Summary
Background
Conventional treatments for patients with type 2 diabetes are often inadequate. We aimed to assess outcomes of diabetes control and treatment risks 2 years after adding Roux-en-Y gastric bypass to intensive lifestyle and medical management.
Methods
We report 2-year outcomes of a 5-year randomised trial (the Diabetes Surgery Study) at four teaching hospitals (three in the USA and one in Taiwan). At baseline, eligible participants had to have HbA1c of at least 8·0% (64 mmol/mol), BMI between 30·0 and 39·9 kg/m2, and type 2 diabetes for at least 6 months, and be aged 30–67 years. We randomly assigned participants to receive either intensive lifestyle and medical management alone (lifestyle and medical management), or lifestyle and medical management plus standard Roux-en-Y gastric bypass surgery (gastric bypass). Staff from the clinical centres had access to data from individual patients, but were masked to other patients’ data and aggregated data until the 2-year follow-up. Drugs for hyperglycaemia, hypertension, and dyslipidaemia were prescribed by protocol. The primary endpoint was achievement of the composite treatment goal of HbA1c less than 7·0% (53 mmol/mol), LDL cholesterol less than 2·59 mmol/L, and systolic blood pressure less than 130 mm Hg at 12 months; here we report the composite outcome and other pre-planned secondary outcomes at 24 months. Analyses were done on an intention-to-treat basis, with multiple imputations for missing data. This study is registered with ClinicalTrials.gov, number NCT00641251, and is still ongoing.
Findings
Between April 21, 2008, and Nov 21, 2011, we randomly assigned 120 eligible patients to either lifestyle and medical management alone (n=60) or with the addition of gastric bypass (n=60). One patient in the lifestyle and medical management group died (from pancreatic cancer), thus 119 were included in the primary analysis. Significantly more participants in the gastric bypass group achieved the composite triple endpoint at 24 months than in the lifestyle and medical management group (26 [43%] vs eight [14%]; odds ratio 5·1 [95% CI 2·0–12·6], p=0·0004), mainly through improved glycaemic control (HbA1c <7·0% [53 mmol/mol] in 45 [75%] vs 4 [24%]; treatment difference −1·9% (−2·5 to −1·4); p=0·0001). 46 clinically important adverse events occurred in the gastric bypass group and 25 in the lifestyle and medical management group (mainly infections in both groups [four in the lifestyle and medical management group, eight in the gastric bypass group]). With a negative binomial model adjusted for site, the event rate for the gastric bypass group was non-significantly higher than the lifestyle and medical management group by a factor of 1·67 (95% CI 0·98–2·87, p=0·06). Across both years of the study, the gastric bypass group had seven serious falls with five fractures, compared with three serious falls and one fracture in the lifestyle and medical management group. All fractures happened in women. Many more nutritional deficiencies occurred in the gastric bypass group (mainly deficiencies in iron, albumin, calcium, and vitamin D), despite protocol use of nutritional supplements.
Interpretation
The addition of gastric bypass to lifestyle and medical management in patients with type 2 diabetes improved diabetes control, but adverse events and nutritional deficiencies were more frequent. Larger and longer studies are needed to investigate whether the benefits and risk of gastric bypass for type 2 diabetes can be balanced.
Funding
Covidien, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Nutrition Obesity Research Centers, and the National Center for Advancing Translational Sciences.
Introduction
Bariatric surgery has been advocated to improve disordered metabolism in patients with type 2 diabetes. Substantial, sustained weight loss produced by bariatric surgery could improve the effectiveness limitations of the cornerstone of diabetes therapy—lifestyle changes aimed at reducing weight by decreasing calorie intake and increasing physical activity. Surgical rearrangement of gut anatomy might also have additional metabolic benefits. Evidence favouring improvement in diabetes outcomes from bariatric surgery is shown in observational studies1,2 and early reports from randomised trials,3–8 including our report of results from the first year of the 5-year Diabetes Surgery Study (DSS).9
Interest in bariatric surgery for diabetes has largely focused on glycaemic benefits of surgery, but full treatment of diabetes needs attention to all aspects of the metabolic disturbance, including control of blood pressure and lipids. Evidence and expert opinion, as explained in the American Diabetes Association (ADA) treatment standards,10 direct diabetes therapy at the triple endpoint of control of glycaemia, systolic blood pressure, and LDL cholesterol. Achievement of triple endpoint control has proved remarkably difficult, with typical population groups reaching this goal roughly only 10% of the time.11,12 However, in the first year of the DSS, 49% of participants randomly assigned to Roux-en-Y gastric bypass reached the triple endpoint goal compared with 19% of the participants randomly assigned to treatment with intensive lifestyle and medical management.9
Questions remain about the persistence of the surgical effect on glycaemic and triple-endpoint control, generalisability of surgical improvements across clinical centres, and full accounting of adverse events. We therefore report here the results at 2 years of the DSS. To address the durability of study interventions we report the results of preplanned secondary analyses related to the triple endpoint, weight changes, medicines used, and adverse events after the full 24-month period of active intervention.
Methods
Study design and participants
This international, multicentre, randomised study was done at four sites: the University of Minnesota (Minneapolis, MN, USA), Columbia University Medical Center (New York, NY, USA), two academic clinics in Taiwan (National Taiwan University Hospital and Min Sheng General Hospital, together referred to as Taiwan in this report), and the Mayo Clinic in Rochester (MN, USA). We obtained institutional review board approval at all sites.
We recruited participants for our study using mailings, radio messages, clinic referrals, and posters. Full details of the inclusion and exclusion criteria are described in the appendix (p 1, 2). Key inclusion criteria included HbA1c of 8·0% (64 mmol/mol) or higher despite at least 6 months care from a doctor for type 2 diabetes, BMI 30·0–39·9 kg/m2, age 30–67 years, and a willingness and ability to accept random assignment and follow the full treatment protocol. Exclusion criteria included any cardiovascular event (such as myocardial infarction or stroke) in the previous 6 months, or current evidence of congestive heart failure or angina pectoris. Participants gave written informed consent at all sites. The 4-year recruitment procedure is described elsewhere.13
Randomisation and masking
We randomly assigned patients within each site to either lifestyle and medical management alone (lifestyle and medical management group) or lifestyle and medical management plus a Roux-en-Y gastric bypass (gastric bypass group), using a random permuted block design and blocks of size two, four, and six. We generated all randomisation assignments in advance using a pseudorandom number generator in SAS (version 9.3). Staff from the clinical centres had access to data for their individual patients, but were masked to data for other patients and to aggregated data until all 2-year follow-up data had been obtained. The aggregate data was not disclosed until the 2-year visits were completed.
