Abstract
In mammals, pluripotent stem cells can give rise to every cell type of embryonic lineage, and hold great potential in regenerative medicine and disease modeling. Guided by the mechanism underlying pluripotency, pluripotent stem cells have been successfully induced through manipulating the transcriptional and epigenetic networks of various differentiated cell types. However, the factors that confer totipotency, the ability to give rise to cells in both embryonic and extra-embryonic lineages, still remain poorly understood. It is currently unknown whether totipotency can be induced and maintained in vitro. In this review, we summarize the current progress in the field, with the aim of providing a foundation for understanding the mechanisms that regulate totipotency.
Keywords: Totipotency, Pluripotency, Epigenetics, Embryonic stem cell, Reprogramming, Somatic cell nuclear transfer (SCNT)
INTRODUCTION
Following fertilization in mammals, the resulting zygote initiates a developmental program that gives rise to a new organism composed of a myriad of different cell types. Cells from very early-stage embryos have the ability to generate both embryonic and extra-embryonic cell types and thereby be defined as totipotent cells (Figure 1). In a strict sense, totipotency denotes the ability of a cell to generate an entire organism. For instance, if separated, each blastomere from a mouse 2-cell embryo is capable of developing into a complete organism [1]. However, mouse blastomeres at the 4- or 8-cell stage have already lost this ability [2]. Therefore, it is believed that mouse zygote and blastomeres of 2-cell stage embryos are the only mouse cells to be strictly totipotent. The success of a single splitting blastomere in giving rise to a whole organism has also been demonstrated in multiple mammalian species, including sheep, rat, cattle, pig, horse and monkey [3-8]. Particularly, a single blastomere from 4- and 8-cell stage embryos is capable of giving rise to live organisms in sheep, cattle and pig [7, 9, 10]. As such, the developmental stages at which cells maintain totipotency seem to be variable among species.
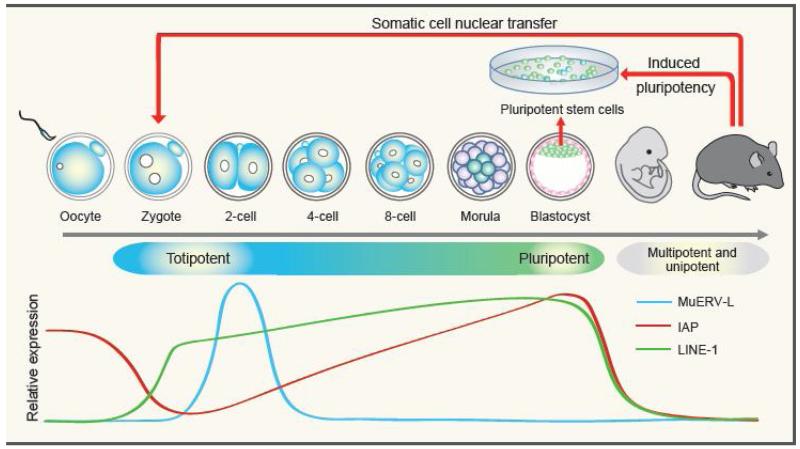
Figure 1. Relationship between development, cell potency and reprogramming.
Development of a mouse begins with fertilization. 1-cell zygotes and blastomeres of 2-cell embryos are totipotent. The inner cell mass (ICM) cells in blastocyst are pluripotent. The transition from totipotent to pluripotent cells takes place between the 4-cell to morula stage. During post-implantation development, some tissue-specific stem cells or progenitor cells remain multipotent, while the majority develop to unipotent and terminally differentiated cells. Differentiated cells can be reprogrammed to totipotent cells through somatic cell nuclear transfer (SCNT) or to pluripotent cells through forced expression of pluripotency-associated master transcription factors (induced pluripotency). Diagrams of the relative abundance of long interspersed nuclear element 1 (LINE-1), intracisternal A-particle (IAP), and murine endogenous retrovirus with leucine tRNA primer (MuERV-L) repeats are shown at the bottom.
Shortly into development, the totipotent cells of an embryo commit to two different cell fates, the embryonic cell lineage (the inner cell mass, ICM) and the extra-embryonic cell lineage (the trophectoderm). This transition takes place between the 4-cell and morula stage in mouse. Cells from ICM can contribute to all cell types of embryonic lineage, but not to cell types of extra-embryonic lineage. Thus, ICM cells are pluripotent instead of totipotent. Embryonic stem cells (ESCs) can be derived from ICM cells and maintain pluripotency in culture [11]. ESCs can contribute to different embryonic lineages when injected into pre-implantation embryos or differentiated in vitro [12]. Since ESCs are capable of self-renewal in culture and have great potential in regenerative medicine, the transcriptional and epigenetic networks regulating their pluripotency have been extensively studied [13-15]. The knowledge gained from these studies not only contributed to the optimization of culturing conditions for maintaining ESC pluripotency, but also led to the discovery of induced pluripotent stem cell (iPSC) through manipulating transcriptional and epigenetic networks [16, 17]. Contrary to pluripotency, our knowledge of totipotency is limited partly due to the small number of totipotent cells present in pre-implantation embryos. Nonetheless, recent studies have revealed some key features of totipotent embryos. Here we review these recent advances, which may serve as the foundation for understanding the mechanisms of totipotency.
