Abstract
Bricklayers may be exposed to several lung carcinogens, including crystalline silica and asbestos. Previous studies that analyzed lung cancer risk among these workers had several study design limitations. We examined lung cancer risk among bricklayers within SYNERGY, a large international pooled analysis of case–control studies on lung cancer and the joint effects of occupational carcinogens. For men ever employed as bricklayers we estimated odds ratios (OR) and 95% confidence intervals (CI) adjusted for study center, age, lifetime smoking history and employment in occupations with exposures to known or suspected lung carcinogens. Among 15,608 cases and 18,531 controls, there were 695 cases and 469 controls who had ever worked as bricklayers (OR: 1.47; 95% CI: 1.28–1.68). In studies using population controls the OR was 1.55 (95% CI: 1.32–1.81, 540/349 cases/controls), while it was 1.24 (95% CI: 0.93–1.64, 155/120 cases/controls) in hospital-based studies. There was a clear positive trend with length of employment (p < 0.001). The relative risk was higher for squamous (OR: 1.68, 95% CI: 1.42–1.98, 309 cases) and small cell carcinomas (OR: 1.78, 95% CI: 1.44–2.20, 140 cases), than for adenocarcinoma (OR: 1.17, 95% CI: 0.95–1.43, 150 cases) (p-homogeneity: 0.0007). ORs were still elevated after additional adjustment for education and in analyses using blue collar workers as referents. This study provided robust evidence of increased lung cancer risk in bricklayers. Although non-causal explanations cannot be completely ruled out, the association is plausible in view of the potential for exposure to several carcinogens, notably crystalline silica and to a lesser extent asbestos.
What's new?
In their work, bricklayers can be exposed to various airborne carcinogens, including crystalline silica and asbestos. Previous studies of cancer risk have not accounted for full employment history or smoking status, and failed to establish a firm relationship between bricklaying and lung cancer. In this study, the authors used data from the largest collection of case-control studies on lung cancer with complete occupational and smoking history existing today, the SYNERGY project. They found clear evidence that lung cancer risk increases in proportion to the length of time spent working as a bricklayer, paving the way for better protection and compensation for those in this occupation.
Keywords: lung neoplasms, case–control studies, bricklayers, occupational health, epidemiology
Of the 11 million workers in the construction industry in the European Union (1990–1993), it has been estimated that more than half were exposed to carcinogenic agents.1 The most common carcinogenic exposure was crystalline silica in the form of quartz dust (19% of the workforce exposed), followed by diesel fumes (6%) and asbestos (5%). Less frequent exposures included cadmium (0.3%), chromium (0.2%) and nickel (0.3%).2 The impact of this industrial sector on the total lung cancer burden is estimated to be substantial.3
Several jobs within the construction sector are known (insulators and pipe coverers, roofers and asphalt workers using coal-tar, painters, truck drivers and operators of excavating machines) or suspected (carpenters) to increase lung cancer risk.4–6 Bricklayers represent a large proportion of construction workers in several countries. Increased lung cancer risks were reported for bricklayers in several studies.7–20 However, most of these studies were registry-based, general population studies or cohort studies using routinely collected information and thus had important drawbacks, including lack of complete job history information and/or smoking data,7–12,17,18,20 or availability of only limited smoking information.13,17 Also the few case–control studies that collected smoking information through interviews had study design limitations. In particular, only one study included (partly) controls selected from the general population (the other two being based on controls selected from pathology or cancer registry records).16 Moreover, two had very small sample sizes leading to imprecise odds ratios estimates;15,16 only one study had a fair number of bricklayers.14 For these reasons, a firm association between working as a bricklayer and lung cancer risk has not yet been established. Lung cancer among bricklayers is therefore not recognized as an occupational disease in most countries (unless exposure to asbestos or specific tasks with high exposure to crystalline silica are clearly documented) and the affected workers are not compensated.
To address this important public health issue, we studied the lung cancer risk for bricklayers within the SYNERGY project, a large international pooled analysis of case–control studies on the joint effects of occupational carcinogens in the development of lung cancer. SYNERGY represents today the largest collection of case–control studies on lung cancer with complete occupational and smoking information. Hence, it provides a unique opportunity to validly assess whether a lung cancer excess exists among bricklayers while taking into account major potential confounders, including smoking, socio-economic status and work in other occupations. Further, its large sample size allows the examination of the pattern of risk with length of exposure and by lung cancer histology.
Material and Methods
Study design
The SYNERGY project (http://SYNERGY.iarc.fr) pooled lung cancer case–control studies from 13 European countries, Canada, Hong Kong and New Zealand. Its primary objective is to study the joint effects of exposure to occupational lung carcinogens including asbestos, crystalline silica, polycyclic aromatic hydrocarbons and nickel and chromium compounds. SYNERGY currently includes 16 population- or hospital-based case–control studies which collected lifetime occupational and smoking history and a cohort-nested case–control study (Supporting Information Tables S1 and S2). The occupational data were coded using the International Standard Industrial Classification of All Economic Activities Rev. 2 (ISIC) and the 1968 International Standard Classification of Occupations (ISCO).4,5 Ethical approvals were obtained in accordance with legislation in each country and by the Institutional Review Board at the International Agency for Research on Cancer (IARC).
The pooled dataset used in this study included 34,139 men (15,608 cases and 18,531 controls) recruited in 1985–2010 (Supporting Information Tables S1 and S2). The multicenter study INCO, coordinated by IARC, included seven studies in Central and Eastern Europe and United Kingdom. They were considered as individual studies in the analyses, for a total of 22 studies/centers. The majority of controls (14,519 or 78.3%) were sampled from general population lists of the respective study bases. Overall, the response rate was higher for cases (81%) than for controls (67%), with large variability across studies. Information was predominantly collected by interviews with the subjects themselves. Next-of-kin respondents were accepted in five studies. Face-to-face interviews were conducted for nearly 90% of the subjects.
Statistical analysis
We analyzed lung cancer risk for bricklayers (ISCO code 9-51.20). Out of 8,904 women within the SYNERGY database, only 6 had ever been employed as bricklayers. Therefore, we restricted statistical analyses to men. For subjects ever employed as bricklayers we calculated odds ratios (OR) and 95% confidence intervals (CI) with unconditional logistic regression models similar to those used in other SYNERGY studies. All models contained the covariates study (22 centers) and log(age). To adjust for cigarette smoking we used the following variables: ever cigarette smoker (yes/no), log(1 + pack-years), time since quitting cigarette smoking (five categories, 0 for never and current smokers, 2−7, 8−15, 16−25, ≥26 years before interview/diagnosis). We also adjusted for smoking (ever/never) of other forms of tobacco only (cigars and pipe). To take into account exposure to lung carcinogens in other jobs we adjusted for ever employment in occupations known (so called “list A”) or suspected (“list B”) to be associated with lung cancer. Precise definitions of the industry/occupations in the lists and the related ISIC/ISCO code can be found elsewhere.4,5 Briefly, list A includes mining and quarrying, iron and steel founding, metal workers, ceramic, refractory bricks, and granite production, asbestos production, shipyard and railroad manufacturing, insulators and pipe coverers, roofers, asphalt workers, coke plant and gas production and painters. Because of the recent inclusion of diesel engine exhausts among lung carcinogens,6 we included in list A also bus and truck drivers and operators of excavating machines. List B includes butchers and meat workers, leather tanners and processors, carpenters, printing, rubber manufacture, glass production, motor vehicle manufacturing and repair, welders, railroad workers, filling station attendants and launderers. Subjects ever exposed to both list A and B occupations were classified in list A, so that the variable had the following values: 0 (never worked in occupations in list A or B), 1 (ever employed in list B but never in list A occupations) and 2 (ever employed in list A occupations). When not otherwise specified, the ORs reported in this article were obtained using the model adjusted for center, age, smoking and lists A/B.