Procedures
The lifestyle and medical management intervention was modelled on successful clinical trials, especially the Diabetes Prevention Program14 and the Look AHEAD trial protocol.15 Participants were instructed to weigh themselves and record eating and exercise behaviours daily, and advised to progressively increase their amount of moderate-intensity physical activity (such as walking) to 325 min per week. Participants met regularly with a dietitian or registered nurse to discuss strategies for weight management and increasing physical activity, including self-monitoring, stimulus control, problem-solving, social support, cognitive behaviour modification, recipe modification, eating away from home, and relapse prevention. Counselling sessions consisted of 24 meetings (one per week) during the first 6 months, one meeting every 2 weeks between months 7 and 9, one meeting per month between months 10 and 15, then one meeting every 3 months either up to 24 months or until a total of 40 modules were completed. The lifestyle and medical management intervention protocol for 12–24 months was similar in both treatment groups. The intervention was provided without charge, except New York participants were required by law to make standard insurance copayments for medicines. Minor modifications were made to the interventions in the USA versus Taiwan to account for differences in language and culture.
Visits with an endocrinologist took place each month for 6 months, then every 3 months (or monthly if not at goal) for the next 6 months, then every 3 months through the second year. Medicines for glycaemic control were added or reintroduced in the following order: metformin, a glucagon-like peptide-1 agonist or dipeptidyl peptidase-4 inhibitor, sulfonylurea or pioglitazone, and insulin. We pursued control of LDL cholesterol with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors first, then ezetimibe if necessary. Blood pressure medicines were used in the following order: angiotensin-converting enzyme inhibitors or angiotensin receptor II blockers, diuretics, β blockers, and additional agents as necessary. If triglyceride concentrations remained higher than 3·39 mmol/L after hyperglycaemia was controlled, fenofibrate or fish oil were added to participants’ diets. Smoking cessation was strongly recommended for all. An angiotensin-converting enzyme inhibitor or angiotensin receptor II blocker was provided for participants with microalbuminuria or macro-albuminuria. Aspirin (81–100 mg daily) was added, consistent with evolving recommendations from the ADA, when not contra indicated. Medicines approved by the US Food and Drug Administration for long-term obesity treatment were used. By protocol, participants in the gastric bypass group were prescribed a multivitamin and other supplements, including calcium, iron, vitamin D, and vitamin B12, irrespective of routine test results, to prevent nutritional deficiencies. All patients received routine testing for nutritional deficiencies and supplements were adjusted as necessary.
The Roux-en-Y surgical technique was standardised across all sites and done with construction of a 20 mL lesser curvature gastric pouch, and a 100 cm biliopancreatic limb. All surgeons committed to following this protocol, which was reviewed at an onsite meeting. The technical skill of each surgeon was assessed by personal observation of the principal surgeon. The study surgeons did all postoperative surgical interventions.
Outcomes
The primary outcome was achievement of the composite triple endpoint16 of HbA1c less than 7·0% (53 mmol/mol), LDL cholesterol less than 2·59 mmol/L and systolic blood pressure lower than 130 mm Hg at the 12-month visit; we now report the durability of the primary outcome at 24 months, a prespecified secondary outcome measure. Additional prespecified secondary outcome measures included weight loss, fasting blood glucose (FBG), HDL cholesterol, C-peptide, and triglyceride concentrations, diastolic blood pressure, waist circumference, and use of medicines to control glycaemia, cholesterol, and blood pressure at both 1 and 2 years. We also report occurrences of diabetes remission to enable comparision with other trials of bariatric surgery that used this measure, although this was not prespecified in the trial protocol because standards for diabetes remission had not been determined at the time of commencement of the study.
Partial remission of diabetes was defined as HbA1c lower than or equal to 6·5% (48 mmol/mol), and full remission as HbA1c lower than or equal to 6·0% (42 mmol/mol), each at every sampling from months 12 to 24 with no medicines for hyperglycaemia. These definitions are in line with the recommendations of an ADA consensus statement17 that identifies an HbA1c threshold of 6·5% (48 mmol/mol) for partial remission, and a healthy HbA1c of 6·0% (42 mmol/mol) for 1 year without any diabetes medicine as full remission.
We obtained data at baseline, medical visits, and lifestyle intervention visits. For each group, baseline measurements were taken at randomisation. The data included measurements of height, weight, blood pressure, waist circumference, information about medicines used, (appendix) and adverse events. Laboratory measurements included blood concentrations of HbA1c, fasting lipid-profile, complete blood cell count, electrolytes, hepatic panel, ferritin, vitamin B1, vitamin B12, vitamin D, parathyroid hormone, calcium, FBG and 90-min post-meal glucose and C-peptide concentrations, and urine microalbumin to creatinine ratios (appendix). Queries about possible adverse events were part of every study visit. A full record of these events over 24 months was obtained and analysed and all events judged to be clinically important were tabulated. Clinically important events were events that needed a medical response; these are different from severe adverse events as defined by the US Food and Drug Administration. Adverse events were reviewed every 6 months by a data safety monitoring board.
Statistical analysis
We estimated the sample size for our study on the basis of the following assumptions: (1) a two-sided significance level of 0·05, (2) 90% power, (3) an alternative hypothesis of success rates on the triple endpoint of 65% in the gastric bypass group versus 30% in the lifestyle and medical management only group. These estimates were based on previous studies. Schauer and colleagues18 reported that 82% of participants with type 2 diabetes who underwent Roux-en-Y surgery had reduced HbA1c concentrations to less than 7·0% more than 12 months after surgery. However, the estimated success rate for the gastric bypass group in this trial was reduced from 82% to 65% because the triple endpoint's definition of success also needs LDL cholesterol to be less than 2·59 mmol/L and systolic blood pressure to be less than 130 mm Hg. The success rate of 30% in the lifestyle management group was based on two studies and the ADA standards of care.19,20
We did the statistical analyses on an intention-to-treat basis with SAS version 9.3. The analysis of adverse events and nutritional deficiencies included all directly observed outcomes from the randomly assigned patients. Distributions with outliers (glucose, triglycerides, C-peptide and post-challenge C-peptide) were capped at the IQR plus or minus 1·5 times the width of the IQR. Values outside the specified range were set equal to the range limit.