MOLECULAR FEATURES OF TOTIPOTENT EMBRYOS
Unique transcriptome
Mature oocytes are arrested at MII phase and are transcriptionally inert. Upon fertilization, the fertilized egg reenters the cell cycle to initiate the embryonic developmental process. To satisfy the requirement of the embryonic developmental process, new transcripts need to be synthesized from the zygotic genome. This process is called zygotic genome activation (ZGA). Mouse ZGA begins at S/G2 phase of 1-cell zygotes and becomes prominent at 2-cell stage [18, 19]. ZGA is essential for embryonic development as embryos will arrest at the 2-cell stage if ZGA is blocked by inhibitors of RNA synthesis [20]. Transcriptome analysis of pre-implantation mouse embryos revealed two major waves of transcriptional activation; with ZGA largely taking place at the 2-cell stage and the second wave occurring from the morula to blastocyst stage [21]. Additionally, a minor wave of ZGA involving about 500 genes is observed at 1-cell stage [22]. However, these early microarray studies may not completely represent de novo synthesized transcripts due to the masking of newly synthesized transcripts by the large pool of maternally stored RNAs. Sequencing nascent transcripts or transcripts derived from the paternal genome using SNP information will reveal precisely which genes are indeed activated in totipotent 1-cell and 2-cell stage embryos.
Activation of transposable elements (TEs) is one feature unique to ZGA. TEs are silenced in most cell types but contribute significantly to the transcriptome of pre-implantation embryos. Several types of TEs are highly and specifically activated during pre-implantation development with different kinetics (Figure 1). Long interspersed nuclear element 1 (LINE-1) repeats are activated at 1-cell stage embryos and remain active throughout pre-implantation development [23-25]. Indeed, activation of LINE-1 has been shown to be important for pre-implantation development [26]. Inhibition of LINE-1 by morpholino-modified antisense oligonucleotides in zygotes causes developmental arrest of embryos at 2- or 4-cell stage. Intracisternal A-particles (IAPs), one of the active transposons of type II endogenous retroviruses, are expressed in oocytes but are degraded after fertilization. These repeats are re-expressed at the 2-cell stage and peak at the blastocyst stage [27, 28]. Murine endogenous retrovirus with leucine tRNA primer (MuERV-L) repeats belong to type III endogenous retroviruses and are specifically expressed at the 2-cell stage. Hundreds of genes express chimeric transcripts with junctions to MuERV-L at the 5′ end, indicating that the long terminal repeats (LTRs) of MuERV-L serve as functional promoters in the activation of a large set of 2-cell specific genes [29]. Despite the observation of dynamic TE expression, the mechanism of regulation and the biological function of these transcripts remain largely unknown.
Another hallmark of ZGA is stage-specific gene expression, where many genes activated in 2-cell stage embryos are undetectable during any other stage of embryonic development. Since many of the 2-cell specific genes are physically close to endogenous retroviruses, transcription of at least a subset of these genes is likely controlled by nearby ERVs [29, 30]. One of the best known 2-cell embryo-specific gene families is the Zscan4 family gene cluster. Zscan4 proteins have been shown to be important for genome stability and telomere elongation [31]. Indeed, depletion of Zscan4 genes has caused severe delay in pre-implantation development with many embryos arrested at the 2-cell stage [32]. With the exception of Zscan4, the function of the majority of the 2-cell specific genes is largely unknown. How ZGA is achieved and whether any of the genes activated during ZGA is required for totipotency remain to be determined. Nevertheless, a complete characterization of the transcripts associated with totipotent cells will be the first step for understanding the mechanism underlying totipotency.
Epigenetic and chromatin features
During pre-implantation development, dramatic epigenetic and chromatin changes take place, including de novo nucleosome assembly, DNA demethylation and dynamic histone modifications. Since totipotency might be linked to the unique epigenetic and chromatin state of totipotent cells, we now summarize the molecular events taking place in totipotent cells.
Loss of DNA methylation
DNA in mammalian cells is subject to methylation at the 5-positon of cytosine (5mC) mostly in the context of CpGs. Recent studies have revealed that DNA methylation is dynamically regulated through active and passive demethylation [33]. Following fertilization, both maternal and paternal genomes are globally demethylated, reaching its lowest levels at the blastocyst stage [34, 35]. Specifically, global loss of 5mC, especially in the paternal genome takes place a few hours after fertilization [36, 37]. This wave of 5mC loss is coupled with 5mC oxidation by the ten eleven translocation 3 (TET3) protein to generate 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) [38-42]. The oxidized 5mC products are lost through DNA replication-dependent dilution [39, 42]. Consistently, the Thymine DNA glycosylase (TDG), the enzyme that removes 5fC and 5caC, is not expressed during pre-implantation development and is not required for this wave of DNA demethylation [43].
Recent studies indicate that DNA replication, instead of Tet3-mediated 5mC oxidation, is a major contributor for the loss of 5mC in zygotes [43, 44]. Given that heterozygous Tet3 mutant offspring lacking maternal Tet3 only shows a modest penetrance of post-implantation developmental failure due to haploinsufficiency [40, 45], Tet3-mediated 5mC oxidation is unlikely to contribute to the totipotent state. Nevertheless, due to the inability of decoupling of the DNA replication and embryonic developmental process, the potential role of this wave of DNA demethylation in the generation of the totipotent state cannot be ruled out. Thus, developing methods to manipulate replication-dependent DNA demethylation, though difficult, may provide an avenue to understand the functional importance of genome-wide DNA demethylation during pre-implantation development and cell potency.