We performed three sets of analyses by length of employment as bricklayers: (i) treating it as a categorical variable (<10, 10–19, 20–29, 30–39, ≥40 years); (ii) using restricted cubic splines (with knots at 10th, 25th, 50th, 75th and 90th percentiles of length of employment, log-transformed) and (iii) using a continuous log-transformed variable.21 We calculated tests for linear trend for categorical length of employment and the OR for continuous length of employment either in the whole population or among bricklayers only: the aim of the latter analysis was to verify if the trend of lung cancer risk was dependent on the inclusion of the zero exposure category (never bricklayers).22, pp. 316–317
We evaluated risk for the main histologic types with polytomous (multinomial) logistic regression models.22, pp. 413–414 In occupational cancer studies the need for adjustment for socioeconomic status (SES) is controversial and it is often advisable to present both crude and SES-adjusted estimates.23 Therefore, we also provided: (i) ORs additionally adjusted for educational level (as a surrogate for SES) and (ii) ORs obtained when using as reference subjects ever employed in other blue collar jobs, defined by the following ISCO codes: 5–5 (building caretakers, charworkers, cleaners and related workers), 5–6 (launderers, dry-cleaners and pressers), 5–81 (firefighters), 6–28 (farm machinery operators), 6–31 (loggers) and all jobs (with the obvious exception of bricklayers) within the major group 7/8/9 (production and related workers, transport equipment operators and labourers).4
To take into account possible heterogeneity in job coding of “bricklayers” across study centers we performed an analysis by combining in a single category the ISCO code 9-51.20 (“Bricklayers”), and the less-specific codes 9-51.90 (“Other bricklayers, stonemasons and tile setters”), 9-59.10 (“Housebuilders”) and 9-59.90 (“Other construction workers”).
Meta-analytic and meta-regression techniques were used to visualize study-specific results, assess heterogeneity and to evaluate dependence of log(OR) on study characteristics, including study design, response rates, recruitment or job history periods and study size.24 In these analyses, only studies for which there were enough cases and controls among bricklayers to calculate the OR could be included (Supporting Information Table S1). To detect asymmetry in the distribution of study-specific log(ORs) around the overall meta-analytic estimate we used the Egger's test. This test, which can be viewed as a statistical analogue to the funnel plot, determines whether the intercept deviates significantly from zero in a regression of the standardized effect estimates against their precision. Although the Egger's test (like a funnel-plot) is mainly known and used to detect publication bias in meta-analyses of published studies, an asymmetry of study-specific estimates may, in general, derive from other types of problems, including the presence of studies with small sample sizes.24
To quantify the confounding bias from cigarette smoking in estimating either the crude OR for bricklayers or the crude relative risk excess (OR − 1),22, pp.53,54 we used the following two formulas22, p. 261:
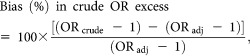 |
where ORcrude is the OR adjusted for study center, age, smoking of other types of tobacco only and lists A/B, and ORadj is the OR additionally adjusted for lifetime cigarette smoking history (ever/never, pack-years and time since quitting).
To evaluate the modifying effect of smoking we performed a number of additional analyses. First, we calculated lung cancer risk only among never cigarette smokers. Second, we evaluated the joint effect (interaction) of working as a bricklayers and cigarette smoking by fitting a unique logistic model including the covariates: bricklayer (ever/never), cigarette smoking (ever/never), study center, log(age), smoking of other types of tobacco only (ever/never) and occupation in lists A/B. Then we evaluated the interaction on the multiplicative scale by adding an interaction term (bricklayer × smoking) and by calculating the likelihood ratio test between the two nested models (with and without interaction).22, pp. 402–407 A similar approach was used to evaluate the modifying effect of exposure in lists A/B occupations. To assess the bricklayers-smoking interaction on the additive scale we calculated the relative excess risk due to interaction (RERI),25 sometimes referred to as the interaction contrast ratio (ICR).22, pp. 298–299 RERI > 0 indicates a more than additive (super-additive) interaction. Confidence limits for RERI were calculated using the method proposed by Zou.26 Finally, we explored the modifying effect of cigarette pack-years by fitting a joint model to obtain ORs for length of employment (dichotomized as <20 and ≥20 years because of small numbers) stratified by cigarette pack-years in three categories: never cigarette smokers and light smokers (<10 pack-years) together; 10 to <35 pack-years; and ≥35 pack-years. ORs were adjusted for center, log(age), smoking of other tobacco products only, years since quitting cigarette smoking and occupation in lists A/B.
Statistical analyses were performed with Stata 13 (StataCorp. 2013. College Station, TX: StataCorp LP.).
Results
Study population
Out of 15,608 cases, 695 (4.5%) had ever been working as a bricklayer. Among the 18,531 controls, 469 were bricklayers (2.5%). In most studies, cases and controls were matched for age, therefore mean age was quite similar between cases and controls, both among bricklayers and non-bricklayers (Table 1). Compared to other subjects, bricklayers had smoked more cigarettes, had lower education level and had been less frequently employed in list B occupations. Smokers of other types of tobacco only (cigars and pipe) were very few. Among bricklayers there was a slightly lower frequency of employment in list A occupations for cases and higher for controls. Squamous cell and small cell carcinomas were more frequent among bricklayers than in the other subjects.
Table 1.