Our results show means and 95% CIs and used imputed data. Multiple imputation was used to address the issue of missing data.21 All outcomes were modelled together with PROC MI in SAS. Information on crossovers was also included in the model. 40 imputations were done. Data from baseline, 12 months, and 24 months were included, along with data every 3 months for the triple endpoint components and percentage weight loss. We did regressions separately for each imputed dataset and then summarised them using PROC MIANALYZE. We analysed dichotomous data with logistic regressions stratified by site, and continuous data with linear regressions adjusted for site. Estimates of percentages, means, and SDs were derived from the imputed database. SDs show estimated population variations and uncertainty about imputed values.
For dichotomous variables, we used logistic regressions for analyses of 24-month changes, with the 24-month value as the outcome and the starting value as an adjusting variable. For continuous variables, the change was computed and used as the outcome in a linear regression.
We compared adverse event counts by treatment group using regressions adjusted by site and assuming a negative binomial distribution. We considered using a zero-inflated model but rejected it because the effect was not significant. We computed p values for differences in nutritional safety outcomes with Fisher's exact test.
This study is registered with ClinicalTrials.gov, number NCT00641251.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The funder reviewed the manuscript before submission but had no role in the decision to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
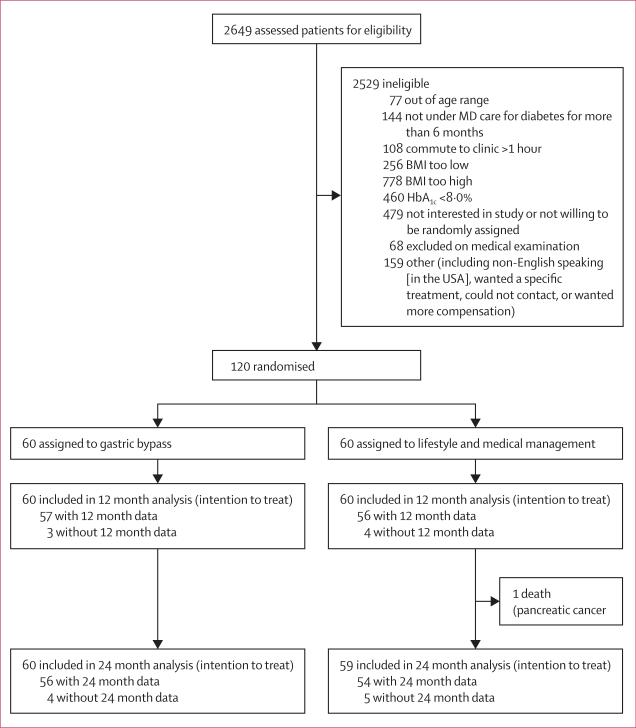
Between April 21, 2008, and Nov 21, 2011, we randomly assigned 120 eligible patients to either lifestyle and medical management alone (n=60) or with the addition of gastric bypass (n=60; figure 1). Baseline characteristics (table 1) were similar across treatment groups, except more participants in the gastric bypass group used insulin than in the lifestyle and medical management group. Three participants assigned to lifestyle and medical management crossed over to the gastric bypass group (one received a bypass after 3 months, two received a bypass shortly after 12 months). Two patients assigned to gastric bypass declined surgery and crossed over to lifestyle and medical management only. These participants were analysed according to the group that they were originally assigned to. Nine (8%) of 120 patients were lost to follow-up by 24 months. 40 imputations were done, meaning that we created 40 versions of the dataset, ran each analysis 40 times, and then combined the results. We verified the results of imputed analyses by performing parallel analyses on only patients with 24 month data available. In all cases, the results were similar (data not shown).
Figure 1.
Trial profile
Table 1.
Baseline characteristics of the intention-to-treat population
| Lifestyle management (n=59) | Gastric bypass (n=60) | |
|---|---|---|
| Age (years) | 49 (8) | 49 (9) |
| Sex | ||
| Male | · · | · · |
| Female | 34 (57%) | 38 (63%) |
| Ethnic origin | ||
| White | 30 (51%) | 33 (55%) |
| East Asian | 17 (29%) | 16 (27%) |
| Black | 6 (10%) | 5 (8%) |
| Hispanic | 4 (7%) | 4 (7%) |
| Native American | 1 (2%) | 2 (3%) |
| Other | 2 (3%) | 0 (0%) |
| BMI (kg/m2) | 34·3 (3·1) | 34·9 (3·0) |
| BMI 30·0–34·9 kg/m2 | 35 (59%) | 36 (60%) |
| Waist circumference (cm) | 113 (12) | 114 (10) |
| Systolic blood pressure (mm Hg) | 132 (14) | 127 (15) |
| Diastolic blood pressure (mm Hg) | 79 (10) | 78 (12) |
| Years since diagnosis of diabetes | 9·1 (5·7) | 8·9 (6·1) |
| Laboratory measurements | ||
| HbA1c (%) | 9·6% (1·2) | 9·6% (1·0) |
| HbA1c (mmol/mol) | 81·4 (13·1) | 81·4 (10·9) |
| LDL cholesterol (mmol/L) | 2·7 (1·1) | 2·7 (0·9) |
| HDL cholesterol (mmol/L) | 1·1 (0·2) | 1·1 (0·03) |
| Triglycerides (mmol/L) | 2·2 (0·9) | 2·1 (0·9) |
| Total cholesterol (mmol/L) | 4·9 (1·2) | 4·7 (1·0) |
| Creatinine (μmol/L) | 69·8 (16·8) | 71·6 (17·7) |
| Fasting C-peptide (nmol/L) | 1·0 (0·5) | 0·9 (0·5) |
| Post-meal C-peptide (nmol/L) | 1·6 (0·7) | 1·4 (0·7) |
| Fasting blood glucose (mmol/L) | 11·4 (2·9) | 11·9 (3·2) |
| Concomitant medicines | ||
| Insulin | 25 (42%) | 37 (62%) |
| Other glycaemia medicines | 56 (95%) | 52 (87%) |
| Medicines for dyslipidaemia | 40 (68%) | 39 (65%) |
| Antihypertensives | 43 (73%) | 41 (68%) |
| Number of medicines for control of glycaemia, dyslipidaemia and blood pressure | 4·4 (1·5) | 4·1 (1·9) |
Data are number (%) or mean (SD).