Chromatin remodeling and asymmetric histone modifications
In addition to loss of DNA methylation, the two pronuclei, particularly the paternal pronucleus, go through drastic remodeling resulting in the replacement of protamines by maternally-stored histones. Interestingly, only the histone variant H3.3, but not H3.1 or H3.2, is used in the repackaging of the paternal genome after removal of protamines [46-50]. The newly assembled paternal chromatin exhibits distinct features from that of the maternal chromatin inherited from oocytes. For example, paternal chromatin is devoid of several histone modifications, including H3K4me3/2, H3K9me3/2/1 and H3K27me3/2/1 [46, 51]. Despite of the establishment of H3K27me3 at the late pronuclei stage in the paternal pronucleus, paternal and maternal pronuclei exhibit visibly distinct H3K27me3 immunostaining patterns, indicating that different parts of the genome are modified in the two pronuclei [51]. Although the level of H3K27me3 is low in paternal chromatin, the Polycomb repression complex 1 (PRC1) is recruited to the paternal pronucleus and plays an important role in the repression of transcription from heterochromatin in male pronucleus [52]. In contrast, H3K9me3, instead of PRC1, is important for heterochromatin repression in the female pronucleus. It is interesting to note that unlike maternal pronucleus-depleted zygotes, paternal pronucleus-depleted zygotes cannot support somatic cell nuclear transfer (SCNT)-mediated reprogramming [53]. This suggests that asymmetric distribution of reprogramming factors in the two pronuclei might be associated with the asymmetric epigenetic status of the two pronuclei. Understanding the epigenetic status of the two pronuclei may reveal important clues for understanding SCNT-mediated reprogramming.
Chromatin organization and mobility
In addition to the dynamic changes in histone and DNA modifications, drastic chromatin reconfiguration takes place during pre-implantation development. Heterochromatin is the best-characterized chromatin domain during this process. In somatic cells, chromocenters can be visualized by staining with DNA dye. However, the chromocenter structure is not visible in early developing embryos until the late 2-cell stage [54, 55]. Instead, in zygotes and 2-cell embryos, the centromeric heterochromatin is packed at the periphery of the Nucleolar Precursor Bodies (NPBs) and forms a ring-shaped structure around NPBs. Remodeling of centromeric heterochromatin starts at 2-cell stage. The centromeric heterochromatin is associated with NPBs at the beginning of the second cell cycle. However, at the end of the second cell cycle, a significant portion of the rims of centromeric heterochromatin begins to form spherical patches, and centromeric heterochromatin starts to form chromocenters at the late 2-cell stage [56]. This NPB association of centromeric heterochromatin correlates with the timing of cells with totipotency. Moreover, centromeric heterochromatin is also relocated to the periphery of NPBs in SCNT embryos [54]. Nucleoplasmin2 (NPM2) is the major protein component of NPBs and is required for sperm chromatin decondensation [57, 58]. Knockout of NPM2 in the oocyte causes failure of pre-implantation development [59], and physical removal of NPBs causes significant retardation of sperm chromatin decondensation [57]. These results suggest that functional NPBs are required for the generation of totipotent zygotes. However, how NPBs participate in chromatin reconfiguration to support the totipotent state remains unknown. Recent studies have also revealed that chromatin of 2-cell embryos has much higher mobility than that of later-stage pre-implantation embryos [60]. This unusual chromatin mobility in 2-cell embryos may be one of the features of the totipotent cell state. Future study should reveal how the high mobility chromatin state is established in 2-cell embryos and how chromatin mobility is functionally linked to totipotency.
TRANSITION FROM TOTIPOTENCY TO PLURIPOTENCY
The earliest cell fate commitment of totipotent embryos results in the generation of Cdx2 positive trophectoderm cells that give rise to extra-embryonic tissues, and Oct4 positive inner cell mass (ICM) cells, which generate the three germ layers of an embryo [61-63]. However, the inner and outer cells of the early blastocyst can still specify in the absence of Cdx2 or Oct4, indicating the existence of other mechanisms regulating initial cell fate specification [61, 63, 64]. For instance, the transcription factor TEAD4 has been shown to be required for trophectoderm lineage specification [65]. Although it is not yet clear how these cell lineage-specific transcription factors are selectively activated for the initial cell fate commitment, some recent studies have started to reveal important clues.
Blastomeres of 2-cell embryos are believed to be identical. Global differences in the H3R26me2 histone arginine methylation pattern can be detected as early the 4-cell stage in different blastomeres [66]. Furthermore, expression of PRDM14, a potential chromatin modifier, is shown to be highly expressed in two of the blastomeres while exhibiting low expression in the other two blastomeres at late 4-cell stage [67]. Although Oct4 protein levels are similar in every cell of a 4-cell stage embryo and that all cells are morphologically indistinguishable, by this point the mobility of Oct4 has already diverged into two distinct patterns [68]. This suggests that chromatin accessibility for Oct4 binding in 4-cell blastomeres is already in two distinct states. Indeed, those cells exhibiting high Oct4 mobility in 4-cell embryos or 8-cell embryos tend to contribute to the trophectoderm, while those cells with low Oct4 mobility contribute to both the trophectoderm and the ICM [68]. How Oct4 mobility is regulated in different 4-cell stage blastomeres remains to be determined. Since these events coincide with the timing when blastomeres exit the totipotent state, differences in chromatin dynamics of the different 4-cell stage blastomeres might be an important factor regulating totipotency [69, 70].
