Abstract
Alpha-2-macroglobulin is an abundant secreted protein that is of particular interest because of its diverse ligand binding profile and multifunctional nature, which includes roles as a protease inhibitor and as a molecular chaperone. The activities of alpha-2-macroglobulin are typically dependent on whether its conformation is native or transformed (i.e. adopts a more compact conformation after interactions with proteases or small nucleophiles), and are also influenced by dissociation of the native alpha-2-macroglobulin tetramer into stable dimers. Alpha-2-macroglobulin is predominately present as the native tetramer in vivo; once purified from human blood plasma, however, alpha-2-macroglobulin can undergo a number of conformational changes during storage, including transformation, aggregation or dissociation. We demonstrate that, particularly in the presence of sodium chloride or amine containing compounds, freezing and/or lyophilization of alpha-2-macroglobulin induces conformational changes with functional consequences. These conformational changes in alpha-2-macroglobulin are not always detected by standard native polyacrylamide gel electrophoresis, but can be measured using bisANS fluorescence assays. Increased surface hydrophobicity of alpha-2-macroglobulin, as assessed by bisANS fluorescence measurements, is accompanied by (i) reduced trypsin binding activity, (ii) increased chaperone activity, and (iii) increased binding to the surfaces of SH-SY5Y neurons, in part, via lipoprotein receptors. We show that sucrose (but not glycine) effectively protects native alpha-2-macroglobulin from denaturation during freezing and/or lyophilization, thereby providing a reproducible method for the handling and long-term storage of this protein.
Introduction
Alpha-2-macroglobulin (α2M) is an ancient component of the innate immune system that is highly conserved in animal species separated by over half a billion years of evolution. The best known function of α2M is its ability to trap covalently a broad spectrum of proteases and facilitate their clearance via interaction with the low-density lipoprotein receptor-related protein (LRP) [1–3]. α2M has also been shown to facilitate the clearance of a diverse range of non-covalently bound ligands including the Alzheimer’s disease-associated amyloid β-peptide (Aβ) [4, 5] and many cytokines and growth factors [6–10]. The binding of α2M to heat-denatured and amyloidogenic peptides and proteins inhibits their aggregation and α2M is found co-localized with misfolded proteins in disease states [11–17]. Thus, α2M is proposed to have important roles in extracellular proteostasis, immune system regulation and tissue remodeling, and has been the subject of over 5000 scholarly articles and reviews since the 1970s [18].
Human α2M is a 720 kDa homotetramer comprised of four 180 kDa subunits. These subunits are paired by disulfide bonds to form covalently-linked dimers, which non-covalently associate to complete the cage-like quaternary structure of α2M [19]. Protease trapping by α2M involves three major steps, namely (i) cleavage of α2M in the ‘bait region’ which contains a large number of different protease cleavage sites [20], (ii) covalent cross-linking of the protease to α2M via a reactive thioester bond [21], and (iii) a dramatic conformational change which causes α2M to become more compact and reveals a cryptic binding site on α2M for LRP [2]. In the absence of proteases, small nucleophilic molecules such as methylamine cause α2M to adopt a structurally similar compact form by reacting with the thioester bond [2, 22]. Several alternative naming systems have been used to describe the two main conformational states of α2M. These include native and transformed [23, 24]; slow and fast, due to the enhanced mobility of the compact form of α2M as assessed by native gel electrophoresis [2, 20]; native and activated [5, 16, 25] and active (or functional) and inactivated [26, 27]. The latter two naming systems can cause confusion given that in the compact state α2M may be considered “activated” in reference to its newly acquired ability to bind to LRP [5] or α2M may be considered “inactivated” in terms of its ability to trap proteases [26, 27]. The term α2M-protease complex is also used to describe α2M after proteases become trapped within the α2M cage [28]. For the purpose of this report the compact conformation of α2M will be distinguished from the native state using the term transformed.
The activities of α2M are typically dependent on whether its conformation is native or transformed, and may also be influenced by dissociation of the native tetramer into stable dimers (Table 1). In biological fluids native α2M is far more abundant than transformed α2M, as the latter is cleared very rapidly via LRP [26, 29, 30]. The physiological relevance of human α2M dimers has yet to be established; it has been demonstrated, however, that human α2M readily forms dimers after exposure to hypochlorite, a potent oxidant produced by activated immune cells [31, 32]. This finding supports the idea that α2M dimers may have specialized importance during inflammation. It has been observed that during cold storage purified α2M can adopt transformed or partially transformed conformations, dissociate into dimers or form high molecular weight aggregates [33–38]. Despite such observations, to our knowledge, no previous study has investigated the effect of commonly used storage conditions on the preservation of purified α2M in its native conformation.
Table 1. Examples of conformational dependent α2M activities.
Many of the activities of α2M are dependent on whether or not the protein is in its native conformation or in its transformed state. Additionally, dissociation of the native α2M tetramer into dimers (that can be induced using several different chemical methods) has also been demonstrated to influence the activities of α2M.
| Function | Native α2M | Transformed α2M | Dissociated α2M dimer | References |
|---|---|---|---|---|
| Protease trapping | Yes | No | No* | [21, 66, 67] |
| Binding to LRP | No | Yes | Yes, providing the treatment does not denature the receptor binding domain* † | [2, 3, 68] |
| Chaperone activity | Yes | Yes, α2M-protease complexes can also prevent protein aggregation by degrading substrates | Yes, enhanced compared to the native α2M* ‡ | [13, 16, 17, 32, 69] |
| Binding to Aβ1–40 or Aβ1–42 peptide | Yes, but to oligomers only, early on the amyloid forming pathway | Yes, binds monomeric and oligomeric Aβ | Yes, binds monomeric and oligomeric Aβ with higher affinity than native α2M* | [17, 32, 45] |
| Binding to TGF-β1 | Yes; KD = 330 ± 130 nM | Yes; KD = 80 ± 11 nM | Markedly reduced compared to native α2M* | [40, 70] |
| Binding to TGF-β2 | Yes; KD = 11 ± 3 nM | Yes; KD = 13 ± 2 nM | Markedly reduced compared to native α2M* | [40, 70] |
| Binding to TNF-α | Only weakly; KD > 1.27 ± 0.17 µM | Only weakly; KD > 0.75 ± 0.10 µM | Markedly increased compared to native α2M* | [40, 70] |
* α2M dimers generated by hypochlorite treatment.
† α2M dimers generated by thiocyanate treatment.
‡ α2M dimers generated by SDS treatment.