Selected characteristics of male lung cancer cases and controls included in the pooled analyses on lung cancer risk among bricklayers, the SYNERGY study, 1985–2010
| Ever bricklayers |
Never bricklayers |
All |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases |
Controls |
Cases |
Controls |
Cases |
Controls |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| All subjects | 695 | 100 | 469 | 100 | 14,913 | 100 | 18,062 | 100 | 15,608 | 100 | 18,531 | 100 |
| Age (years) | ||||||||||||
| Mean (SD) | 62.5 | (9.0) | 63.0 | (9.3) | 62.7 | (9.0) | 62.2 | (9.5) | 62.7 | (9.0) | 62.2 | (9.5) |
| Cigarette smoking | ||||||||||||
| Never | 15 | 2.2 | 107 | 22.8 | 647 | 4.3 | 5,194 | 28.9 | 662 | 4.2 | 5,301 | 28.6 |
| Former (≥2 years) | 336 | 34.0 | 230 | 49.0 | 5,186 | 34.8 | 7,859 | 43.5 | 5,422 | 34.7 | 8,089 | 43.6 |
| Current | 442 | 63.6 | 131 | 27.9 | 8,956 | 60.0 | 4,867 | 26.9 | 9,398 | 60.2 | 4,998 | 27.0 |
| Unknown | 2 | 0.3 | 1 | 0.2 | 124 | 0.8 | 142 | 0.8 | 126 | 0.8 | 143 | 0.8 |
| Cigarette pack-years | ||||||||||||
| Mean/Median (SD) | 45.2/42.0 | (27.7) | 23.3/18.0 | (24.2) | 42.4/38.3 | (29.0) | 20.2/13.2 | (24.1) | 42.6/38.5 | (28.9) | 20.3/13.5 | (24.1) |
| Other tobacco only | ||||||||||||
| Ever | 6 | 0.9 | 12 | 2.6 | 188 | 1.3 | 558 | 3.1 | 194 | 1.2 | 570 | 3.1 |
| Education | ||||||||||||
| None | 37 | 5.3 | 21 | 4.5 | 404 | 2.7 | 242 | 1.3 | 441 | 2.8 | 263 | 1.4 |
| Some primary | 186 | 26.8 | 97 | 20.7 | 2,975 | 19.9 | 2,538 | 14.0 | 3,161 | 20.3 | 2,635 | 14.2 |
| Primary/some secondary | 378 | 54.4 | 263 | 56.1 | 6,598 | 44.2 | 7,078 | 39.2 | 6,976 | 44.7 | 7,341 | 39.6 |
| Secondary/some college | 69 | 9.9 | 71 | 15.1 | 2,577 | 17.3 | 4,170 | 23.1 | 2,646 | 17.0 | 4,241 | 22.9 |
| University | 10 | 1.4 | 13 | 2.8 | 1,456 | 9.8 | 3,066 | 17.0 | 1,466 | 9.4 | 3,079 | 16.6 |
| Unknown | 15 | 2.2 | 4 | 0.8 | 903 | 6.1 | 968 | 5.4 | 918 | 5.9 | 972 | 5.3 |
| Ever employed in list A/B1 | ||||||||||||
| No | 465 | 66.9 | 344 | 73.3 | 9,158 | 61.4 | 12,697 | 70.3 | 9,623 | 61.6 | 13,041 | 70.4 |
| List B | 78 | 11.2 | 26 | 5.5 | 2,073 | 13.9 | 2,185 | 12.1 | 2,151 | 13.8 | 2,211 | 11.9 |
| List A | 152 | 21.9 | 99 | 21.1 | 3,682 | 24.7 | 3,180 | 17.6 | 3,834 | 24.6 | 3,279 | 17.7 |
| Lung cancer morphology | ||||||||||||
| Squamous cell carcinoma | 309 | 44.5 | 6,105 | 40.9 | 6,414 | 41.1 | ||||||
| Small cell carcinoma | 140 | 20.1 | 2,286 | 15.3 | 2,426 | 15.5 | ||||||
| Adenocarcinoma | 150 | 21.6 | 3,864 | 25.9 | 4,014 | 25.7 | ||||||
| Other/unknown | 96 | 13.8 | 2,658 | 17.8 | 2,754 | 17.6 | ||||||
List A/B, occupations known (list A) or suspected (list B) to be associated with lung cancer; SD, standard deviation.
Subjects with previous employment in occupations categorized in list A and B were assigned to list A.
Smoking-adjusted lung cancer risk for bricklayers
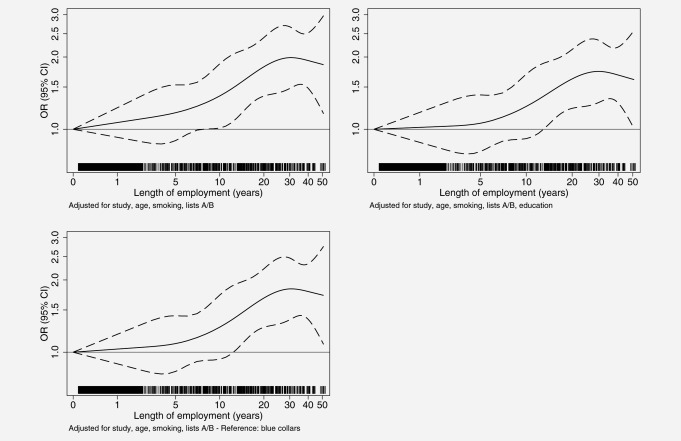
The OR for ever working as a bricklayer adjusted for center, age, smoking and list A and B occupations was 1.47 (95% CI: 1.28–1.68) (Table 2). After adjustment for education the OR was 1.32 (95% CI: 1.14–1.52). Using as reference non-bricklayer blue collar workers, the OR was 1.37 (95% CI: 1.19–1.57). There were clear positive trends of lung cancer risk by categories of length of employment as a bricklayer, with some decrease after 40 years. The restricted cubic spline plots (Fig. 1) show a gradual increase of lung cancer risk until about 25 years and a plateau afterwards. However, in the analyses using continuous length of employment (log-transformed) ORs ranged from 1.14 to 1.19 (Table 2) with no evidence of departure from linearity (p > 0.28 for the quadratic and p > 0.63 for the cubic components of length of employment). The slopes were even higher when analyzing length of employment among bricklayers only. There was an evident association between working as a bricklayer and small cell and squamous cell carcinoma, while the ORs for adenocarcinoma were much smaller (Table 2).
Table 2.
Lung cancer risk among bricklayers, the SYNERGY study, 1985–2010
| Cases | Controls | OR1 | (95% CI) | OR2 | (95% CI) | OR3 | (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| All subjects | 15,608 | 18,531 | ||||||
| Never bricklayers | 14,913 | 18,062 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Never bricklayers, blue collars | 11,750 | 12,275 | 1.00 | (Reference) | ||||
| Ever bricklayers | 695 | 469 | 1.47 | (1.28–1.68) | 1.32 | (1.14–1.52) | 1.37 | (1.19–1.57) |
| Length of employment as bricklayers | ||||||||
| <10 years | 278 | 225 | 1.20 | (0.98–1.47) | 1.10 | (0.90–1.36) | 1.13 | (0.92–1.38) |
| 10–19 years | 111 | 63 | 1.55 | (1.09–2.20) | 1.37 | (0.96–1.94) | 1.43 | (1.01–2.03) |
| 20–29 years | 88 | 55 | 1.73 | (1.17–2.56) | 1.53 | (1.03–2.26) | 1.60 | (1.08–2.37) |
| 30–39 years | 99 | 45 | 2.43 | (1.61–3.66) | 2.12 | (1.40–3.20) | 2.24 | (1.48–3.37) |
| ≥40 years | 89 | 55 | 1.81 | (1.22–2.69) | 1.58 | (1.06–2.35) | 1.68 | (1.13–2.49) |
| Unknown | 30 | 26 | – | – | – | – | – | – |
| Test for linear trend (p-value) | <0.001 | <0.001 | <0.001 | |||||
| Test for linear trend among bricklayers only (p-value) | 0.005 | 0.016 | –1 | |||||
| Length of employment as bricklayers, log (years) | ||||||||
| Among all subjects | 15,578 | 18,505 | 1.19 | (1.13–1.25) | 1.14 | (1.08–1.20) | 1.16 | (1.10–1.22) |
| Among bricklayers only | 665 | 443 | 1.26 | (1.08–1.47) | 1.22 | (1.04–1.44) | –1 | |
| Lung cancer morphology | ||||||||
| Squamous cell carcinoma | 309 | 1.68 | (1.42–1.98) | 1.45 | (1.22–1.72) | 1.53 | (1.29–1.81) | |
| Small cell carcinoma | 140 | 1.78 | (1.44–2.20) | 1.56 | (1.26–1.94) | 1.61 | (1.30–2.00) | |
| Adenocarcinoma | 150 | 1.17 | (0.95–1.43) | 1.11 | (0.91–1.37) | 1.12 | (0.91–1.37) | |
| Test of homogeneity (p-value) | 0.0007 | 0.017 | 0.005 |
Lists A/B, occupations known (list A) or suspected (list B) to be associated with lung cancer; CI, confidence interval; OR1, odds ratio adjusted for study center, age, smoking, and list A/B occupations; OR2, odds ratio adjusted for study center, age, smoking, lists A/B occupations, and education; OR3, odds ratio adjusted for study center, age, smoking, and lists A/B occupations, with blue collar workers as reference.