INDUCTION OF TOTIPOTENCY
Totipotent cells can be generated naturally through fertilization. Alternatively, they can also be generated artificially through somatic cell nuclear transfer (SCNT) (Figure 1) [71-73]. It has been shown that MII oocytes, 1-cell zygote and 2-cell blastomeres all are capable of supporting SCNT to generate an entire organism [53, 74-76], indicating that these cells have the capacity to support totipotency.
Despite the success in generating cloned animals through SCNT in many different species, a common problem associated with SCNT is the low efficiency in generating viable animals. SCNT-mediated cloning efficiency is very low (1-5%) in most species, except for bovine (5-20%) [77]. In the case of mouse, half of SCNT embryos arrest during preimplantation development and only 1-2% of SCNT blastocysts transferred to surrogate mother can eventually give birth to live mice [77]. In the case of human, the developmental potential of SCNT embryos has not yet been tested due to ethical issues. However, the success rate of human ESC line derivation is already low as only 10-25% of SCNT-derived human embryos can reach the blastocyst stage [78, 79]. Treatment with Trichrostatin A(TSA), a histone deacetylase (HDAC) inhibitor, can significantly improve the developmental potential of SCNT embryos [80], suggesting the presence of epigenetic barriers in the genome of the donor nuclei that prevent successful reprogramming. Identifying and overcoming these epigenetic barriers will increase the success rate of totipotency induction.
A recent study not only identified a major epigenetic barrier for SCNT-mediated reprogramming, but also provided a simple solution to overcome the barrier leading to a drastic increase in SCNT cloning efficiency [81]. By comparing the transcriptomes of developing mouse embryos generated by SCNT and in vitro fertilization (IVF), Matoba et al identified genomic regions, dubbed reprogramming resistant regions (RRRs), that failed to be activated in SCNT 2-cell embryos, but were properly activated in IVF embryos. Interestingly, RRRs are enriched for the repressive marker H3K9me3 in donor somatic cells [81]. Importantly, removing H3K9me3 by overexpressing an H3K9me3 demethylase, Kdm4d, in recipient oocytes or by depletion of the H3K9me3-specific methyltransferases, SUV39h1/2, in donor cells not only reactivated most of the RRRs, but also drastically improved the developmental potential of SCNT embryos [81]. Previous studies also indicated that treatment with histone deacetylase (HDAC) inhibitors is able to improve SCNT efficiency [80]. HDAC inhibition and H3K9me3 removal may work on the same pathway, as combined treatment with TSA and Kdm4d overexpression does not yield a synergistic effect on either pre-implantation development of SCNT embryos or the rate of nuclear transfer embryonic stem cell (ntESC) derivation from SCNT embryos [81]. Given that cloning efficiency is still not comparable to IVF following removal of the H3K9me3 barrier, it is likely that additional barriers to SCNT-mediated totipotency exist and are yet to be discovered.
RARE TOTIPOTENT CELLS IN ESC CULTURE
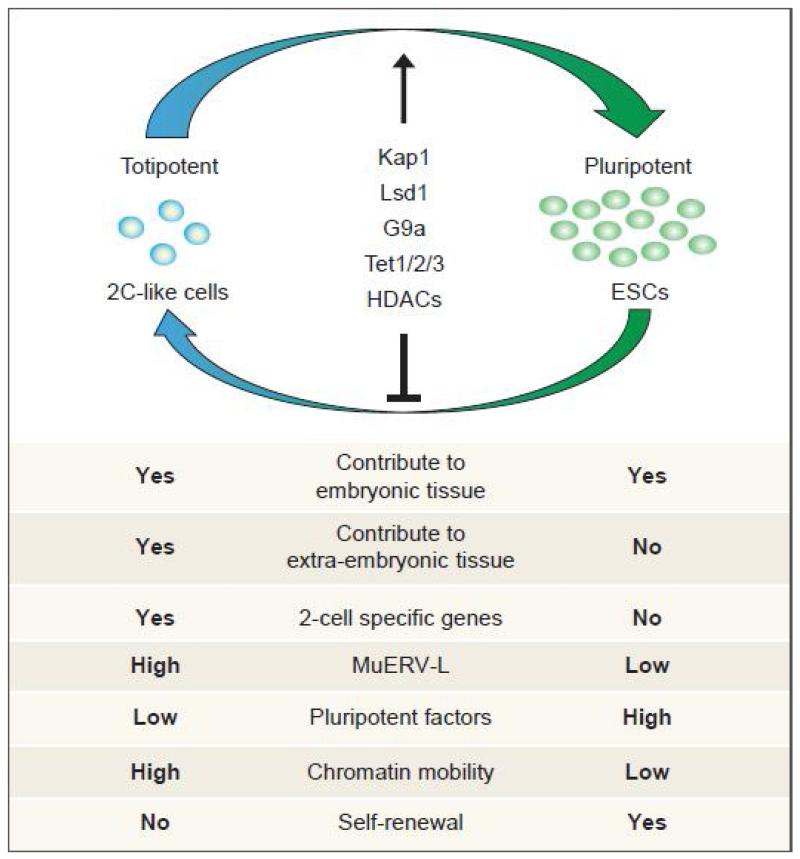
It is believed that, under proper culture conditions, ESCs are capable of indefinite self-renewal and are capable of maintaining a pluripotent state. However, recent studies have revealed that even under these conditions, a rare subpopulation of ESCs (less than 0.5%) expresses much lower levels of Oct4, Nanog, and Sox2 than the majority of ESCs while expressing a group of genes that are only detected in 2-cell mouse embryos. Based on these transcriptional features, they are named 2-cell embryo-like (2C-like) cells (Figure 2) [29]. Similar to the 2-cell mouse embryos, the endogenous retrovirus MuERV-L is highly active in 2C-like cells. In addition, 2C-like cells also exhibit a different epigenetic state compared to ESCs. For example, 2C-like cells exhibit high levels of histone acetylation and H3K4me2 [29]. In addition, 2C-like cells also possess high chromatin mobility observed in totipotent 2-cell embryos [60]. Amazingly, the 2C-like cells can contribute to both embryonic and extraembryonic tissues when injected into pre-implantation embryos [29]. This indicates that 2C-like cells have expanded potency compared to ESCs as ESCs can only contribute to embryonic tissues. Although these studies suggest that 2C-like cells may be totipotent, a definitive conclusion awaits the demonstration that a single 2C-like cell can indeed contribute to both embryonic and extra embryonic tissues as the above study cannot rule out the possibility that the 2C-like cells may contain two cell populations with potential for either embryonic or extra embryonic tissues.