Interestingly, there are significant differences between the reported binding affinities of α2M to a range of ligands [17, 32, 39–45] and also between the reported efficacies of α2M at inhibiting protein aggregation [13, 32]. Given the current limited understanding of the cold-induced conformational changes of native α2M, we have investigated the effect of different buffers and storage conditions on the physical properties of α2M and its key activities. This has enabled us to identify a suitable method for the long-term preservation of α2M in its native state.
Materials and Methods
Ethical approval for human blood collection was obtained from the Human Ethics Committee at the University of Wollongong (HE02/080). All donors gave their consent in writing prior to blood collection. All chemicals were obtained from Sigma-Aldrich (Castle Hill NSW, Australia) unless otherwise stated.
Purification of α2M
Human blood was obtained from healthy consenting volunteers and supplemented with 0.4 mg/mL sodium heparin. The cells were pelleted by centrifugation (1000 x g, 30 min, 4°C) and the blood plasma was collected and further supplemented with EDTA-free Complete Protease Inhibitor Cocktail (Roche Diagnostics Ltd., Castle Hill, Australia), according to the manufacturer’s directions. α2M purification procedures were carried out immediately (i.e. the plasma was not stored or frozen) [46]. 5M NaCl and 1M HEPES buffers, pH 7.2, were added to the plasma to achieve final concentrations of 1 M and 20 mM, respectively, and the plasma was filtered through a 0.22 μm membrane. A HiTrap chelating column (GE Healthcare, Silverwater, Australia) was stripped (stripping buffer; 0.5 mM NaCl, 50 mM EDTA, 20 mM HEPES, pH 7.2), washed using milliQ water, recharged (recharge buffer; 0.1 M ZnSO4), and then equilibrated in binding buffer (1 M NaCl, 20 mM HEPES, pH 7.2). The filtered plasma was loaded onto the column and unbound proteins were removed by extensively washing with binding buffer. Loosely bound proteins were eluted from the column by washing with 20 mM imidazole, 0.5 M NaCl, 20 mM HEPES, pH 7.2, and discarded. The remaining bound protein was eluted with 500 mM imidazole, 0.5 M NaCl, 20 mM HEPES, pH 7.2, and dialyzed against phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4, pH 7.4) supplemented with 0.02% (w/v) sodium azide (PBS/Az). The dialyzed protein (2 ml) was fractionated by gel filtration using a HiPrep 26/60 Sephacryl S-300 gel filtration column (GE Healthcare, bed volume 320 mL) and fractions containing purified α2M were pooled and stored at 4°C. To test the effect of different buffer formulations, α2M was extensively dialyzed against 20 mM phosphate buffer, pH 7.4 or 20 mM Tris, pH 8.0, supplemented with NaCl, sodium azide, sucrose or glycine as described in the text. Selected samples were stored at -20°C with or without prior rapid freezing in liquid nitrogen. To quantify the amount of total protein present in solution, a standard bicinchoninic acid (BCA) assay was used [47]. Where indicated, α2M was lyophilized using an Alpha 1–2 LD plus freeze dryer (Martin Christ GmbH, Osterode am Harz, Germany).
Native polyacrylamide gel electrophoresis (PAGE)
Proteins were diluted in sample buffer (100 mM Tris, 10% (v/v) glycerol, 0.0025% (w/v) bromophenol blue, pH 8.6) and subjected to native PAGE using NuPAGE Novex 3–8% Tris-acetate gels (Life Technologies, Mulgrave, Australia) and Tris-glycine running buffer (25 mM Tris, 192 mM glycine, pH 8.3), according to the manufacturer’s instructions. Gels were stained using InstantBlue (Stratech Scientific, Sydney, Australia). To determine the relative proportions of different proteins present in a sample, the density of the relevant bands on native PAGE were measured using ImageJ software.
Trypsin binding assay
Trypsin activity was measured using a N α-benzoyl-L-arginine 4-nitroanilide hydrochloride (BAPNA) assay [48]. A stock solution of bovine trypsin (38 μM) was prepared in 1 mM HCl. Trypsin (3.8 μM) and α2M (70 nM) were co-incubated in 50 mM Tris, pH 8.0 containing 5 μM CaCl2 (10 min, room temperature (RT)) before soybean trypsin inhibitor (10 μM) was added to the solution. After a further incubation (10 min, RT) 2.5 mM BAPNA was added to the solution and the conversion of BAPNA to p-nitroaniline at 37°C was measured at 405 nm using a POLARstar Omega plate reader (BMG Labtech Ltd., Mornington, Australia).
4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonic acid (bisANS) assay
α2M (170 nM) was incubated, in the absence of ambient light, with bisANS (10 μM; 5 min, RT) before the fluorescence was measured using a POLARstar Omega plate reader using excitation and emission wavelengths of 360 ± 10 and 490 ± 10 nm, respectively.
Protein aggregation assays
Creatine phosphokinase (CPK; 5 μM) was incubated in the presence or absence of α 2M (340 nM) in PBS and heated at 43°C in a FLUOstar OPTIMA platereader (BMG Labtech Ltd.). The turbidity was continuously measured as absorbance at 595 nm.
Tissue culture experiments
SH-SY5Y cells corresponding to a human neuroblastoma cell line, were cultured in DMEM:F-12 (Life Technologies) supplemented with 10% (v/v) fetal bovine serum (Bovogen Biologicals, Keilor East, Australia) and maintained in an incubator at 37°C with humidified air containing 5% (v/v) CO2. The cells were routinely passaged using trypsin/EDTA (1:250, pH 7.0; Life Technologies). Prior to flow cytometry experiments, SH-SY5Y cells were cultured for 48 hr without passage before being detached using 5 mM EDTA in PBS.
Flow cytometry analysis
Receptor-associated protein (RAP) inhibits the binding of ligands, including α2M, to lipoprotein receptors [49]. The recombinant fusion protein glutathione-S-transferase-RAP was purified as previously described [50]. SH-SY5Y cells were detached (as described above) and washed in Hank’s binding buffer (HBB; 137 mM NaCl, 5.4 mM KCl, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 2 mM CaCl2, 2 mM MgCl2, 0.1% (w/v) BSA, pH 7.4) by centrifugation. The cells were then incubated step-wise with the following reagents diluted in HBB (all incubations were for 30 min at 4°C and were separated by centrifugal washing of the cells): (i) RAP (8 μM) or HBB alone; (ii) α2M (100 nM); (iii) rabbit anti-α2M antibody (diluted 1:1000; Dako, North Sydney, Australia), and finally (iv) anti-rabbit IgG-FITC antibody (diluted 1:1000; Chemicon, Boronia, Australia). After the final incubation step, the cells were washed twice before analysis using an LSR-II flow cytometer (Beckton Dickinson, North Ryde, Australia). Viable cells were gated based on propidium iodide exclusion (PI; 3 μM). The acquired data were analyzed using FlowJo7 software (TreeStar Inc., Ashland, OR, USA). Background fluorescence was measured using cells treated as above except without incubation with α2M.
