Abstract
The rotation of the earth and associated alternating cycles of light and dark–the basis of our circadian rhythms–are fundamental to human biology and culture. However, it was not until 1971 that researchers first began to describe the molecular mechanisms for the circadian system. During the last few years, groundbreaking research has revealed a multitude of circadian genes affecting a variety of clinical diseases, including diabetes, obesity, sepsis, cardiac ischemia, and sudden cardiac death. Anesthesiologists, in the operating room and intensive care units, manage these diseases on a daily basis as they significantly impact patient outcomes. Intriguingly, sedatives, anesthetics, and the ICU environment have all been shown to disrupt the circadian system in patients. In the current review we will discuss how newly acquired knowledge of circadian rhythms could lead to changes in clinical practice and new therapeutic concepts.
Circadian Rhythms: ‘External Cues’ vs ‘Internal Clock’
What would happen if there was no light? What would control the biological rhythm of a human being or a plant? Jean-Jacques d’Ortous de Mairan asked this question in 17291 when observing the movement of the leaves of a plant in synchrony with sun light. He noted that a 24-hour pattern in the movement of the leaves of the Mimosa pudica plant continued even when the plant was kept in constant darkness. He concluded that there must be an ‘internal clock’ that provided a circadian rhythm even without the presence of light. More than 200 years later, Jürgen Aschoff applied similar methods to investigations in humans by building an underground “bunker” to isolate human subjects from any external environmental cues.2 Aschoff’s research demonstrated that humans exhibited a persistent 25-hour biological cycle. This 25-hour cycle is similar to previous studies examining the circadian rhythms of blind persons.3 However, it was not until researchers began experimenting with the circadian system in Drosophila melanogaster in the 1970’s, that identification of gene loci such as Clock or Period were identified as important regulators of these processes4 (Figure 1). Results demonstrated that when Clock or Period genes were disrupted in animals, the circadian rhythm was severely compromised in conditions of constant darkness. To emphasize the endogenous self-sustained nature of biological rhythms, Franz Halberg coined the term circadian (Latin: circa=about; dies=day) time to refer to daily rhythms that are endogenously generated.5 As such, any biological process in the body that repeats itself over a period of approximately 24-hours and maintains this rhythm in the absence of external stimuli is termed a circadian rhythm.
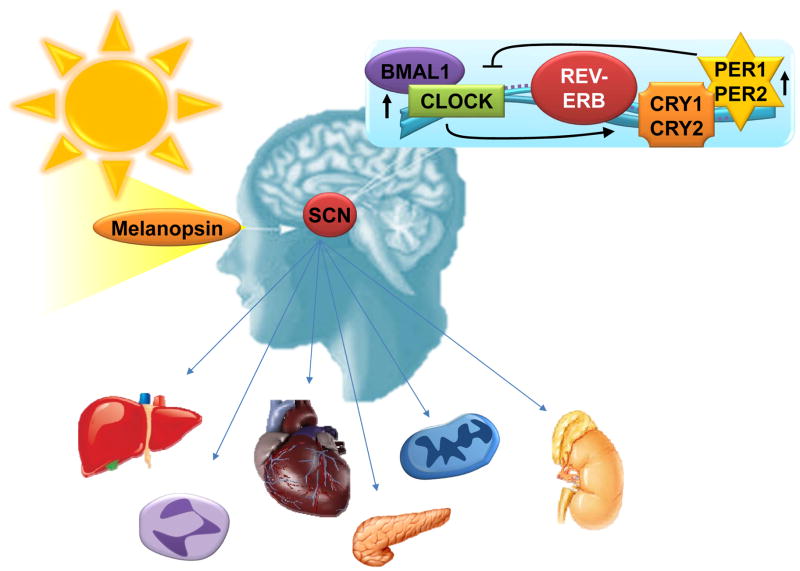
Figure 1. Regulatory mechanisms of the circadian system.
The suprachiasmatic nucleus (SCN) in the brain is the central regulator of circadian rhythmicity. External stimuli such as light determine ‘Zeitgeber’ time. Light via melanopsin receptors in the retinal ganglion cells lead to the transcriptional induction of Clock and Bmal1 which leads in turn to the induction of Rev-Erb, Per1, Per2, Cry1 and Cry2. Via feedback inhibition of Clock and Bmal1 a cycle will be terminated and a new can begin. Hormonal and humoral factors are supposed to control circadian rhythms in peripheral organs.
In contrast, the term “zeitgeber” (German for “time giver” or “synchronizer”) was first used by Jürgen Aschoff to emphasize the existence of exogenous (external) cues that influence the timing of these internal clocks.6 To maintain circadian synchrony, ‘zeitgebers’ induce changes in the concentrations of the molecular components of the clock to levels consistent with the appropriate stage in the 24-hour cycle, a process termed entrainment (Figure 1). Thus, circadian entrainment describes a biological rhythm that is synchronized with the external physical environment. The most dominant zeitgeber appears to be sunlight, not surprising, as it is the strongest and most dependable representation of the time of day.7,8
As such, a hallmark of the mammalian circadian pacemaker – located in the suprachiasmatic nuclei (SCN) within our brain – is its ability to be synchronized by light, thus allowing organisms to adapt to temporal variations in natural light conditions.9 Photic stimuli are transmitted from the retina to target neurons in the brain, where they are transduced to the molecular clockwork.10,11 Light activation of melanopsin receptors11 in the retinal ganglion cells leads to the transcriptional induction of circadian rhythm proteins (such as Clock, Bmal1, Rev-Erb, Period 1, Period 2, Cryptochrome 1 and Cryptochrome 2) and concomitant synchronization. Peripheral tissues display oscillations in circadian rhythm protein expression similar to those of the brain,12,13 likely through secreted signaling molecules (Figure 1).10,14,15
Although it is now accepted that a component of our genome reflects the evolutionary influence of the earth’s daily rotation about its axis,16 little is known about how disturbances in this circadian system affect patient health and clinical outcomes. Further basic and clinical research addressing the importance of circadian rhythms in the context of critical care and anesthesia is highly warranted and necessary to develop a better understanding of complex pathophysiology and novel diagnostic and therapeutic approaches.