Figure 2. Relationship and comparison between ESCs and 2C-like cells.
Embryonic stem cells (ESCs) can cycle in and out of a transient 2-cell embryo-like (2C-like) state. The population of 2C-like cells at a given time point are less than 0.5% under standard ESC culture conditions. The known regulators of the 2C-like state are listed, all of which are repressors. The different cellular and molecular features of 2C-like cells and ESCs are listed at the bottom.
2C-like cells not only share features of totipotent cells but also appear to be required for long-term maintenance of ESCs in culture. Depletion of cells entering the 2C-like state by expression of toxic DTA under the control of LTR of MuERV-L not only compromises ESC self-renewal rate but also causes differentiation bias toward mesoderm and ectoderm cell lineages when the ESCs are subjected to differentiation [29]. However, these cells are still capable of generating high contribution chimaeras even after 20 passages, although their proliferation rate is significantly decreased [29]. Another piece of evidence supporting the importance of entering the 2C-like state for ESC maintenance comes from the study of Zscan4 proteins that are specifically expressed in 2-cell stage mouse embryos. Depletion of Zscan4 in mouse embryos causes a delay in pre-implantation development as well as implantation failure [32]. Interestingly, Zscan4 is also capable of promoting telomere elongation in a telomerase-independent manner in ESCs, as depletion of Zscan4 in this population leads to telomere shortening, genome instability and ultimately, cell collapse [31]. In addition, ESCs with increased expression frequency of Zscan4 can restore and maintain developmental potency in long-term culture [82] and these cells are of higher quality in terms of tetraploid complementation for chimera generation compared to normal ESCs [82]. Nevertheless, it is not yet clear why cycling into the 2C-like state can improve long-term maintenance and pluripotency of ESCs. It is also not yet known whether cycling between ESC and 2C-like states is a regulated or stochastic event and whether the 2C-like state can be stably maintained in vitro.
Since both 2C-like cells and 2-cell embryos are associated with activation of MuERV-L repeats, understanding how MuERV-L repeats are controlled may provide clues to how 2C-like state is regulated. Several studies have shown that MuERV-L can be activated in response to the depletion of a number of epigenetic factors that include Kap1, Lsd1, G9a, GLP, HP1, Rybp, Rex1 and Tet proteins [29, 30, 83-86]. In addition, MuERV-L can also be activated by the treatment of an HDAC inhibitor, TSA [29]. Kap1, a transcriptional co-repressor of Kruppel-associated box domain-zinc finger proteins (KRAB-ZFPs) [87], can bind and repress MuERV-L expression, although how KRAB-ZFP mediates the binding is still unknown [30, 84]. In addition, Lsd1 and HDACs can be recruited by Kap1 to further suppress the transcription of MuERV-L [84]. Lsd1, a histone demethylase, can contribute to transcriptional repression by removing the active transcription mark H3K4me2/1 [88] while HDACs repress gene expression by removing histone acetylation, also a transcription activation mark [89]. In addition to removing histone marks associated with active transcription, adding the repressive H3K9me2 mark by G9a and GLP is also required for efficient suppression of MuERV-L [83, 84, 90]. Consistent with a role of H3K9me2 in repressing MuERV-L, H3K9me2 binding protein, HP1, is required for efficient repression of MuERV-L [83, 91]. In addition to histone modifications, DNA methylation may also contribute to the regulation of MuERV-L. A recent study showed that the Tet proteins affect MuERV-L expression by modulating the chromatin binding of the Kap1 protein [30]. Furthermore, MuERV-L expression can also be modulated by some sequence specific DNA binding proteins, such as RYBP and Rex1 [85, 86], although the mechanism of action is still unknown. It is important to point out that the increased expression of MuERV-L in cells deficient of Kap1, Lsd1, G9a or Tet proteins can be attributed to the increased 2C-like cell population as MuERV-L is specifically activated in 2C-like cells (Figure 2) [29, 30].
