- 92.Postnov YuV., Orlov S.N., Shevchenko A.S., Adler A.M. Altered sodium permeability, calcium binding and Na-K-ATPase activity in the red blood cell membrane in essential hypertension. Pflugers Arch. 1977;371:263–269. doi: 10.1007/BF00586267. [DOI] [PubMed] [Google Scholar]
- 93.Postnov YuV., Orlov S.N. Ion transport across plasma membrane in primary hypertension. Physiol Rev. 1985;65:904–945. doi: 10.1152/physrev.1985.65.4.904. [DOI] [PubMed] [Google Scholar]
- 94.Orlov S.N., Adragna N., Adarichev V.A., Hamet P. Genetic and biochemical determinants of abnormal monovalent ion transport in primary hypertension. Am J Physiol. 1999;276:C511–C536. doi: 10.1152/ajpcell.1999.276.3.C511. [DOI] [PubMed] [Google Scholar]
- 95.Garay R.P., Alda O. What can we learn from erythrocyte Na-K-Cl cotransporter NKCC1 in human hypertension. Pathophysiology. 2007;14:167–170. doi: 10.1016/j.pathophys.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Orlov S.N., Tremblay J., Hamet P. NKCC1 and hypertension: a novel therapeutic target involved in regulation of vascular tone and renal function. Curr Opin Nephrol Hypert. 2010;19:163–168. doi: 10.1097/MNH.0b013e3283360a46. [DOI] [PubMed] [Google Scholar]
- 97.Orlov S.N., Koltsova S.V., Tremblay J., Baskakov M.B., Hamet P. NKCC1 and hypertension: role in the regulation of vascular smooth muscle contractions and myogenic tone. Ann Med. 2012;44:S111–S118. doi: 10.3109/07853890.2011.653395. [DOI] [PubMed] [Google Scholar]
- 98.Bianchi G., Ferrari P., Trizio P. Red blood cell abnormalities and spontaneous hypertension in rats. A genetically determined link. Hypertension. 1985;7:319–325. [PubMed] [Google Scholar]
- 99.Kotelevtsev YuV., Orlov S.N., Pokudin N.I., Agnaev V.M., Postnov YuV. Genetic analysis of inheritance of Na+,K+ cotransport, calcium level in erythrocytes and blood pressure in F2 hybrids of spontaneously hypertensive and normotensive rats. Bull Exp Biol Med. 1987;103:456–458. [PubMed] [Google Scholar]
- 100.Meyer J.W., Flagella M., Sutliff R.L. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl- cotransporter. Am J Physiol. 2002;283:H1846–H1855. doi: 10.1152/ajpheart.00083.2002. [DOI] [PubMed] [Google Scholar]
- 101.Wall S.M., Knepper M.A., Hassel K.A. Hypotension in NKCC1 null mice: role of the kidney. Am J Physiol Ren Physiol. 2006;290:F409–F416. doi: 10.1152/ajprenal.00309.2005. [DOI] [PubMed] [Google Scholar]
- 102.Lee H.-A., Baek I., Seok Y.M. Promoter hypomethylation upregulates Na+-K+-2Cl- cotransporyter 1 in spontaneously hypertensive rats. Biochem Biophys Res Commun. 2010;396:252–257. doi: 10.1016/j.bbrc.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 103.Cho H.-M., Lee H.-A., Kim H.Y., Han H.S., Kim I.K. Expression of Na+,K+-2Cl- cotransporter is epigenetically regulated during postnatal development of hypertension. Am J Hypertens. 2011;12:1286–1293. doi: 10.1038/ajh.2011.136. [DOI] [PubMed] [Google Scholar]
- 104.Mancia G., Grassi G., Giannattasio C., Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 105.Schlaich M.P., Lambert E., Kaye D.M. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 106.Huang B.S., Amin M.S., Leenen F.H.H. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol. 2006;21:295–394. doi: 10.1097/01.hco.0000231398.64362.94. [DOI] [PubMed] [Google Scholar]
- 107.Leenen F.H.H. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–1139. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Judy W.V., Watanabe A.M., Henry P.D., Besch H.R., Murphy W.R., Hockel G.M. Sympathetic nerve activity: role in regulation of blood pressure in the spontaneously hypertensive rats. Circ Res. 1976;38:21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- 109.Allen A.M. Inhibition of the hypothalamic paraventricular nucleus in spontaneousl hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 110.Li D.P., Pan H.L. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 111.Pyner S., Coote J.H. Identification of branching paraventricular neurins of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 112.Li D.P., Pan H.L. Role of GABAA and GABAB receptors in paraventricular nucelus in control sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 113.Li D.P., Pan H.L. Plasticity fo GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–H1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- 114.Ye Z.-Y., Li D.-P., Byun H.S., Li L., Pan H.-L. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8568. doi: 10.1523/JNEUROSCI.1346-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Orlov S.N., Resink T.J., Bernhardt J., Buhler F.R. Na+-K+ pump and Na+-K+ co-transport in cultured vascular smooth muscle cells from spontaneously hypertensive rats: baseline activity and regulation. J Hypertens. 1992;10:733–740. [PubMed] [Google Scholar]
- 116.Jiang G., Cobbs S., Klein J.D., O'Neill W.C. Aldosterone regulates the Na-K-Cl cotransporter in vascular smooth muscle. Hypertension. 2003;41:1131–1135. doi: 10.1161/01.HYP.0000066128.04083.CA. [DOI] [PubMed] [Google Scholar]
- 117.Orlov S.N., Li J.-M., Tremblay J., Hamet P. Genes of intracellular calcium metabolism and blood pressure control in primary hypertension. Seminar Nephrol. 1995;15:569–592. [PubMed] [Google Scholar]
- 118.Hamet P., Orlov S.N., Tremblay J. Intracellular signalling mechanisms in hypertension. In: Laragh J.H., Brenner B.M., editors. Hypertension: Pathophysiology, Diagnosis, and Treatment. 2 ed. Raven Press; New York: 1995. pp. 575–608. [Google Scholar]