The Biology of Light
Earth’s 24-hour rotational cycle with its night and dark periods has existed for more than 4 billion years and has led to the evolution of circadian rhythms in most organisms. Studies in numerous species have demonstrated that daylight is a powerful synchronizer which resets, in an intensity-dependent manner, endogenous circadian pacemakers.17–21
One of the most remarkable and measurable biological effects of light is suppression of melatonin production.22 In 1980, a study demonstrated that bright light, both sunlight and intense artificial light, could suppress melatonin production in humans.23 This research confirmed that humans have biological rhythms cued by sunlight and further, suggested that bright artificial light could be substituted for sunlight in order to experimentally, and perhaps therapeutically, manipulate biological rhythms in humans.
Sunlight has an intensity of greater than 10,000 lux, while ordinary room light is approximately 200 lux. It is compelling that light of different intensities appears to have different effects on the human body. For greater than 4 billion years, life outside of the Earth’s poles and deep oceans has evolved under consistent bright days and dark nights. With the widespread adoption of artificial electric lighting approximately 150 years ago, humans began brightly illuminating their nocturnal environments. Beyond affecting circadian rhythms, recent evidence suggests that exposure to unnatural light cycles increases the risk for cancer24, sleep disturbances25, and mood disorders26. Further, exposure to nighttime light has been linked to changes in metabolism. Shift workers who experience sustained nighttime illumination are at increased risk for cardiovascular disease and elevated body mass index27–30 and nighttime light exposure at home is associated with increased body mass, waist circumference, elevated triglyceride levels, and poor cholesterol balance.31
Impact of Circadian Rhythms on Specific Clinical Disorders
While the analysis of circadian rhythm proteins and their misalignment in humans is difficult to assess, melatonin levels seem to be an excellent surrogate for a ‘normal’ circadian rhythm.32 As such sleep restriction has been shown to affect melatonin levels and circadian rhythms in humans.32 On a molecular level, a mutation of the human circadian rhythm protein Per2 leads to a sleep disorder that is known as ‘familial advanced sleep-phase syndrome (FASPS)’.33 Interestingly, to identify somebody with this disease, tests on melatonin levels after dawn are widely used and validated. 34
Recent research has revealed that sleep duration has decreased in the United States.35 Simultaneously the prevalence of metabolic disease has increased over the same time period.35 Numerous cross-sectional and prospective clinical studies have demonstrated that shorter-duration and poor-quality sleep predicts the development of obesity and type-2 diabetes after age, body-mass-index and various other confounding variables are taken into account.32,36–41 In one study, sleep restriction to four hours for six consecutive nights led to an impaired insulin sensitivity in a glucose challenge test.42 These observations argue for the involvement of circadian rhythms in the pathogenesis of diseases including diabetes and obesity. However, specific knowledge of circadian rhythm proteins and their molecular mechanisms will be required to prove causality.
Disruption of circadian rhythms based on genetic studies, leads to a variety of central nervous system disorders. Examples include the above mentioned sleep disorders (delayed or advanced sleep phase disorder33,43) but also bipolar affective disorders.44 Specific genetic variations in circadian clock genes have been identified for bipolar disease and schizophrenia,45 and genetic polymorphisms in several clock genes have been linked to sleep disorders.46
In addition to central nervous system disorders, epidemiological studies have demonstrated that late-shift and overnight workers have a higher incidence of cardiovascular disease.27,47–52 Evidence of an association between disrupted circadian rhythms and cardiovascular disease has been established in research on blood pressure,53 heart rate,54 endothelial function, 55 and the onset of myocardial infarction and stroke.55,56 These clinical conditions all have distinct circadian patterns and disruption of biological rhythms may in particular contribute to the development of cardiovascular disease.57 Other clinical diseases including cancer, diabetes and metabolic syndrome have also been associated with disrupted circadian rhythms.36–40
Unfortunately, genetic studies on circadian rhythm proteins in relation to those specific diseases are rare. A few polymorphisms of clock genes have been found in humans that are associated with metabolic diseases. For example, polymorphisms in Clock and Bmal1, have been linked to features of the metabolic syndrome. In small sample populations, polymorphisms in the Clock gene have been correlated with predisposition to obesity,58,59 and two Bmal1 haplotypes are associated with type 2 diabetes and hypertension.60 Polymorphisms within other clock core genes (i.e. Per2) have been associated with hypertension and high fasting blood glucose in studies of similar sample size.61
Recently, several genome-wide association studies led to the unexpected discovery that melatonin, a hormone implicated in seasonal and circadian rhythms -as pointed out earlier-, may be important in the regulation of mammalian glucose levels.62,63 Indeed, genetic variants of the melatonin 1B receptor gene (MTNR1B) increase type 2 diabetes risk. In line with their role in type-2 diabetes, MTNR1B is expressed in pancreas cells, and melatonin modulates glucose-stimulated insulin secretion.64 Interestingly, melatonin secretion is reported to be impaired in type 2 diabetic patients65 and the melatonin profile relative to the feeding/fasting cycle is reversed when individuals are subjected to forced dyssynchrony.66 These findings raise the possibility that disruption of circadian systems, either directly at the level of altered clock gene expression, or indirectly through effects on melatonin, may contribute to human metabolic syndrome and cardiovascular disease.
Novel Molecular Mechanisms
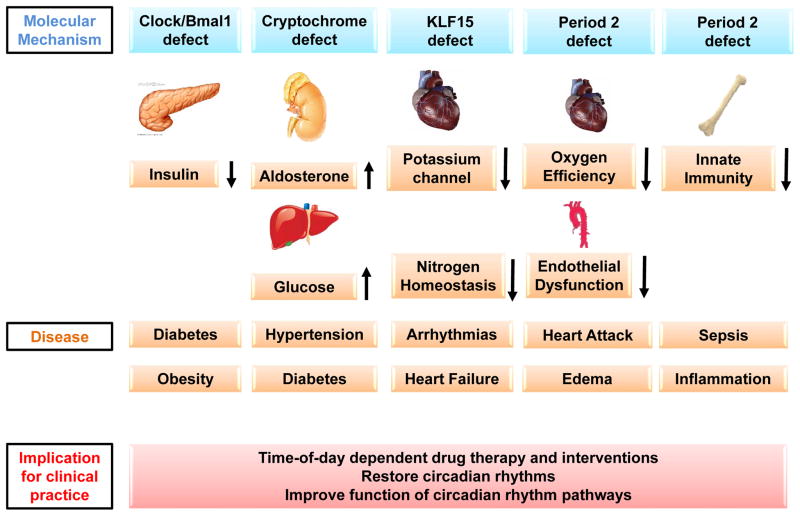
The organ-specific role of circadian rhythm proteins in the development of clinical disease has been an area of intense research over the last few years and significant advances in this field have recently been achieved. When specific circadian rhythm proteins are absent in mice, these animals develop similar medical conditions to those described in late-shift workers or patients with presumably disrupted circadian rhythms (Figure 2).