In addition to 2C-like cells, a small cell population expressing the extraembryonic endoderm marker Hex under 2i culture conditions has also been reported to be capable of contributing to both embryonic and extra-embryonic lineages [92]. Since a single Hex-positive cell is able to contribute to both embryonic and extra-embryonic lineages, Hex-positive cells are considered totipotent. Interestingly, gene expression analysis has indicated that Hex-positive cells have some transcriptional signatures more akin to those of cells of morula- or early blastocyst-stage embryos. Although further studies are needed to fully characterize Hex-positive cells, the identification of 2C-like and Hex-positive cells indicate that rare populations of cells close to different stages of pre-implantation embryos exist in cultured ESCs.
CONCLUDING REMARKS
The molecular mechanisms underlying stem cell pluripotency and cell fate reprogramming have been extensively studied in the past few years. Despite some concerns in using iPS cells, such as incomplete reprogramming, potential of tumorigenesis and immune-incompatibility [93-96], the success of iPS cells in multiple organisms has opened the door to an unlimited cell source for regenerative medicine and disease modeling.
In this review, we discussed the current knowledge relevant to totipotency. The totipotent stage of developing embryos is associated with unique transcriptional and epigenetic states. Understanding the mechanism for generation, maintenance, and exiting the totipotent state should provide more insight into stem cell biology and facilitate the progress of regenerative medicine. In the past several years, great progress has been made in understanding the molecular mechanism of cell plasticity and cell fate transition during preimplantation development. One of the major challenges in the epigenomic study of preimplantation development is the limitation in the number of cells available. As genomic and epigenomic techniques compatible with low-input samples become available, we anticipate great progress in understanding the molecular mechanisms underlying the generation and maintenance of totipotency. Before such technological advances are made, however, 2C-like cells derived from pluripotent ESCs may provide a viable alternative cell source for molecular characterization of the totipotent state. If totipotency can be induced and maintained in vitro, this would substantially facilitate our understanding of fundamental developmental processes, and would hold great potential for regenerative medicine.
ACKNOWLEDGEMENTS
We thank Drs. Azusa Inoue, Shogo Matoba and Luis M. Tuesta for critical reading of the manuscript, and Shinpei Yamaguchi for help in the artwork of Figure 1.
FUNDING
Work in the Zhang lab is supported by National Institutes of Health and Howard Hughes Medical Institute. Y.Z. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Tarkowski AK. Experiments on the Development of Isolated Blastomeres of Mouse Eggs. Nature. 1959;184(4695):1286–1287. doi: 10.1038/1841286a0. [DOI] [PubMed] [Google Scholar]
- 2.Rossant J. Postimplantation development of blastomeres isolated from 4- and 8-cell mouse eggs. J Embryol Exp Morphol. 1976;36(2):283–90. [PubMed] [Google Scholar]
- 3.Willadsen SM. A method for culture of micromanipulated sheep embryos and its use to produce monozygotic twins. Nature. 1979;277(5694):298–300. doi: 10.1038/277298a0. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, et al. Production of identical twins by separating two-cell rat embryos. Gamete Res. 1989;22(3):257–63. doi: 10.1002/mrd.1120220303. [DOI] [PubMed] [Google Scholar]
- 5.Willadsen SM, Polge C. Attempts to produce monozygotic quadruplets in cattle by blastomere separation. Vet Rec. 1981;108(10):211–3. doi: 10.1136/vr.108.10.211. [DOI] [PubMed] [Google Scholar]
- 6.Chan AW, et al. Clonal propagation of primate offspring by embryo splitting. Science. 2000;287(5451):317–9. doi: 10.1126/science.287.5451.317. [DOI] [PubMed] [Google Scholar]
- 7.Saito S, Niemann H. Effects of extracellular matrices and growth factors on the development of isolated porcine blastomeres. Biol Reprod. 1991;44(5):927–36. doi: 10.1095/biolreprod44.5.927. [DOI] [PubMed] [Google Scholar]
- 8.Allen WR, Pashen RL. Production of monozygotic (identical) horse twins by embryo micromanipulation. J Reprod Fertil. 1984;71(2):607–13. doi: 10.1530/jrf.0.0710607. [DOI] [PubMed] [Google Scholar]
- 9.Willadsen SM. The developmental capacity of blastomeres from 4- and 8-cell sheep embryos. Journal of Embryology and Experimental Morphology. 1981;65(1):165–172. [PubMed] [Google Scholar]
- 10.Johnson WH, et al. Production of four identical calves by the separation of blastomeres from an in vitro derived four-cell embryo. Vet Rec. 1995;137(1):15–6. doi: 10.1136/vr.137.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Rossant J. Stem cells and early lineage development. Cell. 2008;132(4):527–31. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143(4):508–25. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva J, Smith A. Capturing pluripotency. Cell. 2008;132(4):532–6. doi: 10.1016/j.cell.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young RA. Control of the embryonic stem cell state. Cell. 2011;144(6):940–54. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tee WW, Reinberg D. Chromatin features and the epigenetic regulation of pluripotency states in ESCs. Development. 2014;141(12):2376–90. doi: 10.1242/dev.096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465(7299):704–12. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Bouniol C, Nguyen E, Debey P. Endogenous Transcription Occurs at the 1-Cell Stage in the Mouse Embryo. Experimental Cell Research. 1995;218(1):57–62. doi: 10.1006/excr.1995.1130. [DOI] [PubMed] [Google Scholar]
- 19.Schultz RM. Regulation of zygotic gene activation in the mouse. Bioessays. 1993;15(8):531–8. doi: 10.1002/bies.950150806. [DOI] [PubMed] [Google Scholar]
- 20.Warner CM, Versteegh LR. In vivo and in vitro effect of alpha-amanitin on preimplantation mouse embryo RNA polymerase. Nature. 1974;248(5450):678–80. doi: 10.1038/248678a0. [DOI] [PubMed] [Google Scholar]