Same as OR1 analysis.
Figure 1.
Association between lung cancer risk and length of employment as bricklayers using restricted cubic splines (knots at 10th, 25th, 50th, 75th and 90th percentiles of length of employment, log-transformed), the SYNERGY study, 1985–2010. Note: A/B, occupations known (list A) or suspected (list B) to be associated with lung cancer; CI, confidence interval; OR, odds ratio. Vertical bars close to the horizontal axis indicate lung cancer cases.
Smoking-adjusted lung cancer risk for bricklayers by study center and type of controls
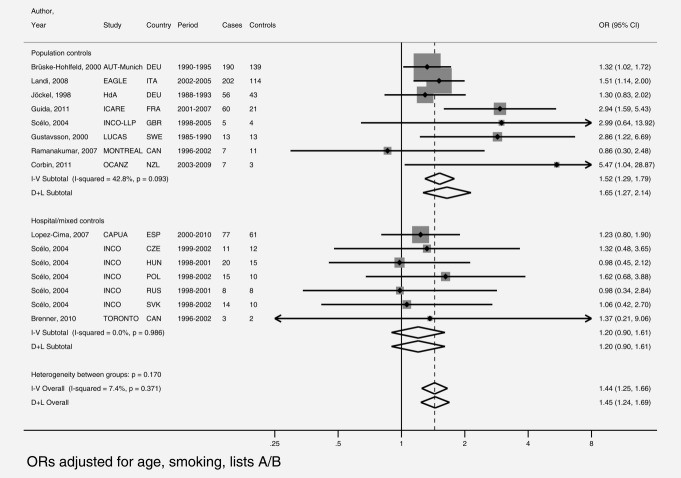
Study-specific smoking-adjusted ORs and meta-analytic estimates, stratified by type of controls, are shown in Figure 2. Fifteen studies, eight using population27–34 and seven using hospital/mixed controls31,35,36 contributed to the overall estimates for bricklayers. The overall fixed- and random-effect OR estimates were 1.44 and 1.45, virtually identical to the maximum likelihood OR (1.47) obtained from logistic regression on the pooled data. The funnel plot (not shown) was rather symmetric and the Egger's test (p = 0.45) confirmed the visual impression of no asymmetry of study-specific log(ORs) around the overall estimate. Two studies (AUT-Munich and EAGLE) included 55.4% of bricklayers. In logistic regressions, exclusion of AUT-Munich gave an OR of 1.53 (95% CI: 1.30–1.80), and excluding EAGLE an OR of 1.43 (95% CI: 1.22–1.68).
Figure 2.
Study-specific and overall meta-analytic odds ratios (OR) for bricklayers, the SYNERGY study, 1985–2010. Note: A/B, occupations known (list A) or suspected (list B) to be associated with lung cancer; CI, confidence interval; D+L, DerSimonian and Laird random effect estimate; I–V, inverse–variance fixed effect estimate.
In population-based studies (Fig. 2, top half) the meta-analytic ORs for bricklayers were 1.52 (fixed-effect) and 1.65 (random-effect), while in studies using hospital/mixed controls (Fig. 2, bottom half) both ORs were 1.20 (p for homogeneity across study type = 0.20 from a meta-regression model).
In studies with population controls (Table 3) all logistic regression ORs were higher than in the overall analysis while showing very similar patterns. In analyses restricted to studies with hospital/mixed controls (Table 4), the overall ORs ranged from 1.18 to 1.24, lung cancer risk by length of employment showed an irregular pattern (with elevated ORs in the category <10 years and after 30 years), and elevated ORs were found for squamous cell and small cell carcinomas, not for adenocarcinoma.
Table 3.
Lung cancer risk among bricklayers in studies using population controls, the SYNERGY study, 1985–2010
| Cases | Controls | OR1 | (95% CI) | OR2 | (95% CI) | OR3 | (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| All men | 11,762 | 14,519 | ||||||
| Never bricklayers | 11,222 | 14,170 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Never bricklayers, blue collars | 8,816 | 9,512 | 1.00 | (Reference) | ||||
| Ever bricklayers | 540 | 349 | 1.55 | (1.32–1.81) | 1.35 | (1.15–1.59) | 1.43 | (1.22–1.68) |
| Length of employment as bricklayers | ||||||||
| <10 years | 229 | 187 | 1.15 | (0.92–1.43) | 1.03 | (0.82–1.29) | 1.07 | (0.86–1.34) |
| 10–19 years | 92 | 43 | 1.85 | (1.23–2.78) | 1.59 | (1.06–2.39) | 1.70 | (1.13–2.55) |
| 20–29 years | 73 | 35 | 2.41 | (1.51–3.87) | 2.08 | (1.30–3.34) | 2.19 | (1.37–3.52) |
| 30–39 years | 76 | 31 | 2.87 | (1.77–4.66) | 2.38 | (1.47–3.85) | 2.61 | (1.61–4.24) |
| ≥40 years | 65 | 45 | 1.83 | (1.17–2.86) | 1.54 | (0.98–2.41) | 1.68 | (1.07–2.63) |
| Unknown | 5 | 8 | – | – | – | – | – | – |
| Test for linear trend (p-value) | <0.001 | <0.001 | <0.001 | |||||
| Test for linear trend among bricklayers only (p-value) | 0.001 | 0.003 | –1 | |||||
| Length of employment as bricklayers, log(years) | ||||||||
| Among all subjects | 11,757 | 14,511 | 1.23 | (1.15–1.30) | 1.17 | (1.10–1.24) | 1.19 | (1.12–1.27) |
| Among bricklayers only | 535 | 341 | 1.38 | (1.16–1.65) | 1.33 | (1.11–1.60) | –1 | |
| Lung cancer morphology | ||||||||
| Squamous cell carcinoma | 233 | 1.77 | (1.46–2.14) | 1.47 | (1.21–1.79) | 1.60 | (1.32–1.95) | |
| Small cell carcinoma | 109 | 1.86 | (1.46–2.37) | 1.58 | (1.24–2.03) | 1.66 | (1.30–2.12) | |
| Adenocarcinoma | 120 | 1.23 | (0.98–1.55) | 1.15 | (0.91–1.46) | 1.18 | (0.93–1.48) | |
| Test of homogeneity (p-value) | 0.004 | 0.06 | 0.02 |
Lists A/B, occupations known (list A) or suspected (list B) to be associated with lung cancer; CI, confidence interval; OR1, odds ratio adjusted for study center, age, smoking, lists A/B; OR2, odds ratio adjusted for study center, age, smoking, lists A/B occupations, education; OR3, odds ratio adjusted for study center, age, smoking, lists A/B occupations, with blue collar workers as reference.
Same as OR1 analysis.