Figure 2. Disruption of the molecular ‘clock’ and disease development.
Numerous resent studies provide evidence that the circadian clock influences the development and progression of disease in an experimental setting. Non-functional circadian rhythm proteins in peripheral organs were linked to very specific disease (e.g. Clock-diabets, Cryptochrome-hypertenstion, Per2-heart attack etc.). These findings indicate that time-of-day dependent drug therapy or interventions might be imperative. However, more important seems to be the restoration of circadian rhythms to improve the function of circadian rhythm pathways such as insulin secretion, inflammation or metabolism.
The Clock protein represents an important pacemaker of the circadian network acting in the SCN (Figure 1). When the Clock gene is deleted in mice, these animals develop ‘late-shift diseases’ including diabetes15 and cardiovascular disease.67 Clock even directly influences the endocrine function of the pancreas. When Clock is specifically deleted from the pancreas, mice are unable to secrete insulin and develop type-1 diabetes (Figure 2).68
Cryptochromes are another group of proteins known to play a role in circadian rhythms and have also been linked to the development of disease. Crypochrome deficient mice exhibited enhanced aldosterone production leading to arterial hypertension69 or disrupted regulation of hepatic gluconeogenesis and insulin resistance (Figure 2).70
Acute myocardial infarction is one of the most well documented acute disease states with a diurnal or circadian incidence.71–73 Investigations into the molecular mechanism of this clinical observation linked the circadian rhythm protein Per2 to myocardial ischemia.13,15,71,74 In a mouse model of myocardial infarction, infarct sizes exhibited a circadian pattern. Furthermore, Per2 deficiency increased the size of myocardial infarcts. In contrast, when Per2 was overexpressed by intense daylight exposure, the size of the necrotic area after myocardial infarction was significantly reduced.13 This study also reported elevated Per2 levels in human heart tissue of patients suffering from ischemic heart failure (Figure 2).13
Further studies have linked Per2 to non-cardiac peripheral ischemia. Lack of the Per2 protein was injurious in a murine model for hind limb ischemia, leading to auto-amputation of the affected legs.75 In addition, Per2 seems to be involved in modulating the inflammatory response to tissue ischemia.71 Together, these findings indicate an important role for the circadian system, and in particular the Per2 protein, in tissue ischemia.
Sudden cardiac arrest, often a consequence of cardiac arrhythmias, also exhibits a circadian pattern. A recent study identified a transcription factor, krueppel like factor 15 (Klf15), as an important regulator of cardiac potassium channels.76 Klf15 was shown to be transcriptionally under the control of the circadian rhythm proteins. Disruption of certain circadian rhythm proteins (Bmal1 or Per2) resulted in marked action potential prolongation due to near complete elimination of the fast component of the transient outward potassium current. As a result, deficiency or excess of Klf15 caused loss of rhythmic QT variation, abnormal repolarization and enhanced susceptibility to ventricular arrhythmias. These findings point to circadian transcription of ion channels as a mechanism for cardiac arrhythmogenesis (Figure 2).
Finally, several recent studies found an association between circadian rhythms and the immune system. In particular, Toll-like receptors (TLRs) seemed to be substantially regulated in a circadian pattern. TLRs are a class of proteins that play a key role in innate and adaptive immunity and have been implicated in several diseases, including sepsis, various immunodeficiencies, atherosclerosis, asthma and myocardial ischemia.77 In this recent study, TLR9 exhibits circadian regulation in mice. This means that the inflammatory response to severe infection, as occurs in sepsis, is likely oscillating in a circadian fashion.78 Other studies have demonstrated that the transcription factor Rev-Erb (Figure 1), an important regulator of the circadian network, also influences the synthesis and secretion of Interleukin-17 from T helper cells.79 Interleukin-17 producing T helper cells are pro-inflammatory immune cells and circadian disruption has been associated with elevated intestinal T(H)17 cell frequencies and increased susceptibility to inflammatory disease (Figure 2).79
Taken together, this group of recently published studies indicate a very important role for the circadian rhythm network and its regulation in the development and progression of various diseases, including diabetes, hypertension, heart disease and sepsis. More specifically, this work has begun to describe the precise functions of the circadian proteins in peripheral organs and the mechanisms by which they may contribute to many of the most important human diseases. Further clinical studies in humans are needed to apply this emerging knowledge to novel diagnostic and therapeutic strategies.