- 119.Piala A.T., Moon T.M., Akella R., He H., Cobb M.H., Goldsmith E.J. Chloride sensing by WNK1 kinase invoolves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gamba G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am J Physiol Ren Physiol. 2004;288:F245–F252. doi: 10.1152/ajprenal.00311.2004. [DOI] [PubMed] [Google Scholar]
- 121.Ponce-Coria J., San-Cristobal P., Kahle K.T. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U. S. A. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gamba G. Regulation of NKCC2 activity by SPAK truncated isoforms. Am J Physiol Ren Physiol. 2014;306:F49–F50. doi: 10.1152/ajprenal.00559.2013. [DOI] [PubMed] [Google Scholar]
- 123.Bergaya S., Faure S., Baudrie V. WNK1 regulates vasoconstriction and blood pressure response to α1-adrenergic stimulation in mice. Hypertension. 2011;58:439–445. doi: 10.1161/HYPERTENSIONAHA.111.172429. [DOI] [PubMed] [Google Scholar]
- 124.Yang S.-S., Lo Y.-F., Wu C.-C. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang Y., Pacary E., Freret T. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidate for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Friso S., Carvajal C.A., Fardella C.E., Oliveri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res. 2015;165:154–165. doi: 10.1016/j.trsl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 127.Orlov S.N. NKCC1 as an epigenetically regulated transporter involved in blood pressure elevation with age. Am J Hypertens. 2011;24:1264. doi: 10.1038/ajh.2011.150. [DOI] [PubMed] [Google Scholar]
- 128.Janardhan V., Qureshi A.I. Mechanisms of ischemic brain injury. Curr Cardiol Rep. 2004;6:117–123. doi: 10.1007/s11886-004-0009-8. [DOI] [PubMed] [Google Scholar]
- 129.Folkow B. Cardiovascular “remodeling” in rat anf human: time axis,extent, and in vivo relevance. Physiology. 2010;25:264–265. doi: 10.1152/physiol.00015.2010. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y., Gutterman D.D. Vascular control in humans: focus on the coronary micocirculation. Basic Res Cardiol. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bidani A., Griffin K.A., Williamson G., Wang X., Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loutzenhiser R., Bidani A.K., Wang X. Systolic pressure and the myogenis response of the afferent arteriole. Acta Physiol Scand. 2004;181:407–413. doi: 10.1111/j.1365-201X.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 133.Khodorov B. Clutamate-induced deregulation of calcium homeostasis and mitochondrial dysfunction in mammalian central neurones. Prog Biophys Mol Biol. 2004;86:279–351. doi: 10.1016/j.pbiomolbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 134.Mongin A.A. Disruption of ionic and cell volume homeostasis in cerebral ischemia: the perferct storm. Pathophysiology. 2007;14:183–193. doi: 10.1016/j.pathophys.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Su G., Kintner D.B., Sun D. Contribution of Na+,K+,Cl- cotransporter to high-K+o-induced swelling and EAA release is astrocytes. Am J Physiol Cell Physiol. 2002;282:C1136–C1146. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- 136.Busse S., Breder J., Dinkel K., Reymann K.G., Schroder U.H. Inhibitors of cation-chloride-cotransporters affect hypoxic/hypoglycemic injury in hyppocampal slices. Brain Res. 2005;1946:116–121. doi: 10.1016/j.brainres.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 137.Su G., Kintner D.B., Flagella M., Shull G.E., Sun D. Astrocytes from Na+,K+.Cl- cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol. 2002;282:C1147–C1160. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- 138.Koltsova S.V., Luneva O.G., Lavoie J.L. HC03-dependent impact of Na+,K+,2Cl- cotransport in vascular smooth muscle excitation-contraction coupling. Cell Physiol Biochem. 2009;23:407–414. doi: 10.1159/000218187. [DOI] [PubMed] [Google Scholar]
- 139.Williams R.S., Benjamin I.J. Protective responses in the ischemic myocardium. J Clin Invest. 2000;106:813–818. doi: 10.1172/JCI11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Danielsson J., Yim P., Rinderspacher A. Chloride chanel blockage relaxes airway smooth muscle and potentiates relaxation by ®-agonists. Am J Physiol Lung Cell Mol Physiol. 2014;307:L273–L282. doi: 10.1152/ajplung.00351.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haglund M.M., Hochman D.W. Furosemide and mannitol suppression of epileptic activity in the human brain. J Neurophysiol. 2005;94:907–918. doi: 10.1152/jn.00944.2004. [DOI] [PubMed] [Google Scholar]
- 142.Klomjai W., Lackmy-Vallee A., Katz R. Changes in spinal inhibitory networks induced by furosemide in humans. J Physiol. 2014;592:2865–2879. doi: 10.1113/jphysiol.2013.265314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hannaert P., Alvarez-Guerra M., Pirot D., Nazaret C., Garay R.P. Rat NKCC2/NKCC1 cotransport selectivity for loop diuretic drugs. Schmiedeb Arch Pharmacol. 2002;365:193–199. doi: 10.1007/s00210-001-0521-y. [DOI] [PubMed] [Google Scholar]
- 144.Delpire E., Lu J., England R., Dull C., Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- 145.Lang F., Vallon V., Knipper M., Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol. 2007;293:C1187–C1208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]