Table 4.
Lung cancer risk among bricklayers in studies using hospital/mixed controls, the SYNERGY study, 1985–2010
| Cases | Controls | OR1 | (95% CI) | OR2 | (95% CI) | OR3 | (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| All men | 3,846 | 4,012 | ||||||
| Never bricklayers | 3,691 | 3,892 | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Never bricklayers, blue collars | 2,934 | 2,763 | 1.00 | (Reference) | ||||
| Ever bricklayers | 155 | 120 | 1.24 | (0.93–1.64) | 1.18 | (0.89–1.58) | 1.19 | (0.90–1.59) |
| Length of employment as bricklayers | ||||||||
| <10 years | 49 | 38 | 1.56 | (0.95–2.57) | 1.52 | (0.92–2.52) | 1.49 | (0.90–2.45) |
| 10–19 years | 19 | 20 | 0.82 | (0.40–1.69) | 0.79 | (0.38–1.62) | 0.80 | (0.39–1.64) |
| 20–29 years | 15 | 20 | 0.74 | (0.35–1.58) | 0.66 | (0.31–1.42) | 0.72 | (0.34–1.54) |
| 30–39 years | 23 | 14 | 1.45 | (0.67–3.16) | 1.45 | (0.66–3.19) | 1.41 | (0.65–3.06) |
| ≥40 years | 24 | 10 | 1.67 | (0.72–3.91) | 1.61 | (0.68–3.80) | 1.63 | (0.69–3.81) |
| Unknown | 25 | 18 | – | – | – | – | – | – |
| Test for linear trend (p-value) | 0.24 | 0.36 | 0.32 | |||||
| Test for linear trend among bricklayers only (p-value) | 0.93 | 0.86 | –1 | |||||
| Length of employment as bricklayers, log (years) | ||||||||
| Among all subjects | 3,821 | 3,994 | 1.07 | (0.96–1.19) | 1.05 | (0.94–1.17) | 1.06 | (0.95–1.18) |
| Among bricklayers only | 130 | 102 | 0.87 | (0.60–1.25) | 0.90 | (0.61–1.30) | –1 | |
| Lung cancer morphology | ||||||||
| Squamous cell carcinoma | 76 | 1.41 | (1.01–1.97) | 1.30 | (0.93–1.83) | 1.33 | (0.95–1.87) | |
| Small cell carcinoma | 31 | 1.59 | (1.02–2.48) | 1.48 | (0.94–2.32) | 1.54 | (0.99–2.41) | |
| Adenocarcinoma | 30 | 0.97 | (0.63–1.51) | 0.99 | (0.63–1.54) | 0.96 | (0.62–1.50) | |
| Test of homogeneity (p-value) | 0.16 | 0.31 | 0.21 |
Lists A/B, occupations known (list A) or suspected (list B) to be associated with lung cancer; CI, confidence interval; OR1, odds ratio adjusted for study center, age, smoking, lists A/B; OR2, odds ratio adjusted for study center, age, smoking, lists A/B occupations, education; OR3, odds ratio adjusted for study center, age, smoking, lists A/B occupations, with blue collar workers as reference.
Same as OR1 analysis.
Evaluation of confounding and effect modification by smoking
The overall OR for bricklayers adjusted for study center, age, smoking of other types of tobacco and occupations in lists A/B (not shown in tables) was 1.66 (95% CI: 1.47–1.88), against an OR also adjusted for lifetime cigarette smoking of 1.47 (Table 2). Hence, in this study not adjusting for cigarette smoking would have produced a confounding bias of the crude OR of +13% (= 100 × [1.66 − 1.47]/1.47), while in terms of crude excess OR the bias from smoking would have been +40% (= 100 × [0.66 − 0.47]/0.47).
The OR for bricklayers among the 5,963 never cigarette smokers was 1.46 (95% CI: 0.82–2.60, from 15 cases and 107 controls among bricklayers). A joint model containing the variables bricklayers, cigarette smoking (ever/never) and their interaction produced the following results: compared to never bricklayers-never cigarette smokers, the lung cancer OR for bricklayers who never smoked cigarette was 1.18, that for ever cigarette smokers never bricklayers was 11.5 and the OR for ever bricklayers ever cigarette smokers was 18.5 (Supporting Information Table S3). There was no indication of interaction on the multiplicative scale (the OR for the interaction term was 1.37 (95% CI: 0.76–2.45; p = 0.28). There was indication of super-additive interaction (RERI = 6.80, 95% CI: 4.36–9.62).
The relationship between length of employment as a bricklayer stratified by cigarette pack-years is shown in Supporting Information Table S4. The increasing trend of ORs with length of employment was evident in each stratum, including light smokers (0 to <10 pack-years). There was no indication of interaction between length of employment and pack-years on the multiplicative scale (p = 0.86).
Evaluation of confounding and effect modification by exposures to carcinogens in other occupations
Among those never employed in list A or B occupations the smoking-adjusted ORs for bricklayers (results not shown in tables) was 1.51 (95% CI: 1.28–1.78, from 465 cases and 344 controls among bricklayers). For those ever employed in list B occupations the OR for bricklayers was 2.40 (95% CI: 1.45–3.99, 78 cases and 26 controls among bricklayers). Finally, the OR for bricklayers was 1.13 (95% CI: 0.85–1.51, 152 cases, 99 controls), among subjects ever employed in list A occupations. Although there was statistical evidence that those three ORs were different (p-interaction = 0.03), in all the analyses the list A/B variable was treated as a confounder and not as a modifier for simplicity of presentation. In general, the confounding effect of lists A/B was small: for example, the OR for bricklayers not adjusted for lists A/B was 1.45 (95% CI: 1.26–1.66), instead of 1.47 (Table 2).
Discussion
In this study we found a smoking-adjusted increased lung cancer risk for bricklayers, with a clear positive association with length of employment. The association was stronger for small cell and squamous cell lung carcinomas. These findings were confirmed after further adjustment for education and in analyses using as reference other blue collar workers.
Effect of study design
Study design had an important impact on OR estimates. Although statistically there was little heterogeneity in OR estimates (p = 0.20), in the eight population-based studies the ORs for bricklayers were 1.55 (adjusted for smoking and lists A/B), 1.35 (after further adjustment for education) and 1.43 (blue collar referents) and a clear positive trend for length of employment was found. The ORs were, respectively, 1.24, 1.18 and 1.19 in the seven studies using hospital/mixed controls (although clearly elevated ORs were found when analyzing squamous cell and small cell carcinomas), with no clear trend with length of employment. To explain these inconsistencies, we examined several subjects' and study characteristics.
Although most studies using hospital controls enrolled only patients with smoking-unrelated diseases and were careful in including several diagnostic groups (Supporting Information Table S2), the proportion of bricklayers among controls was slightly lower in population-based (349/14,519 = 2.4%) compared with hospital-based studies (120/4,012 = 3.0%) (p = 0.036). Therefore, the lower ORs in hospital-based studies can perhaps, at least in part, be attributed to the choice of control diseases. Response rates were higher in hospital-based studies. However, in meta-regression models we did not find evidence of associations of study-specific log(ORs) with response rates among cases (p = 0.83) or controls (p = 0.84), nor was there an association with case–control response ratios (p = 0.59). Also we did not find evidence of a relationship between log(ORs) and response rates (in cases, controls or both) within studies using population (p = 0.92, 0.25 and 0.32, respectively) or hospital/mixed controls (p = 0.79, 0.75 and 0.91, respectively). This would argue against lower response rates in population-based studies being a reason for the different OR estimates between hospital-based and population-based studies. Also, in meta-regression models we did not find associations between study-specific log(ORs) for bricklayers and average recruitment (p = 0.60) or job history periods (p = 0.38). Among studies using hospital/mixed controls, there were ORs close to unity in the three INCO studies performed in Hungary, Russia and Slovakia. These studies were small in size.