Implications for Clinical Practice
An improved understanding of the association between circadian rhythms and specific disease states could lead to significant changes in clinical practice and patient outcomes (Figure 2). Timing of therapeutic interventions, ranging from medication delivery to major surgery,80,81 may be altered based on comprehension of these biological systems. Research in chronotherapeutics82 has already revealed promising results by improving the therapeutic index of several drugs. Chronotherapeutics is defined as medical treatment administered according to a schedule that corresponds to a person’s daily, monthly, seasonal, or yearly biological clock, in order to maximize the health benefits and minimize adverse effects. For example, as a result of circadian variations in cholesterol synthesis (via the diurnal rhythmicity of the rate limiting enzyme HMG-CoA reductase) the efficacy of cholesterol-lowering statin medications is improved when these drugs are taken at bedtime. Interestingly, one of the first wide-scale application of chronotherapeutics was in the 1960s and entailed synthetic corticosteroids.83 However, the results did not necessarily translate to major changes in modern clinical practice or if changes were made they might not be recognized as chronotherapeutics.84,85
Recent studies on blood pressure medication made clear that bedtime administration is more effective than administration during morning hours. In addition, reduction in blood pressure during sleep has been identified as the most significant predictor of decreased cardiovascular risk in patients. As such, patients with chronic kidney disease have a high prevalence of increased blood pressure during sleep and a blunted sleep-time-related blood pressure decline (a ‘non-dipper’ pattern) which is associated with disease severity. Administration of blood pressure lowering drugs at bedtime could therefore lead to significant improvement is this clinical setting.80,86
Inpatient insulin dosing may be optimized by incorporating recent evidence that supports a circadian pattern to insulin secretion and insulin sensitivity87. Further studies are required to determine the appropriate dosing and glucose level associated with best outcomes given the known dysfunction in the circadian system associated with critical illness.88 Similarly, the improved clinical outcomes associated with intermittent versus continuous tube feeding may ultimately be explained by circadian patterns of hepatic metabolism.89
Circadian variation in immune system function may have broad impact in infectious disease states including sepsis and septic shock. The use of corticosteroids remains a common, albeit controversial adjunct therapy for septic shock.90 Sepsis has been shown to be associated with elevated serum concentrations of IL-17, which can in turn be dampened by treatment with hydrocortisone.91 Given the nature of circadian rhythms and a recently described regulation of IL-17 by the circadian clock,79 it is possible that the efficacy of corticosteroids in the management of sepsis could be greatly enhanced by synchronizing administration with these rhythms. Moreover, the clinical chronotherapy of corticosteroids might not only improve desired effects but, at the same time, avoid or minimize their side effects.
While some therapies, including anticoagulants, might already be administered at the correct time of day (we know, for example that in the early morning hours coagulation is enhanced) it remains unclear if these therapies are also effective when the normal circadian system has been disrupted. Successful pharmacotherapy may necessitate moving beyond identifying the optimal time of day for administration of a given drug (Figure 2). Indeed, restoration of a nonfunctional circadian system might be required before a treatment can be most effective. Daylight (and ‘real’ night) simulation on ICUs or novel drugs that could resynchronize the circadian system (Rev-Erb-agonists92) hold promise as emerging therapies to restore circadian function.
Circadian Rhythms in Anesthesia
Analgesics including narcotics and various local anesthetics are used on a daily basis by anesthesiologists. Early on researchers discovered a time of day dependency for morphine-induced analgesia and duration of action for local anesthetics. 93–95
Interestingly, recent reports support a strong association between surgery under general anesthesia and disturbances in circadian rhythms. It is proposed that these disturbances may lead to postoperative sleep and cognitive disorders.96–98 Specific medications used during general anesthesia are thought to play a role in these circadian disruptions. Clinically, many patients experience profound sleep disruption and delirium following surgery and general anesthesia. Managing this effect has the potential to help expedite postoperative recovery and improve postoperative cognitive function. Restoration or resetting of the circadian rhythm following general anesthesia might help to blunt or alleviate symptoms of insomnia, confusion, and post-operative delirium. The prevention of postoperative delirium is critically important given the strong association between post-operative delirium and increased morbidity and mortality.99–104
Circadian Rhythms in Critical Care Medicine
a) Effects on Sleep and Cognitive Function
Critically ill patients suffer from similar disruption in circadian rhythms and the clinical effects of altered sleep-wake cycles and cognitive dysfunction.105–109 Medications utilized in the care of critically ill patients overlap and have similar pharmacology with those used in general anesthesia and thus the clinical effects of altered-sleep wake cycles and cognitive function are similar. Sedative medications utilized by critical care practitioners in the treatment of agitation, delirium and insomnia likely lead to worsening of these disorders.110–112 Benzodiazepines appear to be one of the worst offenders and thus many experts advocate for discontinuing their use in critically ill patients.112–115 Unfortunately, almost all sedative agents are associated with delirium and appear to worsen sleep quality.110,111,116 Dexmedetomidine may be associated with the least risk of developing delirium.117–120
As discussed earlier, endogenous melatonin might play an important role in the development of circadian rhythms associated diseases.62,63 Critically ill patients tend toward altered sleep patterns but also abnormal levels of melatonin. Results on melatonin expression have generated interest in the use of exogenous melatonin and melatonin agonists to improve sleep and cognitive function.104,121 Earlier this year, a promising randomized controlled trial demonstrated effectiveness in the use of a melatonin agonist (Ramelteon) versus placebo in the prevention of delirium.121 In the context of a possible melatonin-circadian rhythm–clinical disease axis, studies on endogenous and exogenous melatonin, pharmacological agonists, and associated genetics could provide important insight into the development and treatment of critical illness.
In general, the hypothalamic-pituitary-adrenal (HPA) axis is also under the control of the circadian rhythm and plays an important role in critical illness and sleep wake patterns.122,123 A prospective, observational study involving 24 critically ill sedated patients demonstrated the 24-hr profiles of blood melatonin or cortisol were greatly disturbed or even abolished compared with the well-known rhythmic 24-hr patterns in healthy control subjects.124 Currently, there are gaps in our understanding of secretory patterns and control of cortisol during illness, and these gaps limit our ability to design optimal therapeutic regimens.123
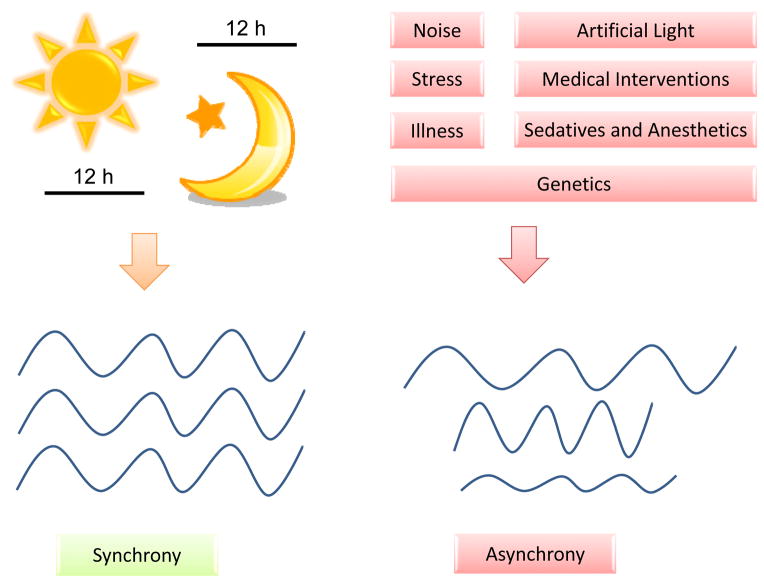
Finally, environmental factors play a substantial role in the disruption of sleep-wake cycles. Critically ill patients in the intensive care unit suffer from more frequent sleep deprivation and sleep disturbances than patients on a general ward.105,106,125 Several factors can contribute to the increase in altered sleep-wake cycles, including noise, patient-care interactions, mechanical ventilation, pain, artificial light, fatigue, stress, delirium and other cognitive dysfunction.126,127 These same factors likely contribute to the increased risk of developing severe circadian rhythm disruptions (Figure 3). Clinical research concentrating on the circadian disruptions and whether interventions to maintain normal sleep-wake can improve patient outcomes are warranted.