Circular dichroism (CD) spectroscopy
CD experiments were performed using a Jasco J-810 spectropolarimeter (JASCO (UK) Ltd, Great Dunmow UK) equipped with a Peltier temperature controller. α2M (270 nM) in sodium phosphate buffer (20 mM, pH 7.4) was analyzed using a 0.1 cm path-length cuvette. For secondary structure analysis, five spectra of each protein sample and of phosphate buffer were recorded between 200 and 250 nm at 20°C (using a scan speed of 50 nm/min with a 1 nm band width and a 4 s response time). The spectra of the samples were averaged and corrected for the signal generated by the buffer alone. In between measurements, the α2M sample was removed from the cuvette and flash frozen in liquid nitrogen. The sample was then thawed, briefly centrifuged and re-analyzed. This sequence was repeated five times and the results were compared to ANS binding measurements of matched α2M samples.
Results and Discussion
Effects of freezing and lyophilization on α2M in PBS/Az
α2M was purified from human blood plasma under non-reducing conditions using a combination of immobilized metal affinity chromatography (IMAC) and size exclusion chromatography. Using this non-denaturing procedure, more than 20 mg of purified native α2M was obtained from 100 ml of blood (the migration of freshly purified α2M in native PAGE is shown in Fig 1A, lane 1). When stored in PBS/Az at 4°C, purified α2M was found to retain its native conformation for 4 months as assessed by its migration on native PAGE (Fig 1A, lane 2). However, after 8 months of storage under the same conditions, purified α2M migrated as a much broader band (Fig 1A, lane 3), suggesting that during prolonged storage some of the protein molecules had altered in structure. Indeed, some molecules appeared to have adopted a more compact structure (migrating slightly faster than the native α2M band), while others had formed smaller species (based on the relative mobility of these species they are likely to be dimers, lower band in lane 3) and still others had formed higher molecular weight species (due to non-covalent self-association of the protein). As a commonly used strategy for long-term storage of proteins involves freezing [51], the protein was analyzed after storage in PBS/Az at -20°C for 10 days; less than 50% of the purified α2M was found to migrate to a position corresponding to native α2M when assessed by native PAGE, the remainder of the protein migrated to a position corresponding to transformed α2M (Fig 1B). The extent of α2M transformation was found to be reduced, but not abolished, by rapid freezing of the protein in liquid nitrogen (-196°C) prior to storage at -20°C (Fig 1B). Rapid freezing of α2M, however, also produced very high molecular weight species that did not readily enter the gel. Analysis of α2M by native PAGE after rapid freezing in liquid nitrogen followed by lyophilization from PBS/Az revealed that a heterogeneous mixture of species was present, which migrated at positions corresponding to native, transformed and aggregated α2M (Fig 1C). Again, under these conditions, less than 50% of the total protein migrated to the same position as native α2M on native PAGE. Also consistent with a loss of native conformation, bisANS fluorescence measurements indicated that when α2M was stored at -20°C or lyophilized it had a markedly increased surface hydrophobicity compared to batch-matched α2M stored in PBS/Az at 4°C (Fig 1D). Lastly, the trypsin binding activity of α2M was reduced by more than 75% after storage at -20°C for 10 days and it was virtually abolished after lyophilization from PBS/Az (Fig 1E). Taken together, the results of these experiments suggest that when stored in PBS/Az at 4°C, α2M retains its native conformation for a period of several months, but that substantial losses of native conformation are incurred during freezing and lyophilization procedures.
Fig 1. The effects of storage temperature on the conformation of purified α2M in PBS/Az.
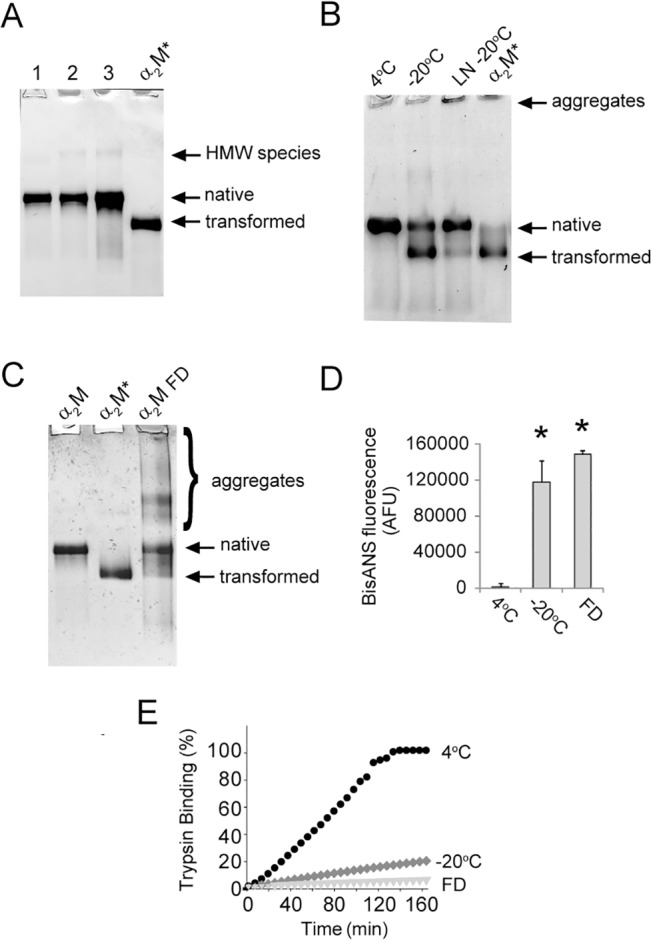
Images of native PAGE (3–8% Tris-acetate) gels showing α2M (A) freshly purified in PBS/Az (lane 1), after storage in PBS/Az (4°C, 4 months) (lane 2) and after storage in PBS/Az (4°C, 8 months) (lane 3). In all samples, there is a small amount of higher molecular weight (HMW) species present; (B) in PBS/Az (4°C or -20°C, 10 days). Also shown is α2M in PBS/Az after rapid freezing in liquid nitrogen (LN) and subsequent storage (-20°C, 10 days), and (C) in PBS/Az (4°C, 2 months) or after freeze-drying (FD) and storage (-20°C, 10 days). In images (A-C) the position of transformed α2M (α2M*; generated by treatment with 400 mM NH4Cl in PBS overnight) is shown. (D) bisANS fluorescence measurements for α2M in PBS/Az (4°C, -20°C, or freeze dried and stored at -20°C, all for 10 days). The results shown are the mean bisANS fluorescence (n = 3±SD) in arbitrary fluorescence units (AFU). (E) Trypsin activity assay showing the conversion of BAPNA to p-nitroaniline by trypsin-α2M complexes. For this assay α2M was stored as described in (D). * Denotes significant increases in bisANS fluorescence as a result of storing native α2M at -20°C, or FD compared to a matched α2M sample stored at 4°C (Student’s t-test p < 0.01).