In summary, it appears that findings from hospital/mixed control studies can be explained by a combination of factors, including choice of control diseases, geographical location (possibly reflecting different exposure patterns) and study size. Although one could question the findings from population-based ORs, we feel the latter are preferable for a number of reasons. First, they are a priori superior in terms of representativeness of the study-base. Second, the results in population-based studies are statistically more precise, being based on a larger sample size (540 cases and 349 controls had ever worked as bricklayers, against 155/120 in hospital-based studies). Third, although population-based studies may suffer from selection bias due lower response rates, we did not find relationships between ORs and response rates in meta-regression analyses, nor after adjustment for education or when using blue collars workers as referents (see discussion on socio-economic status below). Therefore, selection bias is not a likely explanation for the stronger associations in population-based studies. Fourth, and most important, we found a clear positive exposure-response relationship in the population-based studies, which is regarded as a strong argument in favour of causality.21
Evaluation of potential information bias
With regard to possible information bias in our study, it is known that validity and reliability of self-reported job history obtained with an interviewer-administered questionnaire is generally good, especially for jobs held longer, and usually not a source of important recall bias.37–39 In addition, in this study, blind coding of occupations minimized the possibility of differential bias, although a certain degree of non-differential misclassification is unavoidable, most likely leading to a bias towards the null. There are real differences in working practices in the construction sector across countries. Therefore, some difference in the proportions of bricklayers between geographical areas was expected. However, part of the heterogeneity may be due to different coding practices across study centers. For example, some of the studies that did not contribute cases/controls to the overall OR estimates probably coded some bricklayers under less specific ISCO codes, like 9–51.90 (“Other bricklayers, stonemasons and tile setters,” 57/32 exposed cases/controls), 9–59.10 (“Housebuilders,” 274/312 cases/controls) or 9–59.90 (“Other construction workers,” 336/212 cases/controls). After combining bricklayers with those three codes the overall OR was 1.34 (95% CI: 1.21–1.48, 1,304 cases and 997 controls). Although lower because of inclusion of workers performing other tasks, this combined estimate was not very far from that (OR = 1.47) obtained when considering the specific code for bricklayers. Moreover, all other analyses yielded similar results, including a clear positive trend of risk (p < 0.001) with length of employment.
Evaluation of confounding by smoking and exposure to carcinogens in other occupations
In this study, there was positive confounding by cigarette smoking. However, it was taken into account by adjusting for detailed lifetime smoking history. Smoking was adequately modeled by including cumulative exposure (cigarettes pack-years) and time since quitting. Smokers of other types of tobacco only were very few and did not affect much the findings. We found conflicting evidence of an increased lung cancer risk among never cigarette smokers: the OR was 1.46 when fitting a model among never cigarette smokers only and 1.18 when fitting a joint bricklayers-smoking model using the whole dataset. However, the numbers of bricklayers who never smoked were rather small and ORs quite imprecise.
To test the robustness of our results we fitted many other methods to model smoking history available in the literature which use different combinations of cumulative exposure, intensity, duration and time since quitting: they gave almost identical overall ORs (ranging from 1.47 to 1.50). In both population- and hospital/mixed-based studies there were higher ORs for small cell and squamous cell carcinomas, the histologic types most strongly associated with smoking. This might be interpreted as a sign of residual confounding by smoking, a frequent concern in occupational lung cancer studies, although not always justified. However, this pattern is also compatible with the possibility that exposures occurring while working as a bricklayer are associated (like smoking) with different strength with the different lung cancer histological types. The clear positive trend of ORs by length of employment, either in adjusted analyses or in analyses stratified by pack-years, lends more support to the existence of true associations than to residual bias from smoking.
In this study, we were able to take into account exposures to carcinogens in other occupations, even though their confounding effect was small.
Evaluation of effect of socio-economic status
Adjustment for SES is usually advocated for two main reasons23: (i) to reduce non-response bias, because participation is often associated with SES; and (ii) to reduce positive confounding from SES-related life-style factors. Conversely, SES adjustment (or restriction of analysis to blue collars workers) may introduce a negative bias if other occupational exposures cause the cancer under study and if these exposures are strongly correlated with the factor under investigation. This is sometimes referred to as over-adjustment. Notwithstanding the controversial validity of this argument, the ORs adjusted for education or obtained from analyses restricted to blue collar workers were still increased and the positive trends for length of employment were still evident.
Lung cancer risk by length of employment
Although the statistical relationship between lung cancer log(OR) and length of employment as a bricklayer was compatible with a linear increase, we observed a drop in ORs after 40 years (in categorical analyses) and a plateau after about 25 years (in spline analyses). This phenomenon has been repeatedly noted in several occupational epidemiology studies dealing with (but not exclusively) carcinogens. Although several possible explanations have been proposed (e.g., mismeasurement of high exposures, saturation of metabolic pathways, depletion of susceptible individuals and bias resulting from the healthy worker survivor effect), the issue is still unresolved.21
Literature findings
Several studies reported relative risk estimates for lung cancer among bricklayers (Supporting Information Table S5). Three studies showed smoking-unadjusted proportionate mortality ratios (PMR) of 1.18, 1.20 and 1.34.7,18,20 However, in two of them the relative risk was lower (1.09) when blue collar workers were used as referents20 or mortality odds ratios (MOR) were calculated.7 Three registry-based case–control studies reported excesses of lung cancer among bricklayers,8,13,17 but they suffered from a number of limitations, including absence of smoking data8 (or availability of only limited information),13,17 lack of complete job history8,17 and small sample size.13,17 In Nordic countries, the nationwide smoking-unadjusted standardized incidence ratio (SIR) in 1971–1991 for bricklayers was 1.19.9 In a recent (1961–2005) update the estimated SIR was 1.25.11 A nationwide study in Switzerland found a smoking-unadjusted standardized mortality ratio (SMR) of 2.12 and a PMR of 1.58 for masons.10 A large cohort study in Ontario found a smoking-unadjusted SMR of 1.30 in bricklayers, which became 1.53 when excluding places where refractory materials were used.12 Complete job and smoking histories were collected by interview in three case–control studies: the OR estimates ranged from 1.3 to 2.7.14–16 Two of those studies were very small in size.15,16 The only case–control study with a fair sample size included only patients with adenocarcinoma of the lung and hospital controls.14 The relationship with length of employment and time since first employment was examined in a few studies,12–14 and a positive association reported in two.12,14
Exposure to lung carcinogens for bricklayers
Bricklayers may have been exposed to several known or suspected lung carcinogens. The occurrence of pleural cancer excesses in construction workers underlines the importance of exposure to asbestos in this sector.3 However, asbestos exposure was most probably intermittent as it occurred during specific tasks only (e.g., insulation, demolition and building renewal). Indeed, a relatively small fraction (5%) of workers in the construction industry in Europe were considered exposed to asbestos.2 Hexavalent chromium (CrVI) and nickel compounds are contained in cement/concrete, but the estimated fraction of exposed workers in Europe was very low (<1%).2 Moreover, the levels of exposure inhalable cement dust are not usually very high among bricklayers.40
The predominant past (and present) exposure for bricklayers is crystalline silica in the form of quartz dust, which concerns a substantial fraction (almost 20%) of the workforce in the construction industry2 and occurs frequently during several tasks (concrete mixing, cutting, drilling, sandblasting, demolishing and cleaning).41,42 Moreover, industrial hygiene assessments in several countries reported exposure concentrations of respirable crystalline silica for bricklayers above the exposure limit of 0.025 mg/m3 (respirable quartz)42–53 currently recommended by the American Conference of Governmental Industrial Hygienists (ACGIH). A quantitative job-exposure matrix developed for SYNERGY estimated for bricklayers exposures to respirable crystalline silica in 1998 ranging from 0.02 to 0.07 mg/m3.54 In the SYNERGY study six centers (AUT-Munich, CAPUA, EAGLE, HdA, ICARE, INCO, including more than 20,000 subjects in total)27–31,35 had collected data on pneumoconiosis: 78 cases (12 bricklayers) and 39 controls (5 bricklayers) reported to have been diagnosed with silicosis. Radiographic abnormalities and low-grade silicosis (detected through high-resolution CT) have also been reported among construction workers exposed to quartz-containing dust in other studies.55 Reduction of exposure to quartz is regarded as a priority for bricklayers.56
Conclusion
We found an increase in lung cancer risk among bricklayers, with a clear positive association with length of employment. Although non-causal explanations cannot be completely ruled out, the association is plausible in view of the potential for exposure to several carcinogens, notably crystalline silica. Exposure to respirable crystalline silica-containing dust also concerns other workers in the construction industry. The large number of workers in the construction industry suggests that a focus on the work environment, in particular the monitoring and control of respirable crystalline silica-containing dust, may provide a further opportunity for lung cancer prevention.