One patient in the lifestyle and medical management group died (from pancreatic cancer), thus 119 were included in the primary analysis (60 in the gastric bypass group, 59 in the lifestyle and medical management group). Significantly more participants in the gastric bypass group achieved the composite triple endpoint at 24 months than in the lifestyle and medical management group (26 [43%] vs eight [14%]; odds ratio [OR] 5·1 [95% CI 2·0–12·6], p=0·0004, table 2). More participants in both groups achieved the triple endpoint at 12 months than at 24 months (table 2). When we assessed the three triple endpoint components as dichotomous variables, a significant treatment effect was noted only with HbA1c, because more patients in the gastric bypass group achieved HbA1c less than 7·0% (53 mmol/mol) than patients in the lifestyle and medical management group (table 2).
Table 2.
Efficacy outcomes
| Lifestyle and medical management (n=59) |
Gastric bypass (n=60) |
Treatment difference at 24 months |
|||||
|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 12 months | 24 months | Odds ratio (95% CI) | Estimated difference (95% CI) | p value | |
| Primary outcome | |||||||
| Composite primary endpoint met | 11 (19%) | 8 (14%) | 28 (47%) | 26 (43%) | 5·1 (2·0–12·6) | · · | 0·0004 |
| Dichotomous outcomes | |||||||
| HbA1c <7·0% (<53 mmol/mol) | 18 (31%) | 14 (24%) | 44 (73%) | 45 (75%) | 10·4 (4·3–25·2) | · · | <0·0001 |
| LDL cholesterol <2·59 mmol/L | 41 (69%) | 40 (68%) | 47 (78%) | 44 (73%) | 1·3 (0·6–3·0) | · · | 0·50 |
| Systolic blood pressure <130 mm Hg | 45 (76%) | 41 (69%) | 49 (82%) | 48 (80%) | 1·8 (0·7–4·5) | · · | 0·20 |
| HbA1c ≤6·0% (<42 mmol/mol) | 5 (8%) | 4 (7%) | 26 (43%) | 23 (38%) | 9·7 (2·8–34·0) | · · | 0·0004 |
| Partial remission of diabetes* | 0 (0%) | 0 (0%) | 0 (0%) | 25 (42%) | NA | <0·0001 | |
| Full remission of diabetes* | 0 (0%) | 0 (0%) | 0 (0%) | 15 (25%) | NA | <0·0001 | |
| Fasting blood glucose <5·55 mmol/L | 8 (14%) | 10 (17%) | 26 (43%) | 22 (37%) | 2·8 (1·1–7·4) | · · | 0·034 |
| Systolic blood pressure <140 mm Hg | 53 (90%) | 50 (85%) | 56 (93%) | 54 (90%) | 1·8 (0·5–6·4) | · · | 0·36 |
| Continuous outcomes | |||||||
| Glycaemia | |||||||
| HbA1c (%) | 7·8% (2·3) | 8·4% (2·9) | 6·4% (1·6) | 6·5% (1·6) | · · | –1·9 (–2·5 to –1·4) | <0·0001 |
| HbA1c (mmol/mol) | 61·7 (25·1) | 68·3 (31·7) | 46·4 (17·5) | 47·5 (17·5) | · · | –20·8 (–27·3 to –15·3) | <0·0001 |
| Fasting C-peptide (nmol/L) | 0·8 (0·7) | 0·8 (0·8) | 0·6 (0·5 ) | 0·6 (0·5 ) | · · | –0·3 (–0·4 to –0·1) | 0·004 |
| Post-meal C-peptide (nmol/L) | 1·6 (1·3) | 1·4 (1·5) | 1·3 (1·0) | 1·3 (1·1) | · · | –0·1 (–0·4 to 0·3) | 0·70 |
| Fasting blood glucose (mmol/L) | 8·7 (5·7) | 8·9 (6·2) | 6·3 (3·5) | 6·2 (2·9) | · · | –2·7 (–3·9 to –1·6) | <0·0001 |
| Serum lipids | |||||||
| LDL cholesterol (mmol/L) | 2·3 (1·3) | 2·3 (1·4) | 2·1 (1·0) | 2·2 (1·2) | · · | –0·1 (–0·5 to 0·2) | 0·39 |
| HDL cholesterol (mmol/L) | 1·1 (0·4) | 1·1 (0·5) | 1·3 (0·5) | 1·3 (0·5) | · · | 0·2 (0·1–0·3) | 0·002 |
| Total cholesterol (mmol/L) | 4·2 (1·7) | 4·5 (2·0) | 4·0 (1·3) | 4·0 (1·6) | · · | –0·4 (–0·9 to –0·03) | 0·042 |
| Triglycerides (mmol/L) | 1·8 (1·3) | 2·0 (1·5) | 1·2 (0·9) | 1·2 (1·0) | · · | –0·8 (–1·1 to –0·5) | <0·0001 |
| Blood pressure | |||||||
| Systolic blood pressure (mm Hg) | 124 (17) | 125 (22) | 117 (22) | 120 (23) | –5·5 (–10·7 to –0·3) | · · | 0·04 |
| Diastolic blood pressure (mm Hg) | 74 (13) | 75 (14) | 68 (14) | 70 (15) | –5·7 (–9·1 to –2·2) | · · | 0·001 |
| Weight | |||||||
| BMI (kg/m2) | 31·7 (5·4) | 31·8 (6·7) | 26·0 (5·2) | 26·5 (5·4) | –5·3 (–6·7 to –3·8) | · · | <0·0001 |
| Weight loss (%) | 7·5% (12·2) | 7·3% (14·9) | 25·6% (13·3) | 23·8% (13·9) | 17% (13–20) | · · | <0·0001 |
| Reduction in waist circumference (%) | 8·3% (14·7) | 8·0% (15·8) | 23·9% (14·8) | 22·9% (15·8) | 15% (11–19) | · · | <0·0001 |
| Concomitant medicines | |||||||
| Insulin | 25 (42%) | 27 (46%) | 11 (18%) | 12 (20%) | 0·25 (0·10–0·61) | · · | 0·0025 |
| Other glycaemic medicines | 57 (97%) | 53 (90%) | 21 (35%) | 25 (42%) | 0·06 (0·02, 0.21) | · · | <0·0001 |
| Medicines for dyslipidaemia | 40 (68%) | 43 (73%) | 26 (43%) | 27 (45%) | 0·30 (0·13–0·65) | · · | 0·0024 |
| Antihypertensives | 42 (71%) | 37 (63%) | 23 (38%) | 25 (42%) | 0·36 (0·16–0·83) | · · | 0·016 |
| Number of medicines prescribed for control of glycaemia, dyslipidaemia and blood pressure | 4·8 (3·1) | 4·5 (3·1) | 1·8 (2·7) | 1·9 (2·7) | –2·6 (–3·3 –2·0) | · · | <0·0001 |
Data are n (%) or mean (SD). We estimated all values with multiple imputations for missing data. NA=not applicable.