Effects of NaCl on the conformation of frozen α2M
During freezing of a solvent, salts such as NaCl are partitioned to the aqueous phase and, therefore, markedly increase in concentration. Partitioning of salts and other buffer components in this manner can dramatically alter the solution pH and destabilize proteins [52]. Many biological studies are done in PBS, which contains a physiologically relevant concentration of NaCl (150 mM), and we therefore tested whether or not NaCl was a major factor promoting changes to native α2M upon freezing. We prepared matched samples of native α2M in 20 mM phosphate buffer, pH 7.4, containing 0 or 150 mM NaCl, and analyzed these by native PAGE following storage for 20 days at -20°C. The results suggested that the presence of NaCl produced a small increase (less than 5%) in the proportion of α2M transformed under these conditions (Fig 2A). This effect was marginal compared to that observed when α2M was stored in PBS/Az at -20°C, which resulted in around 50% of α2M being in the transformed state after just 10 days (Fig 1C). In a separate experiment, we confirmed that sodium azide, often used as a biocide to prevent bacterial growth in samples, promoted the transformation and aggregation of α2M during storage at -20°C, but not during storage at 4°C for several months (data not shown).
Fig 2. The effect of NaCl on frozen α2M preparations.
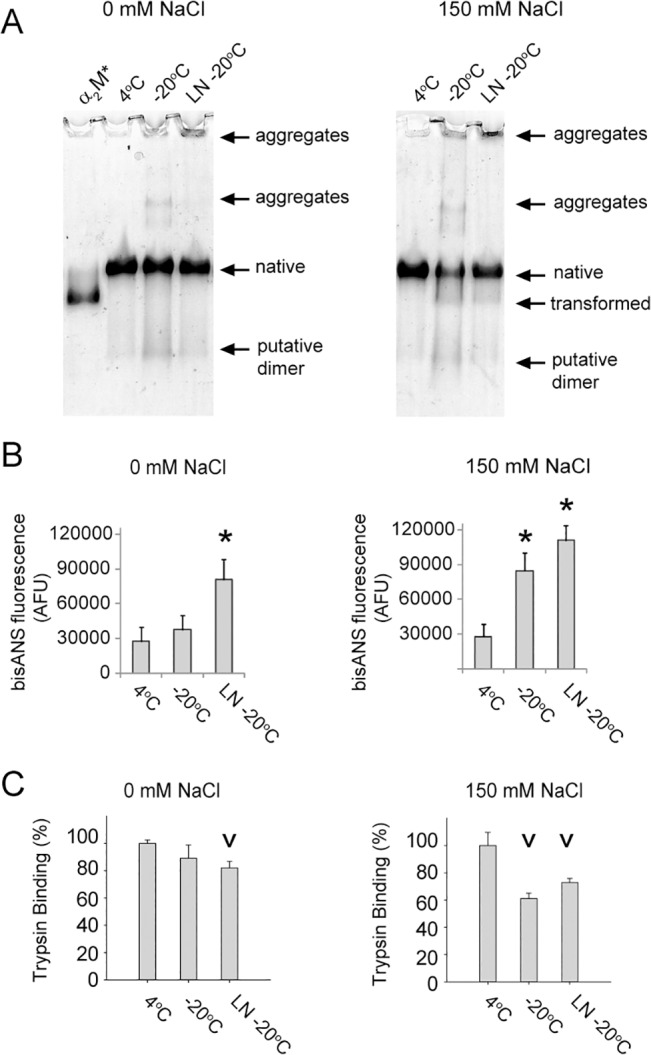
(A) Images of native PAGE (3–8% Tris-acetate) gels showing α2M stored in 20 mM sodium phosphate buffer, pH 7.4, in the presence or absence of 150 mM NaCl (4°C or -20°C, 20 days). LN indicates that the sample was rapidly frozen in liquid nitrogen prior to storage at -20°C. Also shown is the position of α2M* (generated by treatment with 400 mM NH4Cl in PBS overnight). (B) Corresponding bisANS fluorescence measurements for α2M as described in (A). The results shown are the mean bisANS fluorescence (n = 3±SD) in AFU. (C) Trypsin activity assay showing the rate of BAPNA conversion to p-nitroaniline by trypsin-α2M complexes generated using α2M as described in (A). The results shown are the mean BAPNA conversion rates (n = 3±SD). * Denotes significant increases in bisANS fluorescence of α2M stored at -20°C compared to a matched sample stored at 4°C. v Denotes significant decreases in the rate of BAPNA conversion to p-nitroaniline by trypsin-α2M complexes generated using α2M stored at -20°C compared to a matched sample stored at 4°C (both Student’s t-test p < 0.01).
Storage of α2M for 20 days in the absence or presence of NaCl at -20°C resulted in a small degree of dissociation of α2M as well as some aggregation (the combined visible changes were less than 5%; Fig 2A). Although the quantities of smaller species and aggregates visible by native PAGE were similar between the two samples, the amount of native α2M remaining in the sample supplemented with NaCl was reduced by around 50%. Visual inspection of this latter sample revealed that some of the protein had precipitated from solution. Regardless of whether or not NaCl was present, the extent of aggregation and dissociation of α2M in phosphate buffer was reduced by rapidly freezing α2M using liquid nitrogen prior to storage at -20°C, although, as previously observed in this study, this process generated some very high molecular weight species that were retained in the wells of the gel (Fig 2A). After 20 days of storage at -20°C in the presence of NaCl, the surface hydrophobicity of α2M was significantly increased compared to that of a matched sample stored at 4°C as measured by bisANS fluorescence (Fig 2B). This increase did not occur, however, when the α2M was stored under the same conditions in the absence of NaCl (despite some visible changes in the migration of α2M on native PAGE; Fig 2B). Rapid freezing of α2M (which appears to protect against aggregation and transformation that is visible by native PAGE analysis in both the presence and absence of NaCl) did not protect α2M preparations from increases in surface hydrophobicity during storage at -20°C (Fig 2B).