Acknowledgments
The authors thank Véronique Luzon (IARC) for pooling and managing data.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
References
- 1.Kauppinen T, Toikkanen J, Pedersen D, et al. Occupational exposure to carcinogens in the European Union. Occup Environ Med. 2000;57:10–8. doi: 10.1136/oem.57.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll T, Nelson DI, Steenland K, et al. The global burden of disease due to occupational carcinogens. Am J Ind Med. 2005;48:419–31. doi: 10.1002/ajim.20209. [DOI] [PubMed] [Google Scholar]
- 3.Hutchings SJ, Rushton L. Occupational cancer in Britain. Industry sector results. Br J Cancer. 2012;107(Suppl 1):S92–S103. doi: 10.1038/bjc.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int J Occup Environ Health. 1998;4:236–40. doi: 10.1179/oeh.1998.4.4.236. [DOI] [PubMed] [Google Scholar]
- 5.Mirabelli D, Chiusolo M, Calisti R, et al. Database of occupations and industrial activities that involve the risk of pulmonary tumors (Italian) Epidemiol Prev. 2001;25:215–21. [PubMed] [Google Scholar]
- 6.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Diesel and gasoline engine exhausts and some nitroarenes. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 7.Dong W, Vaughan P, Sullivan K, et al. Mortality study of construction workers in the UK. Int J Epidemiol. 1995;24:750–7. doi: 10.1093/ije/24.4.750. [DOI] [PubMed] [Google Scholar]
- 8.Calvert GM, Luckhaupt S, Lee SJ, et al. Lung cancer risk among construction workers in California, 1988–2007. Am J Ind Med. 2012;55:412–22. doi: 10.1002/ajim.22010. [DOI] [PubMed] [Google Scholar]
- 9.Andersen A, Barlow L, Engeland A, et al. Work-related cancer in the Nordic countries. Scand J Work Environ Health. 1999;25(Suppl 2):1–116. [PubMed] [Google Scholar]
- 10.Minder CE, Beer-Porizek V. Cancer mortality of Swiss men by occupation, 1979–1982. Scand J Work Environ Health. 1992;18(Suppl 3):1–27. [PubMed] [Google Scholar]
- 11.Pukkala E, Martinsen JI, Lynge E, et al. Occupation and cancer—follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein MM, Verma DK. Mortality among Ontario members of the International Union of Bricklayers and Allied Craftworkers. Am J Ind Med. 2005;47:4–9. doi: 10.1002/ajim.20116. [DOI] [PubMed] [Google Scholar]
- 13.Baccarelli A, Tretiakova M, Gorbanev S, et al. Occupation and lung cancer risk in Leningrad Province, Russia. Med Lav. 2005;96:142–54. [PubMed] [Google Scholar]
- 14.De Stefani E, Boffetta P, Brennan P, et al. Occupational exposures and risk of adenocarcinoma of the lung in Uruguay. Cancer Causes Control. 2005;16:851–6. doi: 10.1007/s10552-005-2819-4. [DOI] [PubMed] [Google Scholar]
- 15.Matos EL, Vilensky M, Mirabelli D, et al. Occupational exposures and lung cancer in Buenos Aires, Argentina. J Occup Environ Med. 2000;42:653–9. doi: 10.1097/00043764-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Schoenberg JB, Stemhagen A, Mason TJ, et al. Occupation and lung cancer risk among New Jersey white males. J Natl Cancer Inst. 1987;79:13–21. [PubMed] [Google Scholar]
- 17.Zahm SH, Brownson RC, Chang JC, et al. Study of lung cancer histologic types, occupation, and smoking in Missouri. Am J Ind Med. 1989;15:565–78. doi: 10.1002/ajim.4700150509. [DOI] [PubMed] [Google Scholar]
- 18.Wang E, Dement JM, Lipscomb H. Mortality among North Carolina construction workers, 1988–1994. Appl Occup Environ Hyg. 1999;14:45–58. doi: 10.1080/104732299303412. [DOI] [PubMed] [Google Scholar]
- 19.Haldorsen T, Andersen A, Boffetta P. Smoking-adjusted incidence of lung cancer by occupation among Norwegian men. Cancer Causes Control. 2004;15:139–47. doi: 10.1023/B:CACO.0000019485.74818.d6. [DOI] [PubMed] [Google Scholar]
- 20.Robinson C, Stern F, Halperin W, et al. Assessment of mortality in the construction industry in the United States, 1984–1986. Am J Ind Med. 1995;28:49–70. doi: 10.1002/ajim.4700280105. [DOI] [PubMed] [Google Scholar]
- 21.Steenland K, Deddens JA. A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology. 2004;15:63–70. doi: 10.1097/01.ede.0000100287.45004.e7. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 23.Richiardi L, Barone-Adesi F, Merletti F, et al. Using directed acyclic graphs to consider adjustment for socioeconomic status in occupational cancer studies. J Epidemiol Community Health. 2008;62:e14. doi: 10.1136/jech.2007.065581. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context, 2nd edn. London: BMJ Publishing Group, 2001.