Partial remission of diabetes is defined here as HbA1c ≤6·5% (≤48 mmol/mol) and full remission of diabetes is defined here as HbA1c ≤6·0% (≤42 mmol/mol) and no use of antihyperglycaemic medicines, each for the previous 12-month period.
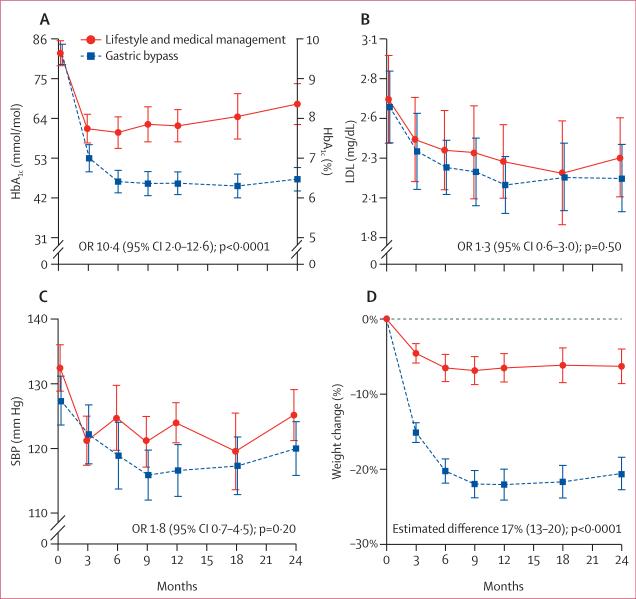
Patients in the gastric bypass group lost more weight than patients in the lifestyle and medical management group; the groups differed in weight loss by 17 percentage points (95% CI 13–20) at 24 months. Weight loss was maintained in both groups between 12 and 24 months. The mean HbA1c was maintained between 12 and 24 months in the gastric bypass group, whereas mean HbA1c increased from 12 and 24 months in the lifestyle and medical management group (table 2, figure 2). Mean fasting glucose was significantly lower in the gastric bypass group, and fasting C-peptide concentrations at 24 months were significantly lower than baseline for the gastric bypass group (p<0·0001), but not for the lifestyle and medical management group (p=0·52). 15 (25%) of patients in the gastric bypass group had full remission of diabetes and 25 (42%) of patients in this group had partial remission, whereas no patients in the lifestyle and medical management group had remission.
Figure 2.
Key efficacy outcomes for (A) HbA1c, (B) LDL cholesterol, (C) systolic blood pressure, and (D) weight change from baseline
SBP=systolic blood pressure. Error bars represent 95% CIs.
Patients in the gastric bypass group had lower mean blood pressure than patients in the lifestyle and medical management group at 24 months (figure 2), but there was no difference between groups in the proportion of patients with systolic blood pressure lower than 130 mm Hg (table 2). The proportion of patients with systolic blood pressure lower than 130 mm Hg without antihypertensive medicines increased by 32 percentage points (20% to 52%) from baseline with gastric bypass compared with 14 percentage points (14% to 28%) with lifestyle and medical management (OR 3·7, 95% CI 1·4–9·6, p=0·0046). LDL cholesterol con centrations were not different between groups at 24 months, but more participants who underwent gastric bypass obtained LDL cholesterol lower than 2·59 mmol/L without medicines for dyslipidaemia (figure 2). Overall, seven patients (12%) in the gastric bypass group but none in the lifestyle and medical management group obtained the composite triple endpoint at 24 months without medicines for glycaemia, LDL cholesterol, or blood pressure.
Table 3 shows clinically important adverse events that occurred during the first 2 years of the study (all 120 enrolled and assigned participants were included in the analysis of adverse events and nutritional deficiencies). Per 1000 person-years of exposure, the gastric bypass group had 407 adverse events, and the lifestyle and medical management group had 233. With a negative binomial model adjusted for site, the event rate for the gastric bypass group differed from that in the lifestyle and medical management group by a factor of 1·67 (95% CI 0·98–2·87, p=0·06). Although most of the first-year adverse events in the gastric bypass group were directly related to surgery, the 19 events for year 2 included nine gastrointestinal events and four serious falls, three of which led to bone factures. Across both years of the study, the gastric bypass group had seven serious falls with five fractures, compared with three serious falls and one fracture in the lifestyle and medical management group. All fractures happened in women. Additionally, eight infections occurred in the gastric bypass group compared with four in the lifestyle and medical management group.
Table 3.