- 21.Hamatani T, et al. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6(1):117–31. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, et al. Inferring the choreography of parental genomes during fertilization from ultralarge-scale whole-transcriptome analysis. Genes Dev. 2013;27(24):2736–48. doi: 10.1101/gad.227926.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kano H, et al. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes & Development. 2009;23(11):1303–1312. doi: 10.1101/gad.1803909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peaston AE, et al. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev Cell. 2004;7(4):597–606. doi: 10.1016/j.devcel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Inoue A, Matoba S, Zhang Y. Transcriptional activation of transposable elements in mouse zygotes is independent of Tet3-mediated 5-methylcytosine oxidation. Cell Res. 2012;22(12):1640–1649. doi: 10.1038/cr.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beraldi R, et al. Expression of LINE-1 retroposons is essential for murine preimplantation development. Mol Reprod Dev. 2006;73(3):279–87. doi: 10.1002/mrd.20423. [DOI] [PubMed] [Google Scholar]
- 27.Pikó L, Hammons MD, Taylor KD. Amounts, synthesis, and some properties of intracisternal A particle-related RNA in early mouse embryos. Proceedings of the National Academy of Sciences. 1984;81(2):488–492. doi: 10.1073/pnas.81.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poznanski AA, Calarco PG. The expression of intracisternal A particle genes in the preimplantation mouse embryo. Developmental Biology. 1991;143(2):271–281. doi: 10.1016/0012-1606(91)90077-g. [DOI] [PubMed] [Google Scholar]
- 29.Macfarlan TS, et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487(7405):57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu F, et al. Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev. 2014;28(19):2103–19. doi: 10.1101/gad.248005.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalzman M, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464(7290):858–63. doi: 10.1038/nature08882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falco G, et al. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol. 2007;307(2):539–50. doi: 10.1016/j.ydbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156(1-2):45–68. doi: 10.1016/j.cell.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rougier N, et al. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 1998;12(14):2108–13. doi: 10.1101/gad.12.14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith ZD, et al. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature. 2012;484(7394):339–44. doi: 10.1038/nature10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer W, et al. Demethylation of the zygotic paternal genome. Nature. 2000;403(6769):501–2. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 37.Oswald J, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10(8):475–8. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 38.Iqbal K, et al. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(9):3642–7. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science. 2011;334(6053):194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu TP, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477(7366):606–10. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 41.Wossidlo M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 42.Inoue A, et al. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res. 2011;21(12):1670–6. doi: 10.1038/cr.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo F, et al. Active and passive demethylation of male and female pronuclear DNA in the Mammalian zygote. Cell Stem Cell. 2014;15(4):447–58. doi: 10.1016/j.stem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, et al. Tet3 and DNA Replication Mediate Demethylation of Both the Maternal and Paternal Genomes in Mouse Zygotes. Cell Stem Cell. 2014;15(4):459–470. doi: 10.1016/j.stem.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue A, et al. Haploinsufficiency, but Not Defective Paternal 5mC Oxidation, Accounts for the Developmental Defects of Maternal Tet3 Knockouts. Cell Reports. 10(4):463–470. doi: 10.1016/j.celrep.2014.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Heijden GW, et al. Asymmetry in Histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mechanisms of Development. 2005;122(9):1008–1022. doi: 10.1016/j.mod.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Torres-Padilla ME, et al. Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol. 2006;50(5):455–61. doi: 10.1387/ijdb.052073mt. [DOI] [PubMed] [Google Scholar]
- 48.Lin CJ, et al. Hira-mediated H3.3 incorporation is required for DNA replication and ribosomal RNA transcription in the mouse zygote. Dev Cell. 2014;30(3):268–79. doi: 10.1016/j.devcel.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue A, Zhang Y. Nucleosome assembly is required for nuclear pore complex assembly in mouse zygotes. Nat Struct Mol Biol. 2014;21(7):609–616. doi: 10.1038/nsmb.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiyama T, et al. Dynamic replacement of histone H3 variants reprograms epigenetic marks in early mouse embryos. PLoS Genet. 2011;7(10):e1002279. doi: 10.1371/journal.pgen.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos F, et al. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol. 2005;280(1):225–36. doi: 10.1016/j.ydbio.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 52.Puschendorf M, et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat Genet. 2008;40(4):411–420. doi: 10.1038/ng.99. [DOI] [PubMed] [Google Scholar]
- 53.Liu W, et al. Asymmetric reprogramming capacity of parental pronuclei in mouse zygotes. Cell Rep. 2014;6(6):1008–16. doi: 10.1016/j.celrep.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Martin C, et al. Genome restructuring in mouse embryos during reprogramming and early development. Dev Biol. 2006;292(2):317–32. doi: 10.1016/j.ydbio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Probst AV, et al. Structural differences in centromeric heterochromatin are spatially reconciled on fertilisation in the mouse zygote. Chromosoma. 2007;116(4):403–15. doi: 10.1007/s00412-007-0106-8. [DOI] [PubMed] [Google Scholar]
- 56.Aguirre-Lavin T, et al. 3D-FISH analysis of embryonic nuclei in mouse highlights several abrupt changes of nuclear organization during preimplantation development. BMC Dev Biol. 2012;12:30. doi: 10.1186/1471-213X-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoue A, et al. Involvement of mouse nucleoplasmin 2 in the decondensation of sperm chromatin after fertilization. Biol Reprod. 2011;85(1):70–7. doi: 10.1095/biolreprod.110.089342. [DOI] [PubMed] [Google Scholar]