Figure 3. Factors influencing the circadian system.
While a regular day/night cycles with dark, quite nights are supposed to be the basis of a well synchronized circadian rhythm in humans, noise, interventions, artificial light and drugs on ICUs have proven to disrupt a functional circadian system and might lead to disease progression.
b) Effects on Sepsis and Disease Progression
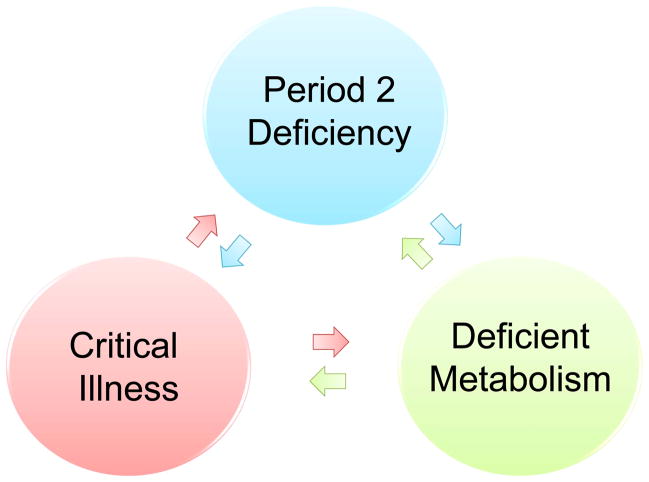
Critically ill patients, and in particular patients diagnosed with sepsis, have been shown to exhibit a very distinct metabolic phenotype.128 These patients experience dysfunction in mitochondria, endothelia, NO synthesis and pyruvate dehydrogenase.128 Previous studies have established that the above defects are all associated with a dysfunction of the circadian rhythm protein Per2 (Figure 4).13,71,75 Two recent clinical trials demonstrate disrupted circadian system function in patients with severe sepsis as demonstrated by urinary 6-sulfatoxy-melatonin production.129,130 This dysfunction was not observed in critically ill patients without sepsis.129 In a rat model of sepsis, survival improved in animals maintained on a circadian light/dark cycle.131 Thus, restoration of a circadian rhythm with normal Per2 levels could be beneficial in restoring the metabolic disruption associated with sepsis (Figure 4).
Figure 4. The link between critical illness and the circadian system.
Sepsis can lead to a disrupted function of mitochondria, nonfunctional NO synthesis and deficient glycolytic pathways. Period 2 (Per2) deficiency has been associated with such metabolic alterations in mice. Therefore one could hypothesize, that restoration of a circadian rhythm with a functional Per2 protein might help to restore a nonfunctional metabolic phenotype in sepsis and thereby alleviating illness and disease progression.
Therapy
a) General Considerations
Restoration of an intact circadian system is likely complex given the multifaceted etiology for these disruptions. Genetic factors (polymorphisms in core clock genes), medications (anesthetics, benzodiazepines), and environmental factors (noise, artificial lighting) can all result in disruption of the circadian system. Biologically, circadian rhythms are controlled by a cyclical expression of circadian genes. Mutations in these genes could result in a modification or disruption of the circadian oscillator and therefore it is important to analyze genetic factors that may contribute to circadian disruption.132 The discovery of novel genes involved in circadian rhythm-related diseases will open up new opportunities for therapy and can serve as biomarkers for identification and prognosis (Figure 5).
Figure 5. Model of disease development.
Several environmental and genetic factors can cause disruption of the circadian rhythms. This disruption could contribute to multifactorial disease such as cardiovascular disease, a metabolic syndrome or progression of critical illness.
As above, avoidance of offending medications and improved environmental conditions may prevent the development and/or increase the native ability of the body to maintain or restore an intact circadian system. Clinical outcome research is warranted to test this hypothesis. Studies in ICU and non-ICU patients have demonstrated a decreased incidence in delirium and improved sleep/wake cycles with non-pharmacological and environmental treatments.133,134 Treatments may be very simple and cost-effective, including earplugs and eye masks135–137 and early mobilization.138,139 Future trials will help clinicians navigate the risks and benefits of interventions that may disrupt sleep and circadian function and develop guidelines for most effective patient care.
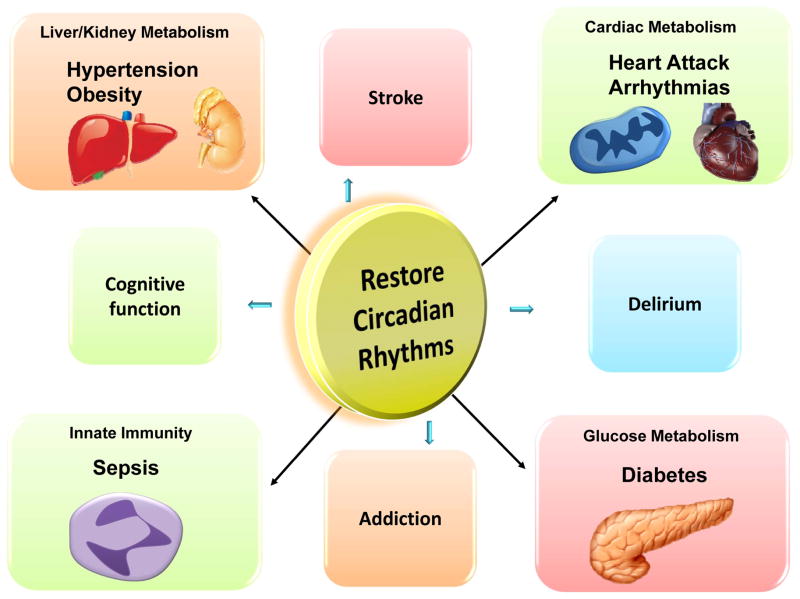
Beyond prevention of circadian disruption, it may be that enhancement of normal circadian rhythmicity has a role to play in disease states like sepsis. Recent efforts led to the discovery of specific enhancers of the circadian rhythm proteins. Compounds such as a ‘RevErb’ agonist92 were shown to enhance the amplitude of circadian rhythmicity and - even more important- were able to improve metabolism in mice.92,140 In addition to restoration of circadian cycles, these medications may become promising drugs in the treatment of diabetes, myocardial ischemia, sepsis and other circadian associated illnesses (Figure 6).