In the absence of NaCl and without rapid freezing in liquid nitrogen, native α2M appeared to tolerate storage in 20 mM phosphate buffer, pH 7.4 at -20°C, for 20 days; however, after prolonged storage under the same conditions the surface hydrophobicity of the preparation increased 8-fold (Fig 2B). All α2M preparations with increased surface hydrophobicity also had significantly decreased trypsin binding activity, consistent with the protein losing its native conformation during storage (Fig 2C). Several cycles of rapid freezing and thawing in phosphate buffer did not greatly alter the migration of α2M on native PAGE or induce change to its secondary structure as assessed by CD spectroscopy (Fig 3A and 3B). Despite the minimal variation between the samples when assessed by native PAGE analysis and CD spectroscopy, bisANS fluorescence measurements indicated that exposed hydrophobicity was increased following rapid freezing and thawing (Fig 3C). Therefore, the results show that it is possible to generate α2M preparations with markedly different hydrophobicity that are largely indistinguishable by native PAGE analysis.
Fig 3. The effect of freezing and thawing on the structure and surface hydrophobicity of α2M.
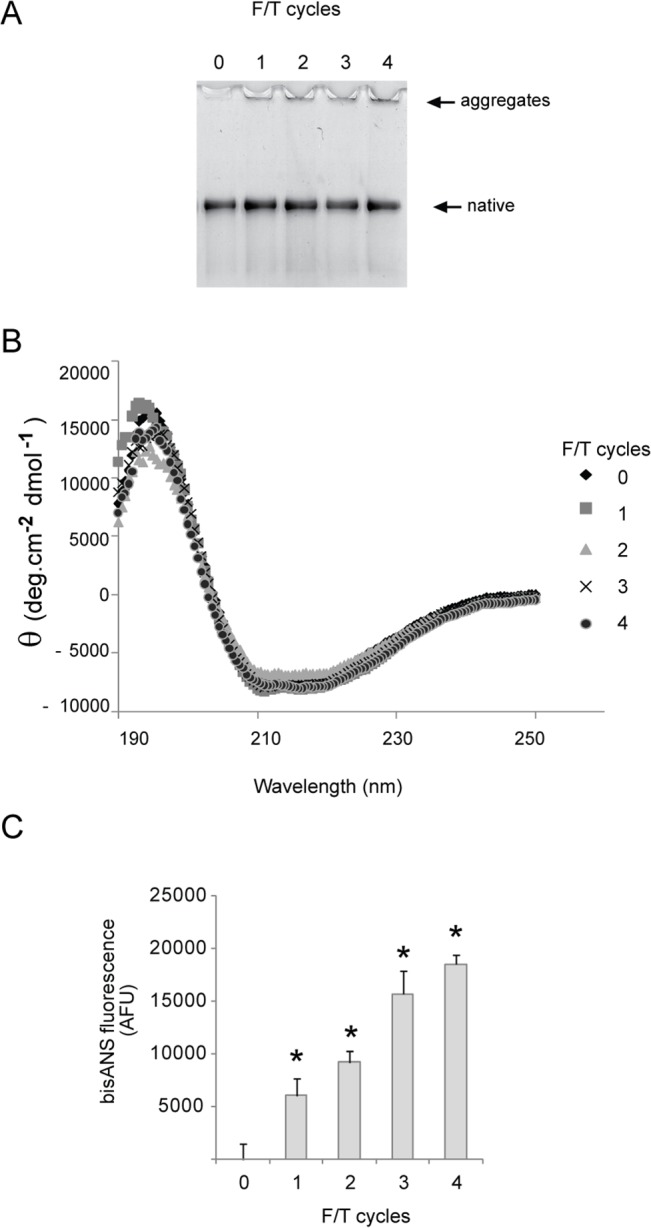
(A) Image of a native PAGE (3–8% Tris-acetate) gel showing the migration of α2M in 20mM sodium phosphate buffer, pH 7.4 after 0–4 cycles of rapid freezing in liquid nitrogen followed by thawing at 37°C (F/T cycles). (B) CD spectra of α2M as described in (A). (C) BisANS fluorescence measurements (excitation 360 nm, emission 490 nm) for α2M as described in (A). * Denotes significant increases or decreases in soluble α2M, bisANS fluorescence or trypsin binding compared to a matched α2M sample stored at 4°C (Student’s t-test p < 0.01).
Effects of freezing-induced conformational changes on α2M interactions with mammalian cells
Transformation of the native α2M tetramer into its compact form, or in some cases its dissociation into dimers, reveals a cryptic binding site for the endocytic receptor LRP (Table 1). To examine the effect of changes induced by freezing on the binding of α2M to SH-SY5Y cells which express LRP [4], we used a range of storage conditions to generate α2M preparations that contained different proportions of transformed α2M (assessed by native PAGE) and different degrees of surface hydrophobicity (assessed by bisANS fluorescence measurements) (Fig 4A and 4B, respectively). The results show that the total cell surface binding of α2M corresponds more closely with the surface hydrophobicity of the preparation than with the degree of transformation (Fig 4C).
Fig 4. The effect of storage at -20°C on the ability of α2M to bind to SH-SY5Y cells.
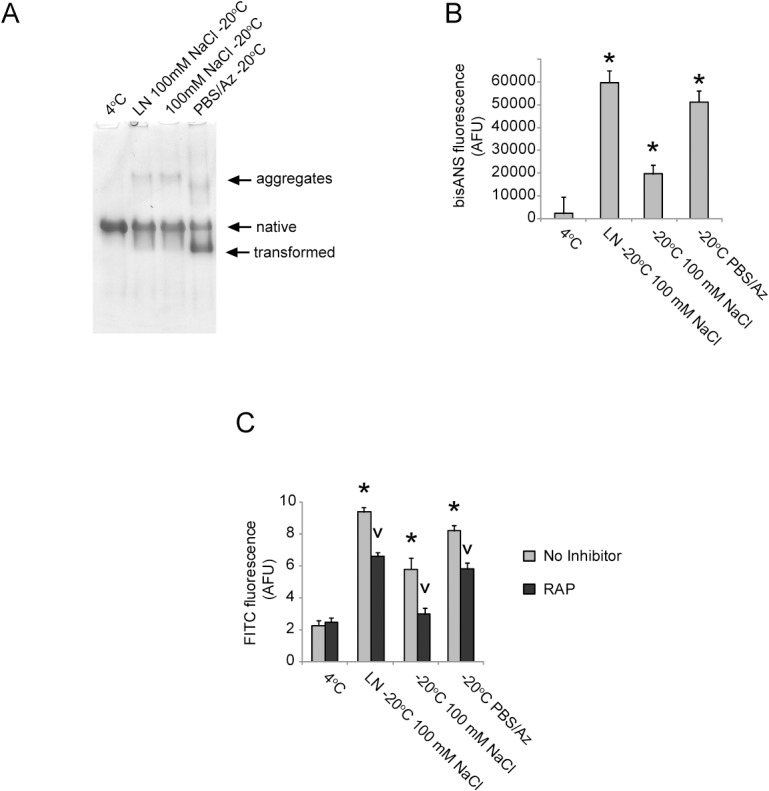
(A) Image of a native PAGE (3–8% Tris-acetate) gel showing α2M stored in 20 mM sodium phosphate buffer, pH 7.4 containing 100 mM NaCl or in PBS/Az (4°C or -20°C, 10 days). LN indicates that the sample was rapidly frozen in liquid nitrogen prior to storage at -20°C. (B) Corresponding bisANS fluorescence measurements for α2M stored as described in (A). The results shown are the mean values of bisANS fluorescence (n = 3±SD) in AFU. (C) Flow cytometry analysis showing the binding of α2M preparations stored as described in (A) to SH-SY5Y cells. The results shown are the composite geometric mean values of FITC fluorescence for 5000 viable cells (n = 3 ± SD) in AFU and are adjusted for background fluorescence. * Denotes significant increases in cell surface binding of α2M stored at -20°C compared to a batch matched sample stored at 4°C. v Denotes significant decreases in cell surface binding of α2M as a result of pre-incubation of the cells with RAP.