Clinically important adverse events associated with the interventions
| Lifestyle and medical management (n=59) |
Gastric bypass (n=60) |
|||||
|---|---|---|---|---|---|---|
| Baseline to 12 months | 12–24 months | Total | Baseline to 12 months | 12–24 months | Total | |
| Surgical complications | ||||||
| Distal anastomotic leak leading to sepsis, brain injury, and amputation below the knee | · · | · · | · · | 1 | · · | 1 |
| Proximal anastomotic leak | · · | · · | · · | 1 | · · | 1 |
| Anastomotic ulcer | · · | 1* | 1 | 2 | 1 | 3 |
| Anastomotic stricture | · · | · · | · · | 2 | · · | 2 |
| Wound infection | · · | · · | · · | 1 | · · | 1 |
| Wound haematoma | · · | · · | · · | 1 | · · | 1 |
| Pouch gastritis | · · | · · | · · | 1 | · · | 1 |
| Small-bowel obstruction | · · | · · | · · | 2 | · · | 2 |
| Gastrointestinal | ||||||
| Acute pancreatitis | 1 | 2 | 3 | · · | · · | · · |
| Pancreatic carcinoma | 2 | · · | 2 | · · | · · | · · |
| Cholelithiasis | · · | · · | · · | · · | 1 | 1 |
| Abdominal pain | 1 | 1 | 2 | 4 | 2 | 6 |
| Reflux oesophagitis | 2 | · · | 2 | · · | 3 | 3 |
| Duodenitis | 1 | · · | 1 | · · | · · | · · |
| Cardiovascular | ||||||
| Deep venous thrombosis | · · | · · | · · | · · | 1 | 1 |
| Congestive heart failure | · · | 1 | 1 | · · | · · | · · |
| Renal | ||||||
| Nephrolithiasis | 1 | · · | 1 | 1 | · · | 1 |
| Metabolic | ||||||
| Diabetic ketoacidosis | · · | 1 | 1 | · · | · · | · · |
| Musculoskeletal | ||||||
| Loss of body strength | · · | · · | · · | 1 | 1 | |
| Amputation below the knee | · · | · · | · · | 1 | · · | 1 |
| Toe amputation | · · | · · | · · | 1 | · · | 1 |
| Neurological | ||||||
| Herniated spinal disc with foot drop | · · | · · | · · | 1 | · · | 1 |
| Multiple sclerosis | · · | · · | · · | · · | 1 | 1 |
| Partial third cranial nerve palsy | 1 | · · | 1 | · · | · · | · · |
| Psychiatric | ||||||
| Depression | · · | 1 | 1 | · · | · · | · · |
| Suicide attempt | 1 | · · | 1 | · · | · · | · · |
| Miscellaneous | ||||||
| Fall with fracture | 1 | · · | 1 | 2 | 3 | 5 |
| Fall with other injury | 2 | · · | 2 | 1 | 1 | 2 |
| Hypertension with admission to hospital | · · | · · | · · | 1 | · · | 1 |
| Unwanted pregnancy | · · | · · | · · | 1 | · · | 1 |
| Abnormal uterine bleeding | 1 | · · | 1 | · · | · · | · · |
| Infections | 2 | 2 | 4 | 3 | 5 | 8 |
| Totals | 16 | 9 | 25 | 27 | 19 | 46 |
··=no data
Participant was randomly assigned to lifestyle and medical management but obtained a Roux-en-Y gastric bypass outside the study.
Over 24 months, biochemical abnormalities and nutritional deficiencies (table 4) were noted more frequently in the gastric bypass group; mainly a greater incidence of anaemia, low serum ferritin, hypoalbuminaemia, hypocalcaemia, raised parathyroid hormone, and low vitamin D. Iron deficiency was seen more often in women in the gastric bypass group; 29 (48%) participants in the gastric bypass group, and all three of the participants originally assigned to lifestyle and medical management who crossed over, took iron supplements. 49 nutritional deficiencies were noted in the gastric bypass group during the second year, compared with 14 in the lifestyle and medical management group.
Table 4.
Biochemical abnormalities and nutritional deficiencies associated with the interventions
| Lifestyle and medical management (n=59) |
Gastric bypass (n=60) |
|||||
|---|---|---|---|---|---|---|
| At baseline | Baseline to 12 months | 12–24 months | At baseline | Baseline to 12 months | 12–24 months | |
| Blood haemoglobin <55 mmol/L | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | 3 (5%) | 5 (9%) |
| Serum abnormalities | ||||||
| Low ferritin | 2 (3%) | 4 (7%) | 0 (0%) | 1 (2%) | 8 (14%) | 11 (20%)* |
| Low albumin | 1 (2%) | 5 (9%) | 3 (6%) | 4 (7%) | 14 (25%)† | 8 (14%) |
| Low vitamin B1‡ | 1 (2%) | · · | · · | 5 (11%) | 12 (31%) | 3 (8%) |
| Low serum vitamin B12 | 0 (0%) | 3 (6%) | 2 (4%) | 2 (3%) | 1 (2%) | 0 (0%) |
| Low calcium | 0 (0%) | 2 (4%) | 1 (2%) | 3 (5%) | 11 (19%)† | 8 (14%)† |
| High parathyroid hormone§ | 3 (5%) | 0 (0%) | 2 (3%)§ | 4 (7%) | 1 (2%) | 10 (17%)† |
| Low vitamin D¶ | 21 (49%) | 22 (66%) | 17 (50%) | 31 (70%) | 24 (59%) | 21 (53%) |
| Vitamin D¶ <6·7 nmol/L | 12 (28%) | 6 (15%) | 5 (15%) | 15 (34%) | 11 (27%) | 7 (18%) |
| Total number of abnormalities, excluding low vitamin B1‡ | 18 (31%) | 21 (38%) | 14 (28%) | 29 (48%) | 49 (86%) | 49 (88%) |
p<0·01 for treatment difference.
p<0·05 for treatment difference.
Vitamin B1 was not assayed for patients in Taiwan or after baseline for patients receiving lifestyle and medical management.
One participant was confirmed to have primary hyperparathyroidism.
25-hydroxy vitamin D; vitamin D was not assayed for patients in Taiwan.
Participants were counted in both timeperiods if an abnormality persisted; we determined the results in the clinical laboratories at each study site; unless otherwise specified, we determined abnormalities on the basis of the local laboratory's reference range; not all participants had all tests. Different denominator numbers were available for each test.
Discussion
In our study, the benefit of adding Roux-en-Y gastric bypass to intensive lifestyle and medical management in patients with type 2 diabetes persisted through 2 years, and the triple endpoint was reached in 43% of participants who received gastric bypass compared with 14% of those given lifestyle and medical management alone (panel). The difficulty of attaining the triple endpoint goal, with its potential metabolic improvements, in patients with type 2 diabetes is well documented,11,12 so this substantial improvement 2 years after gastric bypass surgery is encouraging. The ADA triple endpoint is aimed at treating the key attributes of the metabolic disturbance of diabetes, with the goal of reducing risk of macrovascular and microvascular complications. Notably, the gastric bypass group needed fewer medicines to achieve triple endpoint goals, underscoring the apparent metabolic benefit of surgery.