- 25.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–20. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou GY. On the estimation of additive interaction by use of the four-by-two table and beyond. Am J Epidemiol. 2008;168:212–24. doi: 10.1093/aje/kwn104. [DOI] [PubMed] [Google Scholar]
- 27.Brüske-Hohlfeld I, Mohner M, Pohlabeln H, et al. Occupational lung cancer risk for men in Germany: results from a pooled case-control study. Am J Epidemiol. 2000;151:384–95. doi: 10.1093/oxfordjournals.aje.a010218. [DOI] [PubMed] [Google Scholar]
- 28.Landi MT, Consonni D, Rotunno M, et al. Environment And Genetics in Lung cancer Etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC Public Health. 2008;8:203. doi: 10.1186/1471-2458-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jockel KH, Ahrens W, Jahn I, et al. Occupational risk factors for lung cancer: a case-control study in West Germany. Int J Epidemiol. 1998;27:549–60. doi: 10.1093/ije/27.4.549. [DOI] [PubMed] [Google Scholar]
- 30.Guida F, Papadopoulos A, Menvielle G, et al. Risk of lung cancer and occupational history: results of a French population-based case-control study, the ICARE study. J Occup Environ Med. 2011;53:1068–77. doi: 10.1097/JOM.0b013e318229ab2e. [DOI] [PubMed] [Google Scholar]
- 31.Scelo G, Constantinescu V, Csiki I, et al. Occupational exposure to vinyl chloride, acrylonitrile and styrene and lung cancer risk (Europe) Cancer Causes Control. 2004;15:445–52. doi: 10.1023/B:CACO.0000036444.11655.be. [DOI] [PubMed] [Google Scholar]
- 32.Gustavsson P, Jakobsson R, Nyberg F, et al. Occupational exposure and lung cancer risk: a population-based case-referent study in Sweden. Am J Epidemiol. 2000;152:32–40. doi: 10.1093/aje/152.1.32. [DOI] [PubMed] [Google Scholar]
- 33.Ramanakumar AV, Parent ME, Siemiatycki J. Risk of lung cancer from residential heating and cooking fuels in Montreal, Canada. Am J Epidemiol. 2007;165:634–42. doi: 10.1093/aje/kwk117. [DOI] [PubMed] [Google Scholar]
- 34.Corbin M, McLean D, Mannetje A, et al. Lung cancer and occupation: a New Zealand cancer registry-based case-control study. Am J Ind Med. 2011;54:89–101. doi: 10.1002/ajim.20906. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Cima MF, Gonzalez-Arriaga P, Garcia-Castro L, et al. Polymorphisms in XPC, XPD, XRCC1, and XRCC3 DNA repair genes and lung cancer risk in a population of northern Spain. BMC Cancer. 2007;7:162. doi: 10.1186/1471-2407-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner DR, Hung RJ, Tsao MS, et al. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siemiatycki J, Richardson L, Boffetta P. Occupation. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3rd edn. New York: Oxford University Press; 2006. pp. 322–54. [Google Scholar]
- 38.McGuire V, Nelson LM, Koepsell TD, et al. Assessment of occupational exposures in community-based case-control studies. Annu Rev Public Health. 1998;19:35–53. doi: 10.1146/annurev.publhealth.19.1.35. [DOI] [PubMed] [Google Scholar]
- 39.Ahrens W. Retrospective assessment of occupational exposures in case-control studies. Landsberg: Ecomed Verlagsgesellschaft; 1999. [Google Scholar]
- 40.Peters S, Thomassen Y, Fechter-Rink E, et al. Personal exposure to inhalable cement dust among construction workers. J Environ Monit. 2009;11:174–80. doi: 10.1039/b812357h. [DOI] [PubMed] [Google Scholar]
- 41.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Silica, some silicates, coal dust, and para-aramid fibrils. Lyon, France: International Agency for Research on Cancer; 1997. [PMC free article] [PubMed] [Google Scholar]
- 42.Tjoe Nij E, Hilhorst S, Spee T, et al. Dust control measures in the construction industry. Ann Occup Hyg. 2003;47:211–8. doi: 10.1093/annhyg/meg023. [DOI] [PubMed] [Google Scholar]
- 43.Rappaport SM, Goldberg M, Susi P, et al. Excessive exposure to silica in the US construction industry. Ann Occup Hyg. 2003;47:111–22. doi: 10.1093/annhyg/meg025. [DOI] [PubMed] [Google Scholar]
- 44.Yassin A, Yebesi F, Tingle R. Occupational exposure to crystalline silica dust in the United States, 1988–2003. Environ Health Perspect. 2005;113:255–60. doi: 10.1289/ehp.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nash NT, Williams DR. Occupational exposure to crystalline silica during tuckpointing and the use of engineering controls. Appl Occup Environ Hyg. 2000;15:8–10. doi: 10.1080/104732200301773. [DOI] [PubMed] [Google Scholar]
- 46.Flanagan ME, Seixas N, Becker P, et al. Silica exposure on construction sites: results of an exposure monitoring data compilation project. J Occup Environ Hyg. 2006;3:144–52. doi: 10.1080/15459620500526552. [DOI] [PubMed] [Google Scholar]
- 47.Tjoe Nij E, Hohr D, Borm P, et al. Variability in quartz exposure in the construction industry: implications for assessing exposure-response relations. J Occup Environ Hyg. 2004;1:191–8. doi: 10.1080/15459620490424528. [DOI] [PubMed] [Google Scholar]
- 48.Lofgren D. Slica exposure for concrete workers and masons. Appl Occup Environ Hyg. 1993;8:832–6. [Google Scholar]
- 49.Chisholm J. Respirable dust and respirable silica concentration from construction activities. Indoor Built Environ. 1999;8:94–106. [Google Scholar]
- 50.Linch KD. Respirable concrete dust—silicosis hazard in the construction industry. Appl Occup Environ Hyg. 2002;17:209–21. doi: 10.1080/104732202753438298. [DOI] [PubMed] [Google Scholar]
- 51.Linch KD, Miller WE, Althouse RB, et al. Surveillance of respirable crystalline silica dust using OSHA compliance data (1979–1995) Am J Ind Med. 1998;34:547–58. doi: 10.1002/(sici)1097-0274(199812)34:6<547::aid-ajim2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 52.Huizer D, Spee T, Lumens M, et al. Exposure to respirable dust and crystalline silica in bricklaying education at Dutch vocational training centers. Am J Ind Med. 2010;53:628–34. doi: 10.1002/ajim.20823. [DOI] [PubMed] [Google Scholar]
- 53.Lumens ME, Spee T. Determinants of exposure to respirable quartz dust in the construction industry. Ann Occup Hyg. 2001;45:585–95. [PubMed] [Google Scholar]
- 54.Peters S, Vermeulen R, Portengen L, et al. Modelling of occupational respirable crystalline silica exposure for quantitative exposure assessment in community-based case-control studies. J Environ Monit. 2011;13:3262–8. doi: 10.1039/c1em10628g. [DOI] [PubMed] [Google Scholar]
- 55.Meijer E, Tjoe Nij E, Kraus T, et al. Pneumoconiosis and emphysema in construction workers: results of HRCT and lung function findings. Occup Environ Med. 2011;68:542–6. doi: 10.1136/oem.2010.055616. [DOI] [PubMed] [Google Scholar]
- 56.Boschman JS, van der Molen HF, Sluiter JK, et al. Occupational demands and health effects for bricklayers and construction supervisors: a systematic review. Am J Ind Med. 2011;54:55–77. doi: 10.1002/ajim.20899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information