- 58.Inoue A, Aoki F. Role of the nucleoplasmin 2 C-terminal domain in the formation of nucleolus-like bodies in mouse oocytes. FASEB J. 2010;24(2):485–94. doi: 10.1096/fj.09-143370. [DOI] [PubMed] [Google Scholar]
- 59.Burns KH, et al. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science. 2003;300(5619):633–6. doi: 10.1126/science.1081813. [DOI] [PubMed] [Google Scholar]
- 60.Boskovic A, et al. Higher chromatin mobility supports totipotency and precedes pluripotency in vivo. Genes Dev. 2014;28(10):1042–7. doi: 10.1101/gad.238881.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strumpf D, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132(9):2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 62.Palmieri SL, et al. Oct-4 Transcription Factor Is Differentially Expressed in the Mouse Embryo during Establishment of the First Two Extraembryonic Cell Lineages Involved in Implantation. Developmental Biology. 1994;166(1):259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 63.Mitsui K, et al. The Homeoprotein Nanog Is Required for Maintenance of Pluripotency in Mouse Epiblast and ES Cells. Cell. 2003;113(5):631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 64.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 65.Yagi R, et al. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134(21):3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- 66.Torres-Padilla ME, et al. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007;445(7124):214–8. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burton A, et al. Single-Cell Profiling of Epigenetic Modifiers Identifies PRDM14 as an Inducer of Cell Fate in the Mammalian Embryo. Cell Reports. 2013;5(3):687–701. doi: 10.1016/j.celrep.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 68.Plachta N, et al. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat Cell Biol. 2011;13(2):117–23. doi: 10.1038/ncb2154. [DOI] [PubMed] [Google Scholar]
- 69.Fujimori T, et al. Analysis of cell lineage in two- and four-cell mouse embryos. Development. 2003;130(21):5113–5122. doi: 10.1242/dev.00725. [DOI] [PubMed] [Google Scholar]
- 70.Piotrowska-Nitsche K, et al. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005;132(3):479–490. doi: 10.1242/dev.01602. [DOI] [PubMed] [Google Scholar]
- 71.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–40. [PubMed] [Google Scholar]
- 72.Wilmut I, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Osorio N, et al. Reprogramming mammalian somatic cells. Theriogenology. 2012;78(9):1869–86. doi: 10.1016/j.theriogenology.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 74.Kang E, et al. Nuclear reprogramming by interphase cytoplasm of two-cell mouse embryos. Nature. 2014;509(7498):101–4. doi: 10.1038/nature13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wakayama T, et al. Mice cloned from embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96(26):14984–9. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Egli D, et al. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature. 2007;447(7145):679–85. doi: 10.1038/nature05879. [DOI] [PubMed] [Google Scholar]
- 77.Ogura A, Inoue K, Wakayama T. Recent advancements in cloning by somatic cell nuclear transfer. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20110329. doi: 10.1098/rstb.2011.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tachibana M, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153(6):1228–38. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamada M, et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510(7506):533–6. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- 80.Kishigami S, et al. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun. 2006;340(1):183–9. doi: 10.1016/j.bbrc.2005.11.164. [DOI] [PubMed] [Google Scholar]
- 81.Matoba S, et al. Embryonic Development following Somatic Cell Nuclear Transfer Impeded by Persisting Histone Methylation. Cell. 2014;159(4):884–895. doi: 10.1016/j.cell.2014.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amano T, et al. Zscan4 restores the developmental potency of embryonic stem cells. Nat Commun. 2013;4:1966. doi: 10.1038/ncomms2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maksakova IA, et al. Distinct roles of KAP1, HP1 and G9a/GLP in silencing of the two-cell-specific retrotransposon MERVL in mouse ES cells. Epigenetics Chromatin. 2013;6(1):15. doi: 10.1186/1756-8935-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Macfarlan TS, et al. Endogenous retroviruses and neighboring genes are coordinately repressed by LSD1/KDM1A. Genes Dev. 2011;25(6):594–607. doi: 10.1101/gad.2008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hisada K, et al. RYBP represses endogenous retroviruses and preimplantation- and germ line-specific genes in mouse embryonic stem cells. Mol Cell Biol. 2012;32(6):1139–49. doi: 10.1128/MCB.06441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guallar D, et al. Expression of endogenous retroviruses is negatively regulated by the pluripotency marker Rex1/Zfp42. Nucleic Acids Res. 2012;40(18):8993–9007. doi: 10.1093/nar/gks686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedman JR, et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10(16):2067–78. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 88.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–53. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12(5):599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 90.Tachibana M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16(14):1779–91. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lachner M, et al. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410(6824):116–20. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 92.Morgani SM, et al. Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Rep. 2013;3(6):1945–57. doi: 10.1016/j.celrep.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohnishi K, et al. Premature Termination of Reprogramming In Vivo Leads to Cancer Development through Altered Epigenetic Regulation. Cell. 2014;156(4):663–677. doi: 10.1016/j.cell.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–77. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]