The benefit of surgery remained constant during the second year despite minor reductions in endpoint achievement in both groups. These small reductions in endpoint achievement in both treatment groups over the second year underscore the difficulty in maintaining full metabolic control in patients with diabetes.
Weight loss was substantially greater at 2 years for participants who had a gastric bypass, even compared with the sustained weight loss in the lifestyle and medical management group. The stable weights across the second year of the trial suggest sustained treatment benefit in both groups. Furthermore, waist circumference—a measure of intra-abdominal adiposity—was consistent with the weight outcomes. The results from the third year of the STAMPEDE trial7 show nearly similar maintenance of weight loss compared with the first year, although the lifestyle and medical management group in our study lost more weight after 1 year (7·5%) than the corresponding group in STAMPEDE (4·3%).
Most of the improvement in triple endpoint metabolic control in the gastric bypass group was attributable to better glycaemic control, because 75% of the gastric bypass group reached the goal of HbA1c less than 7·0% (53 mmol/mol) versus 24% of the lifestyle and medical management group by 24 months. However, only seven patients (12%) who underwent gastric bypass achieved the triple endpoint goal without medicines. Gastric bypass was associated with significant improvements in most glycaemic measures, including FBG and fasting C-peptide. These findings are consistent with other randomised trials of bariatric surgery for diabetes,3–8 where the focus has been mainly on glycaemic control. In the report of 3 year outcomes of the STAMPEDE trial7 comparing Roux-en-Y gastric bypass and sleeve gastrectomy to medical treatment, 65% of patients who had Roux-en-Y bypass had HbA1c less than 7·0% (53 mmol/mol) compared with 40% of patients treated medically.
In our study, only 25% of the gastric bypass group achieved full remission, and 42% achieved partial remission, less than reported in other trials. Results from randomised trials 1 year after bariatric surgery3–5,27 have shown that 50% to 90% of patients had partial remission (HbA1c lower than 6·5% [48 mmol/mol]) without diabetes medicines for at least 1 year). A study by Mingrone and colleagues6 reported that 75% had partial remission at 2 years in the gastric bypass group. The STAMPEDE trial7 showed that 35% of the gastric bypass group had HbA1c lower than 6·0% (42 mmol/mol) without diabetes medicines at 3 years. Compared with our study, diabetes remission at 2 years and later was also more frequent in the Swedish Obesity Study in patients with a mean diabetes duration of 2·9 years, but the criterion for remission was FBG rather than HbA1c.2 Additionally, the Swedish Obesity Study used several types of surgery. Possible explanations for the lower rate of remission in our study include greater severity of disease (mean HbA1c 9·6% [81 mmol/mol]) and longer duration of diabetes (8·9 years in our population),22 although other randomised trials with higher rates of remission have included participants with average diabetes durations of 6·4–10·4 years.3–6,27 Additionally, our definition of remission was more rigorous than in some other studies, needing sustained HbA1c less than the target without any diabetes medicines at all visits from 12 to 24 months.
Glycaemic control deteriorated in the lifestyle and medical management group despite sustained weight loss, although improved glycaemic control in the gastric bypass group was sustained. The reason for deterioration in glycaemic control despite stable weight control in the lifestyle and medical management group is unclear, but might suggest greater improvements in weight loss in the first year, difficulty in maintaining compliance, or progression of diabetes.
At baseline, 47% of our participants were not hypertensive (data not shown), although 71% were on antihypertensive medicines. The addition of gastric bypass to lifestyle and medical management did not significantly improve attainment of systolic blood pressure less than 130 mm Hg, but when blood pressure was assessed as a continuous variable, both systolic and diastolic blood pressure were significantly improved in the gastric bypass group. Overall, the evidence suggests that surgery leads to a slight improvement in blood pressure, but no benefit for LDL cholesterol.
The frequency and severity of clinically important adverse events in the gastric bypass group were higher than reported in other trials of surgery for type 2 diabetes.3–8 This result might be because of more rigorous surveillance and regular biochemical monitoring of potential nutritional deficiencies in our study. The occurrence of adverse events remained higher in the surgical group during the second year than in the lifestyle and medical management group, including expected events such as abdominal pain and unexpected events such as falls with bone fractures, and infections. Biochemical abnormalities and nutritional deficiencies were unsurprisingly common in the gastric bypass group. Despite protocol requirements for supplementation, we were often not able to correct deficiencies with mineral and vitamin supplements, perhaps because of poor compliance. Of particular concern is the increased risk of calcium deficiency, vitamin D deficiency, secondary hyper parathyroidism, osteoporosis and fracture rate in the gastric bypass group. Nutritional deficiencies are likely to be a greater challenge in general practice, outside a well controlled trial and where compliance is likely to be lower.
Supplementary Material
Panel: Research in context.
Systematic review
During planning of the study, we identified reports of diabetes improvement and remission after bariatric surgery, but no randomised controlled trials testing the role of bariatric surgery in type 2 diabetes. We searched PubMed for English-language articles published up to Nov 20, 2014, with the terms “bariatric surgery”, “diabetes”, and “clinical trial”. Since 2008, seven reports of randomised trials of bariatric surgery for diabetes have been published,3–9 six specifically testing the role of Roux-en-Y gastric bypass,3–7,9 including our report of the results from the first year of the Diabetes Surgery Study.9 Ours is the only study with the diabetes control triple endpoint of glycaemia, blood pressure, and lipids; all other reports have focused on a main glycaemic endpoint. Some systematic reviews and meta-analyses addressed the role of bariatric surgery in diabetes treatment.22–26 All trials and reviews showed improvement in glycaemic control after surgery, and several suggested a substantial proportion of patients that undergo surgery have remission of diabetes after gastric bypass—50%–90% at 1 year and up to 75% at 2 years. The accounting of adverse events has generally not been fully detailed in studies published so far.