Figure 6. Restoration of circadian rhythms – potential for treatment.
Recent improvements in our understanding of how circadian rhythms impact disease development including diabetes, hypertension, heart ischemia, stroke, sepsis or delirium, exhibits the potential to discover novel therapies for critically ill patients or those undergoing general anesthesia. Restoration of circadian rhythms might be key to treat associated disease.
b) Light Therapy
Given the primary importance of light in the control of circadian rhythms,7,8 it can be postulated that exposure to light might be preventative or therapeutic in the treatment of circadian related illnesses. Artificial light is not as effective as sunlight in entraining the circadian oscillator due to its significantly lower intensity.23,141 and even if timed appropriately, may result in chronic circadian misalignment.141 Evidence demonstrates an association between artificial indoor lighting a deregulation of hormonal rhythms.142 Although studies from our group indicated daylight exposure in animals increased the expression of Per2 in the heart, ultimately resulting in cardio-protection, the exact timing and intensity of this exposure is unclear. Studies in animals have shown that either constant darkness or constant light were worse in an animal model for sepsis when compared to regular day/night cycles.131 These results underline the potential importance of maintaining natural day-night cycles in humans. Many intensive care units in the United States and Europe have been rebuilt to increase exposure to natural light and some new ICUs in Germany (Heart Center or ICU Charite, Berlin, Gemany) have incorporated advanced noise reduction and daylight simulation. Further clinical trials on the use of natural or artificial light to prevent or treat circadian related illness are needed (Figure 6). These studies will have to define the length (12 vs 16 h) of daylight, the wavelength (blue light vs full spectrum light) and the light intensity (4, 000 vs 14,000 lux) that might be beneficial in treating illness. Finally, it remains to be seen whether such treatments will be able to prevent or restore a nonfunctional circadian rhythm network.
Summary
The circadian system influences multiple human biochemical and physiologic variables including sleep/wake cycles, body temperature, hormone secretion, metabolism and our immune systems. Recent improvements in our understanding of how circadian rhythms impact important human disease states may enable the discovery of novel therapies for critically ill patients or those undergoing general anesthesia. Future research will need to include genetic data, as well as pharmacologic and environmental factors in the maintenance and restoration of human circadian rhythms. Understanding the influence of disrupted circadian rhythms on outcomes in critical care and anesthesia and researching new therapeutic interventions might significantly improve the care we provide our patients.
Acknowledgments
Source of financial support for the work:
National Heart, Lung, and Blood Institute (NIH-NHLBI) Grant 1K08HL102267 to T.E.
The present research work is supported by National Heart, Lung, and Blood Institute Grant 1K08HL102267 to TE.
References
- 1.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 2.Aschoff J. Circadian Rhythms in Man. Science. 1965;148:1427–32. doi: 10.1126/science.148.3676.1427. [DOI] [PubMed] [Google Scholar]
- 3.Arendt J, Aldhous M, Wright J. Synchronisation of a disturbed sleep-wake cycle in a blind man by melatonin treatment. Lancet. 1988;1:772–3. doi: 10.1016/s0140-6736(88)91586-3. [DOI] [PubMed] [Google Scholar]
- 4.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–6. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen G, Halberg F, Halberg J, Schwartzkopff O, Cugini P. Remembering the father of chronobiology and chronomics: franz halberg, MD (5 july 1919 - 9 june 2013) Clin Ter. 2013;164:I–VI. [PubMed] [Google Scholar]
- 6.Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–8. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol. 2007;17:R44–5. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Jakubcakova V, Oster H, Tamanini F, Cadenas C, Leitges M, van der Horst GT, Eichele G. Light entrainment of the mammalian circadian clock by a PRKCA-dependent posttranslational mechanism. Neuron. 2007;54:831–43. doi: 10.1016/j.neuron.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendova Z, Sumova S. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res. 2006;55:623–32. doi: 10.33549/physiolres.930849. [DOI] [PubMed] [Google Scholar]
- 13.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–82. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–3. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 15.Bonney S, Hughes K, Harter PN, Mittelbronn M, Walker L, Eckle T. Cardiac Period 2 in myocardial ischemia: Clinical implications of a light dependent protein. Int J Biochem Cell Biol. 2013;45:667–71. doi: 10.1016/j.biocel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson DE. Are you having a good day: a passing nicety or a fundamental question in the intensive care unit? Crit Care Med. 2012;40:344–5. doi: 10.1097/CCM.0b013e318232d2e0. [DOI] [PubMed] [Google Scholar]
- 17.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–9. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang AM, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE, Czeisler CA. Human responses to bright light of different durations. J Physiol. 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 22.Illnerova H, Vanecek J. Response of rat pineal serotonin N-acetyltransferase to one min light pulse at different night times. Brain Res. 1979;167:431–4. doi: 10.1016/0006-8993(79)90841-2. [DOI] [PubMed] [Google Scholar]
- 23.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 24.Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–70. doi: 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohyama J. A newly proposed disease condition produced by light exposure during night: asynchronization. Brain Dev. 2009;31:255–73. doi: 10.1016/j.braindev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Driesen K, Jansen NW, Kant I, Mohren DC, van Amelsvoort LG. Depressed mood in the working population: associations with work schedules and working hours. Chronobiol Int. 2010;27:1062–79. doi: 10.3109/07420528.2010.489877. [DOI] [PubMed] [Google Scholar]
- 27.van Amelsvoort LG, Schouten EG, Kok FJ. Impact of one year of shift work on cardiovascular disease risk factors. J Occup Environ Med. 2004;46:699–706. doi: 10.1097/01.jom.0000131794.83723.45. [DOI] [PubMed] [Google Scholar]
- 28.Parkes KR. Shift work and age as interactive predictors of body mass index among offshore workers. Scand J Work Environ Health. 2002;28:64–71. doi: 10.5271/sjweh.648. [DOI] [PubMed] [Google Scholar]
- 29.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 30.Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health. 2005;47:89–95. doi: 10.1539/joh.47.89. [DOI] [PubMed] [Google Scholar]