Approximately 20–50% of the cell surface binding of the frozen α2M preparations was inhibited by pre-incubating cells with the lipoprotein receptor inhibitor RAP. Surprisingly, the level of RAP-inhibited α2M binding did not closely correspond with the quantity of transformed α2M detected by native PAGE. This finding was clearly demonstrated by comparing the extent of binding inhibited by RAP of α2M stored in PBS/Az at -20°C (which induced ca. 50% of the α2M to migrate on native PAGE similarly to transformed α2M) to that of α2M stored in phosphate buffer supplemented with 100 mM NaCl at -20°C after rapid freezing in liquid nitrogen (which induced less than 5% of the α2M to migrate on native PAGE similar to transformed α2M). In both cases, ca. 20% of the cell surface binding of the α2M appeared to be attributable to lipoprotein receptors. Collectively, the results suggest that loss of the native α2M conformation resulting from storage at -20°C increases its binding to the cell surface via both lipoprotein receptor-dependent and lipoprotein receptor-independent mechanisms (e.g. direct binding to the cell membrane or binding to alternative receptors). The precise mechanisms responsible for the lipoprotein receptor-independent binding of α2M following freezing are unknown; these may, however, include interactions that are mediated by hydrophobicity.
Effects of freezing-induced conformational changes on α2M chaperone activity
The activity of molecular chaperones is typically dependent on hydrophobic interactions between the chaperone and the client protein [53]. We, therefore, tested the chaperone activity of α2M preparations that were largely indistinguishable by native PAGE analysis (Fig 5A), but had significantly different levels of exposed hydrophobic surfaces as assessed by bisANS fluorescence measurements (Fig 5B). Using creatine phosphokinase (CPK), a model client protein that readily aggregates and precipitates when incubated at 43°C, we found that storage at -20°C increased the ability of α2M to inhibit the precipitation of CPK (Fig 5C). At a molar ratio of α2M-to-CPK of 1:15, α2M stored in 20 mM phosphate buffer, pH 7.4 at 4°C for 1 month, had virtually no effect on the precipitation of CPK over a period of 8 hrs. In contrast, under the same conditions a matched α2M preparation that had been rapidly frozen in liquid nitrogen and then stored at -20°C for 1 month, reduced the precipitation of CPK by ca. 50% (Fig 5C).
Fig 5. The effect of storage at -20°C on α2M chaperone activity.
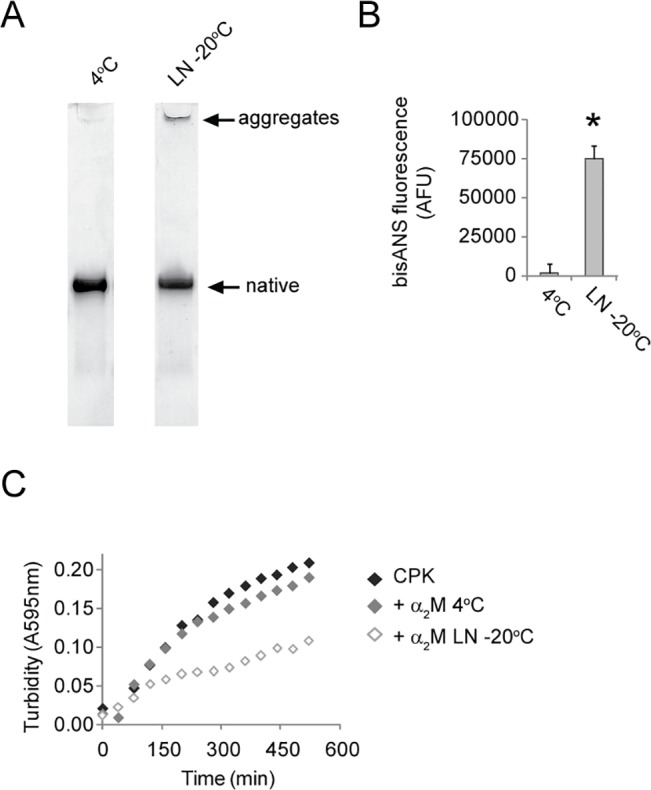
(A) Images of native PAGE (3–8% Tris-acetate) analyses of α2M stored in 20 mM phosphate buffer, pH 7.4 (4°C or -20°C, 1 month). The latter sample was rapidly frozen in LN prior to storage at -20°C. (B) Corresponding bisANS fluorescence measurements for the α2M samples described in (A). The results shown are the values of the mean bisANS fluorescence (n = 3±SD) in AFU. * Denotes significantly increased bisANS fluorescence as a result of storage at -20°C (Student’s t-test p < 0.01) (C) Turbidity measurements of CPK aggregation in the presence or absence of α2M which had been stored at 4°C or -20°C as described in (A). The data are from individual measurements and are representative of several different experiments.
Effects of glycine and sucrose on lyophilized α2M
Many commercial samples of human α2M are in a lyophilized form, although the exact formulations used (i.e. buffers, stabilizers and excipients) can vary significantly. We assessed the composition of one such α2M preparation lyophilized from 20 mM Tris, 130 mM glycine, 80 mM trehalose pH 8.0, by native PAGE analysis. After reconstitution, the α2M was found to contain a heterogeneous mixture of species, mostly corresponding to transformed or partially transformed α2M (Fig 6A). Similar analysis of α2M purified from fresh human plasma (as described in the Materials and Methods), which was rapidly frozen in liquid nitrogen and lyophilized from 20 mM Tris pH 8.0, supported the conclusion that α2M is induced to transform when frozen then lyophilized in Tris buffer (Fig 6B), and that either the stabilizers added to the preparation were insufficient to preserve the native conformation of α2M or the α2M had been induced to adopt a non-native conformation prior to lyophilization. Similar to native α2M stored in 20 mM sodium phosphate, pH 7.4, the native α2M stored in 20 mM Tris pH 8.0, appeared to be stable when stored at 4°C for several months (Fig 6C).