Interpretation
The 2-year results of our Diabetes Surgery Study show that Roux-en-Y gastric bypass significantly improves attainment of the diabetes control triple endpoint, mainly by improving glycaemic goal achievement. Because intensive medical and lifestyle treatment was applied throughout the 2 study years and first-year weightloss was maintained in the lifestyle and medical management group, the surgical benefit is well defined. Although mean HbA1c was lower in the patients who received bariatric surgery than those who received lifestyle and medical management alone, most surgery patients did not reach the triple primary endpoint goal. Additionally, of patients who underwent gastric bypass in our study, 42% had partial remission and 25% had full remission of their diabetes, which contrasts with other studies. Adverse events were more frequent in the surgical group, as were nutritional deficiencies, despite protocol-based supplementation. Larger and longer trials will be needed to fully assess the role of bariatric surgery as a treatment for type 2 diabetes; however, on the basis of our results, the expectation for patients with type 2 diabetes is that gastric bypass surgery can probably reduce disease severity but not induce remission, and also substantially increases the risk of adverse events.
Acknowledgments
The Diabetes Surgery Study was supported by Covidien, Mansfield, Massachusetts, who provided funds for the following five centres: University of Minnesota, Minneapolis, MN, USA; Mayo Clinic, Rochester, MN, USA; Columbia University, New York, NY, USA; National Taiwan University Hospital, Taipei, Taiwan; Min-Sheng General Hospital, Taoyuan, Taiwan. These studies were supported partly by NIH/National Institute of Diabetes and Digestive and Kidney Diseases Nutrition Obesity Research Centers (grant number P30 DK050456), and partly by grants to Columbia University from the National Center for Advancing Translational Sciences, NIH (formerly the National Center for Research Resources; numbers UL1 TR000040 andUL1 RR024156. Nyra Wimmergren (University of Minnesota, Minneapolis, MN, USA), Shu-Chun Chen, and Meng-Chieh Chen (National Taiwan University) were study coordinators. David Nelson (Minnesota VA Health Care System, Minneapolis, MN, USA), Victor J Stevens (Center for Health Research, Portland, OR, USA), and J Michael Gonzalez-Campoy (Minnesota Center for Obesity, Metabolism and Endocrinology, Eagan, MN, USA) were members of the Data Safety Monitoring Board. We thank Merrie J Harrison, and Alain DuChene, Terri Schultz, and Greg Thompson (University of Minnesota Data Coordinating Center) and Stanley E Williams (University of Minnesota) for their contributions.
Footnotes
Contributors
JEC, QW, and AJT had full access to all data in the study and were responsible for the integrity of the data and the accuracy of the analysis. SI, CJB, JK, JEC, WBI III, AJT, DBL, RWJ, LA, KB, JPB, and W-JL conceived the idea and designed the study. JK, W-JL, CJB, AJT, KC, LA, WBI III, AV, L-MC, MGS, PSL, MDJ, JPB, AEO, and JPB obtained the data. JEC, CJB, JK, WBI III, AJT, QW, DBL, LA, AV, MGS, MDJ, analysed and interpreted the data. CJB, SI, JK, JEC, WBI III, AJT, DBL, RWJ, MGS, MDJ, and JPB drafted the manuscript. CJB, JK, W-JL, JEC, WBI III, AJT, DBL, KC, LA, AV, L-MC, MGS, PSL, MDJ, and JPB revised the manuscript for content. JEC, AJT, and QW did the statistical analysis. SI obtained funding. JK, W-JL, JEC, CJB, AJT, DBL, KC, LA, WBI III, AV, L-MC, MGS, PSL, MDJ, and JPB gave administrative, technical, or material support. SI, JK, JEC, WBI III, CJB, AJT, AV, L-MC, MGS, MDJ, and JPB supervised the study. HAB, JLS, AEO were study coordinators.
Declaration of interests
SI serves on an advisory board member for Novo Nordisk, USGI, and Medica, consults for Metamodix, and receives grant support from Covidien, EnteroMedics, and ReShape Medical. JK has received institutional grant support from Covidien, and personal support for expert testimony and participation in the Speaker's Bureau for Takeda. JEC has received institutional and personal grant support from Covidien and National Institutes of Health (NIH), and travel expenses and personal support for the US Food and Drug Administration advisory panel on pulmonary drugs. CJB has received institutional grant support from Covidien and consulting support from Novo Nordisk and EneroMedics. AJT has received salary support from Covidien for the Diabetes Surgery Study, and supplemental salary support from the Minnesota Obesity Center. LA has received institutional grant support from Covidien. AV has recieved consulting support from Genentech, Sanofi-Aventis, and Novartis; and grant support from Biokier, Novartis, and GI Dynamics. PSL has received institutional grant support from Covidien. MDJ has received institutional grant support from Covidien, and consulting support from Novo Nordisk, Eisai, Genentech and Takeda. JLS has received fees from ReShape Medical. HAB received NIH support for the Look AHEAD study. All other authors declare no competing interests.
References
- 1.Carlsson LMS, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 2.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 3.Halperin F, Ding S- A, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149:716–26. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149:707–15. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract. 2013;101:50–56. doi: 10.1016/j.diabres.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 7.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med. 2014;370:2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 9.Ikramuddin S, Korner J, Lee W-J, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–49. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Standards of medical care in diabetes–2014. Diabetes Care 2014. 37(suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 11.Bertoni AG, Clark JM, Feeney P, et al. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complicat. 2008;22:1–9. doi: 10.1016/j.jdiacomp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Wong K, Glovaci D, Malik S, et al. Comparison of demographic factors and cardiovascular risk factor control among US adults with type 2 diabetes by insulin treatment classification. J Diabetes Complicat. 2012;26:169–74. doi: 10.1016/j.jdiacomp.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas AJ, Bainbridge HA, Schone JL, et al. Recruitment and screening for a randomized trial investigating Roux-en-Y gastric bypass versus intensive medical management for treatment of type 2 diabetes. Obes Surg. 2014;24:1875–80. doi: 10.1007/s11695-014-1280-4. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pi-Sunyer X, Blackburn G, Brancati F, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of medical care in diabetes2008. Diabetes Care. 2008;31(suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–35. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–10. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 21.Little RJA, Rubin DB. Statistical analysis with missing data. John Wiley & Sons; London: 2014. [Google Scholar]
- 22.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 24.Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149:1323–29. doi: 10.1001/jamasurg.2014.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309:2250–61. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 26.Müller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg. 2015;261:421–29. doi: 10.1097/SLA.0000000000001014. [DOI] [PubMed] [Google Scholar]
- 27.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.