- 31.Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013;98:337–44. doi: 10.1210/jc.2012-2874. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen J, Wright KP., Jr Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. 2010;2:9–18. doi: 10.2147/NSS.S7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 34.Keijzer H, Smits MG, Duffy JF, Curfs LM. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18:333–9. doi: 10.1016/j.smrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–3. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 2008;16:1887–93. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 40.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 43.Ptacek LJ, Jones CR, Fu YH. Novel insights from genetic and molecular characterization of the human clock. Cold Spring Harb Symp Quant Biol. 2007;72:273–7. doi: 10.1101/sqb.2007.72.017. [DOI] [PubMed] [Google Scholar]
- 44.Albrecht U. Circadian clocks and mood-related behaviors. Handb Exp Pharmacol. 2013:227–39. doi: 10.1007/978-3-642-25950-0_9. [DOI] [PubMed] [Google Scholar]
- 45.Cuninkova L, Brown SA. Peripheral circadian oscillators: interesting mechanisms and powerful tools. Ann N Y Acad Sci. 2008;1129:358–70. doi: 10.1196/annals.1417.005. [DOI] [PubMed] [Google Scholar]
- 46.He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Jr, Rossner MJ, Nishino S, Fu YH. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325:866–70. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kivimaki M, Virtanen M, Elovainio M, Vaananen A, Keltikangas-Jarvinen L, Vahtera J. Prevalent cardiovascular disease, risk factors and selection out of shift work. Scand J Work Environ Health. 2006;32:204–8. doi: 10.5271/sjweh.1000. [DOI] [PubMed] [Google Scholar]
- 48.Harma M. Shift work and cardiovascular disease--from etiologic studies to prevention through scheduling. Scand J Work Environ Health. 2001;27:85–6. doi: 10.5271/sjweh.593. [DOI] [PubMed] [Google Scholar]
- 49.Boggild H, Knutsson A. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health. 1999;25:85–99. doi: 10.5271/sjweh.410. [DOI] [PubMed] [Google Scholar]
- 50.Akerstedt T, Knutsson A, Alfredsson L, Theorell T. Shift work and cardiovascular disease. Scand J Work Environ Health. 1984;10:409–14. doi: 10.5271/sjweh.2302. [DOI] [PubMed] [Google Scholar]
- 51.Akerstedt T, Knutsson A. Cardiovascular disease and shift work. Scand J Work Environ Health. 1997;23:241–2. doi: 10.5271/sjweh.216. [DOI] [PubMed] [Google Scholar]
- 52.Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–5. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–5. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malik M, Farrell T, Camm AJ. Circadian rhythm of heart rate variability after acute myocardial infarction and its influence on the prognostic value of heart rate variability. Am J Cardiol. 1990;66:1049–54. doi: 10.1016/0002-9149(90)90503-s. [DOI] [PubMed] [Google Scholar]
- 55.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–8. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 56.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–90. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 57.Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–5. doi: 10.1038/nm0106-54. discussion 55. [DOI] [PubMed] [Google Scholar]
- 58.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–62. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 59.Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–15. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 60.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–7. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lonnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 63.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49:1619–28. doi: 10.1007/s00125-006-0273-9. [DOI] [PubMed] [Google Scholar]
- 66.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan X, Jiang XC, Hussain MM. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation. 2013;128:1758–69. doi: 10.1161/CIRCULATIONAHA.113.002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 70.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–6. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One. 2013;8:e71493. doi: 10.1371/journal.pone.0071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braunwald E. On circadian variation of myocardial reperfusion injury. Circ Res. 2012;110:6–7. doi: 10.1161/CIRCRESAHA.111.260265. [DOI] [PubMed] [Google Scholar]
- 73.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–32. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med. 2013;19:345–54. doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, Liao JK. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–73. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–9. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eckle T, Eltzschig HK. Toll-like receptor signaling during myocardial ischemia. Anesthesiology. 2011;114:490–2. doi: 10.1097/ALN.0b013e31820a4d78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–61. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–30. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–51. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 81.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16:462–7. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chassard D, Duflo F, de Queiroz Siqueira M, Allaouchiche B, Boselli E. Chronobiology and anaesthesia. Curr Opin Anaesthesiol. 2007;20:186–90. doi: 10.1097/ACO.0b013e328136c55e. [DOI] [PubMed] [Google Scholar]
- 83.Smolensky MH, Reinberg A. The chronotherapy of corticosteroids: practical application of chronobiologic findings to nursing. Nurs Clin North Am. 1976;11:609–19. [PubMed] [Google Scholar]
- 84.Zhu LL, Zhou Q, Yan XF, Zeng S. Optimal time to take once-daily oral medications in clinical practice. Int J Clin Pract. 2008;62:1560–71. doi: 10.1111/j.1742-1241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 85.De Giorgi A, Mallozzi Menegatti A, Fabbian F, Portaluppi F, Manfredini R. Circadian rhythms and medical diseases: does it matter when drugs are taken? Eur J Intern Med. 2013;24:698–706. doi: 10.1016/j.ejim.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 86.Hermida RC, Ayala DE, Smolensky MH, Mojon A, Fernandez JR, Crespo JJ, Moya A, Rios MT, Portaluppi F. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol. 2013;9:358–68. doi: 10.1038/nrneph.2013.79. [DOI] [PubMed] [Google Scholar]
- 87.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35:416–21. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 88.Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, Wilinska ME, Nodale M, Mangat J, Evans ML, Burnstein R, Hovorka R. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care. 2013;17:R159. doi: 10.1186/cc12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.MacLeod JB, Lefton J, Houghton D, Roland C, Doherty J, Cohn SM, Barquist ES. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57–61. doi: 10.1097/01.ta.0000249294.58703.11. [DOI] [PubMed] [Google Scholar]