Fig 6. The effect of lyophilization from Tris buffer on purified α2M.
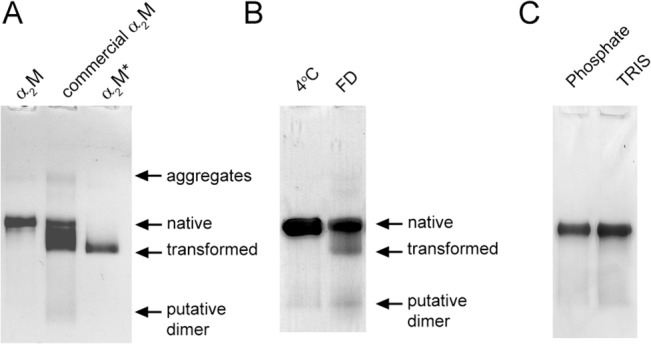
Images of native PAGE (3–8% Tris-acetate) gels showing the migration of (A) reconstituted α2M that had been lyophilized from 20 mM Tris, 130 mM glycine, 80 mM trehalose, pH 8.0 and (B) α2M stored in solution at 4°C in 20 mM Tris, pH 8.0 for 2 months or following reconstitution after it had been lyophilized from 20 mM Tris, pH 8.0 and stored at -20°C for 7 days. As references, the positions of native and transformed α2M are also shown in (A). (C) Matched α2M samples in 20 mM phosphate, pH 7.4 or 20 mM Tris, pH 8.0 stored at 4°C for 2 months. Both samples contained 0.02% (w/v) sodium azide.
In the absence of other buffer constituents (i.e. salt and sodium azide), lyophilization of α2M from 20 mM phosphate buffer, pH 7.4, did not transform the protein; however, a fraction of the protein formed higher molecular weight species (consistent with protein self-association) and a further fraction dissociated into smaller species that migrated on native PAGE similarly to urea-dissociated α2M dimers (Fig 6A). Given that sucrose and glycine are commonly added as stabilizers for commercially available lyophilized α2M preparations, we tested their ability to preserve α2M in its native state during freezing and drying [51, 52, 54]. Native PAGE analysis showed that the addition of either sucrose or glycine to α2M prior to lyophilization reduced its subsequent aggregation and dissociation; however, when glycine was present some high molecular mass species were still visible in the wells of the gel (Fig 7A). Quantification of the amount of soluble protein recovered after reconstitution of the lyophilized sample using a bicinchoninic acid (BCA) assay indicated 85% recovery of the protein (Fig 7B). The addition of sucrose (25–100 mM) or glycine (at a mass ratio of α2M-to-glycine of 1:1 or 1:2) increased the recovery of the lyophilized α2M to nearly 100%. In contrast, using a mass ratio of α2M:glycine of 1:4 did not significantly improve the recovery of lyophilized α2M compare to a matched sample lyophilized from buffer alone (Fig 7B). Consistent with the degree of aggregation assessed by native PAGE analysis, sucrose, but not glycine, preserved the exposed hydrophobicity of the lyophilized α2M preparations at the level of matched α2M samples stored at 4°C (Fig 7C). As observed for α2M preparations stored at -20°C (Fig 2B and 2C), increased hydrophobicity corresponded to decreased trypsin binding activity of lyophilized α2M preparations (Fig 7D).
Fig 7. The effect of sucrose or glycine on the preservation of native α2M characteristics after lyophilization.
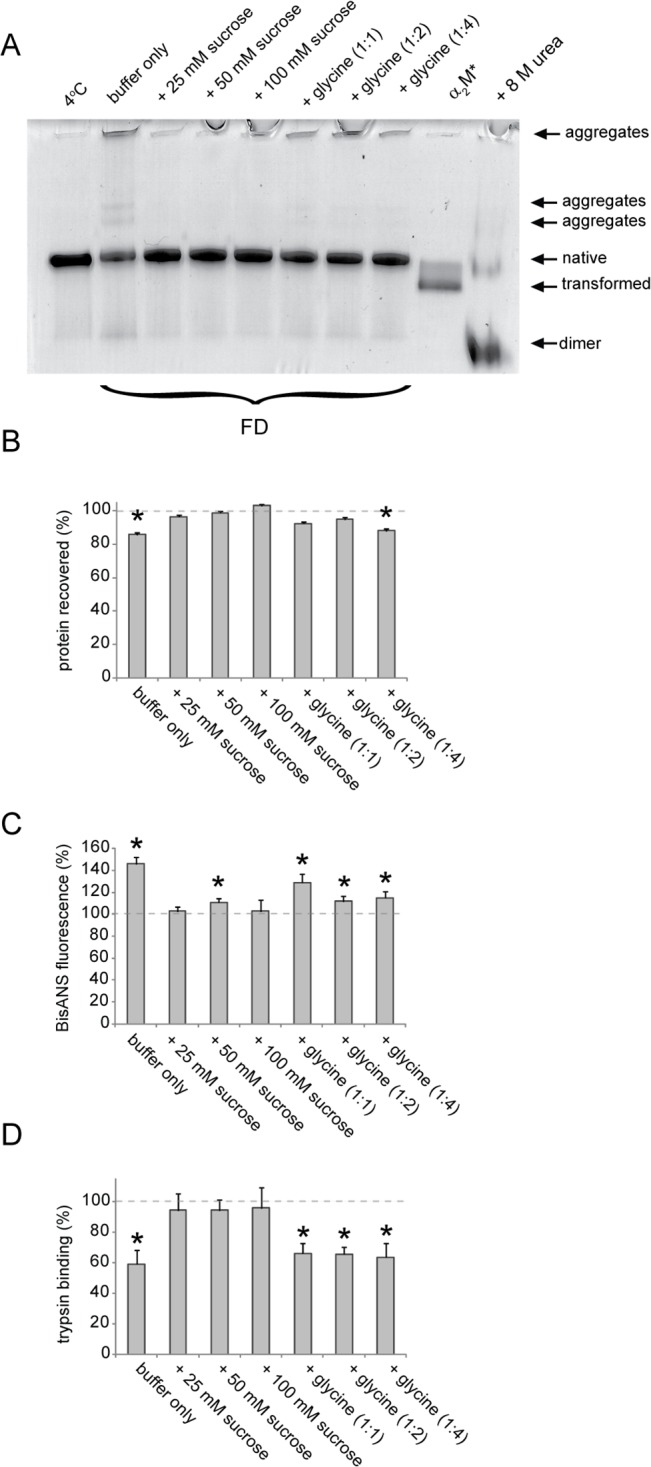
(A) Image of a native PAGE (3–8% Tris-acetate) gel showing α2M stored in 20 mM phosphate buffer, pH 7.4 (4°C, 2 months) or after lyophilization and storage at -20°C for 7 days prior to reconstitution. α2M was lyophilized from buffer only, with sucrose present at the indicated concentrations, or with glycine at the indicated mass ratios (α2M-to-glycine). As references, the positions of native α2M, transformed α2M and dimeric α2M (generated by incubation with 8 M urea) are shown. (B) Recovery of soluble α2M after lyophilization from the conditions described in (A) as assessed by the BCA assay. (C) Corresponding bisANS fluorescence measurements for α2M after lyophilization from the conditions described in (A). The results shown are the values of the mean bisANS fluorescence (n = 3±SD) in AFU.