- 90.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J, Group CS. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 91.Bosmann M, Meta F, Ruemmler R, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Regulation of IL-17 family members by adrenal hormones during experimental sepsis in mice. Am J Pathol. 2013;182:1124–30. doi: 10.1016/j.ajpath.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lutsch EF, Morris RW. Light reversal of a morphine-induced analgesia susceptibility rhythm in mice. Experientia. 1971;27:420–1. doi: 10.1007/BF02137286. [DOI] [PubMed] [Google Scholar]
- 94.Morris RW, Lutsch EF. Susceptibility to morphine-induced analgesia in mice. Nature. 1967;216:494–5. doi: 10.1038/216494a0. [DOI] [PubMed] [Google Scholar]
- 95.Reinberg A, Reinberg MA. Circadian changes of the duration of action of local anaesthetic agents. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:149–52. doi: 10.1007/BF00499924. [DOI] [PubMed] [Google Scholar]
- 96.Guo X, Kuzumi E, Charman SC, Vuylsteke A. Perioperative melatonin secretion in patients undergoing coronary artery bypass grafting. Anesth Analg. 2002;94:1085–91. doi: 10.1097/00000539-200205000-00006. table of contents. [DOI] [PubMed] [Google Scholar]
- 97.Dispersyn G, Pain L, Challet E, Touitou Y. General anesthetics effects on circadian temporal structure: an update. Chronobiol Int. 2008;25:835–50. doi: 10.1080/07420520802551386. [DOI] [PubMed] [Google Scholar]
- 98.Karkela J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46:30–6. doi: 10.1034/j.1399-6576.2002.460106.x. [DOI] [PubMed] [Google Scholar]
- 99.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, Fang YF, Shieh MH, Kuo HP. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–9. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 100.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 101.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg T, Pickkers P. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med. 2012;40:112–8. doi: 10.1097/CCM.0b013e31822e9fc9. [DOI] [PubMed] [Google Scholar]
- 103.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, Investigators B-IS. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bellapart J, Boots R. Potential use of melatonin in sleep and delirium in the critically ill. Br J Anaesth. 2012;108:572–80. doi: 10.1093/bja/aes035. [DOI] [PubMed] [Google Scholar]
- 105.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 106.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 107.Bienert A, Bartkowska-Sniatkowska A, Wiczling P, Rosada-Kurasinska J, Grzeskowiak M, Zaba C, Tezyk A, Sokolowska A, Kaliszan R, Grzeskowiak E. Assessing circadian rhythms during prolonged midazolam infusion in the pediatric intensive care unit (PICU) children. Pharmacol Rep. 2013;65:107–21. doi: 10.1016/s1734-1140(13)70969-1. [DOI] [PubMed] [Google Scholar]
- 108.Gehlbach BK, Chapotot F, Leproult R, Whitmore H, Poston J, Pohlman M, Miller A, Pohlman AS, Nedeltcheva A, Jacobsen JH, Hall JB, Van Cauter E. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35:1105–14. doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 110.Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Crit Care Clin. 2006;22:313–27. vii. doi: 10.1016/j.ccc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 111.Bourne RS, Mills GH. Sleep disruption in critically ill patients--pharmacological considerations. Anaesthesia. 2004;59:374–84. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 112.Trompeo AC, Vidi Y, Locane MD, Braghiroli A, Mascia L, Bosma K, Ranieri VM. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77:604–12. [PubMed] [Google Scholar]
- 113.McPherson JA, Wagner CE, Boehm LM, Hall JD, Johnson DC, Miller LR, Burns KM, Thompson JL, Shintani AK, Ely EW, Pandharipande PP. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41:405–13. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–6. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R American College of Critical Care M. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 116.Kondili E, Alexopoulou C, Xirouchaki N, Georgopoulos D. Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study. Intensive Care Med. 2012;38:1640–6. doi: 10.1007/s00134-012-2623-z. [DOI] [PubMed] [Google Scholar]
- 117.Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–17. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 118.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG, Group SS. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 119.Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Jr, Dittus R, Ely EW. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dasta JF, Kane-Gill SL, Pencina M, Shehabi Y, Bokesch PM, Wisemandle W, Riker RR. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38:497–503. doi: 10.1097/CCM.0b013e3181bc81c9. [DOI] [PubMed] [Google Scholar]
- 121.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H, Group D-J. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71:397–403. doi: 10.1001/jamapsychiatry.2013.3320. [DOI] [PubMed] [Google Scholar]
- 122.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 123.Gibbison B, Angelini GD, Lightman SL. Dynamic output and control of the hypothalamic-pituitary-adrenal axis in critical illness and major surgery. Br J Anaesth. 2013;111:347–60. doi: 10.1093/bja/aet077. [DOI] [PubMed] [Google Scholar]
- 124.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24:45–61. doi: 10.1080/07420520601142569. [DOI] [PubMed] [Google Scholar]
- 125.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–18. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 126.Toublanc B, Rose D, Glerant JC, Francois G, Mayeux I, Rodenstein D, Jounieaux V. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148–54. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 127.Chan MC, Spieth PM, Quinn K, Parotto M, Zhang H, Slutsky AS. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med. 2012;40:246–53. doi: 10.1097/CCM.0b013e31822f0abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin. 2010;26:567–75. x–xi. doi: 10.1016/j.ccc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–40. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 130.Verceles AC, Silhan L, Terrin M, Netzer G, Shanholtz C, Scharf SM. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med. 2012;38:804–10. doi: 10.1007/s00134-012-2494-3. [DOI] [PubMed] [Google Scholar]
- 131.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–32. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
- 132.Rosato E, Tauber E, Kyriacou CP. Molecular genetics of the fruit-fly circadian clock. Eur J Hum Genet. 2006;14:729–38. doi: 10.1038/sj.ejhg.5201547. [DOI] [PubMed] [Google Scholar]
- 133.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 134.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM., Jr A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 135.Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16:R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112:89–95. doi: 10.1093/bja/aet304. [DOI] [PubMed] [Google Scholar]
- 137.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14:R66. doi: 10.1186/cc8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–42. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 140.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–7. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–2. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 142.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]