Conclusion
Despite the fact that functional activities of α2M are crucially dependent on its conformation (Table 1), the susceptibility of α2M to conformational changes during storage [33, 35–37] has often been overlooked in studies of α2M function. The present study, however, indicates that freezing of native α2M can result in a loss of protease-trapping activity together with enhanced chaperone activity and increased binding to cells via lipoprotein receptors or by other unidentified mechanisms. All of these effects appear to be associated with an increase in the surface hydrophobicity of α2M, which may occur independently of α2M adopting the transformed conformation that is known to be more hydrophobic than the native state [55].
We also demonstrate in this study that changes in α2M structure that are undetectable by native PAGE analysis can significantly alter its activities. This finding has broad significance because native PAGE analysis is currently the preferred method used to verify the integrity of native α2M preparations before conducting functional analyses. Our results strongly suggest that it is not safe to assume that α2M is in the native conformation on the basis of native PAGE analysis alone. This conclusion may explain why contradictory results regarding the activities of native α2M have been published; particularly in studies involving hydrophobic ligands (see Introduction). The current study also enables us to propose a strategy to monitor structural changes of stored α2M preparations. This strategy involves the periodic assessment of the surface exposed hydrophobicity of the protein using bisANS fluorescence measurements.
Since their initial characterization in the 1950-60s, bisANS and the related dye 1-anilinonapthalene-8-sulfonate (ANS) have become popular tools for studying surface hydrophobicity and protein aggregation (reviewed in [56]). Both compounds are weakly fluorescent in aqueous solution, but become highly fluorescent when bound to apolar surfaces. It has previously been shown that ANS fluorescence measurements can be used to monitor freezing-induced perturbations of tertiary protein structure with high sensitivity [57, 58]. In the absence of cryoprotectants, an increase in ANS binding induced by freezing is a general property of proteins, but large multi-subunit proteins tend to be more susceptible to destabilization when frozen compared to small monomeric proteins [58]; this latter conclusion is likely to be the result of subunit dissociation. Although the results of this study support that dissociation of α2M into dimers may be a contributing factor, it may not be the only structural modification responsible for the increased surface hydrophobicity of frozen or lyophilized α2M preparations. Nevertheless, dissociation of non-covalently associated subunits has been demonstrated to influence the chaperone activity of α2M and a number of other chaperone proteins [32, 59, 60]; freezing-induced subunit dissociation should, therefore, be closely monitored in chaperone studies.
Prolonged storage of proteins whilst maintaining their physicochemical properties is a complex problem and many investigations about the mechanisms by which proper storage can be achieved have been reported [51, 52, 61–63]. Given the important relationship between structural changes in α2M and its functional properties, we have tried to identify, biologically appropriate, storage conditions for this protein. To improve their long-term stability, proteins are commonly stored at or below -20°C, either in solution or in a lyophilized form [51]. Rapid freezing of α2M was found to provide some protection against structural changes that were visible by native PAGE analysis (Figs 1C and 2A), although transformation, self-association and dissociation of α2M was still observed during prolonged storage at -20°C after rapid freezing. Furthermore, supercooling of α2M in liquid nitrogen generated some high molecular weight species with significantly enhanced surface hydrophobicity compared to the native protein (Fig 2A and 2B). Together these data support the conclusion that, in the absence of a suitable cryoprotectant, rapid freezing of α2M is not a suitable strategy for preserving its native conformation.
Under the conditions used in this study we found that glycine only marginally improved the solubility of lyophilized α2M, and did not preserve its trypsin binding activity (Fig 7). In contrast, the addition of sucrose to α2M preparations appears to offer a reliable method for preserving the native structure and activity of α2M during prolonged storage in solution at temperatures below -20°C and during lyophilization. During freezing (in solution) sucrose affords cryoprotection by being preferentially excluded from contact with the surface of the proteins, thereby leaving the protein preferentially hydrated [64]; whereas during lyophilization, sucrose prevents damage to proteins by forming hydrogen bonds with the dried protein in place of the lost water [65]. Given that many biological functions of proteins are mediated by hydrophobic interactions, great care must be taken when storing proteins in frozen or lyophilized forms for in vitro studies. We have identified that α2M is acutely sensitive to both freezing and lyophilization and that freezing-induced conformational changes significantly influence several of its key activities. While some of these conformational changes result in the visibly altered migration of α2M on native PAGE, bisANS fluorescence measurements provide a far more sensitive indication of the degree to which the native conformation of α2M has been compromised during storage. Our results, therefore, indicate that, in addition to native PAGE analysis, measurement of the surface hydrophobicity of α2M should be adopted as a standard quality control measure for functional studies of this protein.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a Early Career Fellowship from the National Health and Medical Research Council GNT1012521 (A.R.W.); Wellcome Trust Programme Grant (J.R.K., C.M.D.) 094425/Z/10/Z; and Samsung GRO Grant (M.R.W.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Barrett AJ, Starkey PM. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973;133(4):709–24. Epub 1973/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256(15):8134–9. Epub 1981/08/10. . [PubMed] [Google Scholar]
- 3. Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265(29):17401–4. Epub 1990/10/15. . [PubMed] [Google Scholar]
- 4. Fabrizi C, Businaro R, Lauro GM, Fumagalli L. Role of alpha2-macroglobulin in regulating amyloid beta-protein neurotoxicity: protective or detrimental factor? J Neurochem. 2001;78(2):406–12. Epub 2001/07/20. . [DOI] [PubMed] [Google Scholar]
- 5. Narita M, Holtzman DM, Schwartz AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69(5):1904–11. Epub 1998/02/12. . [DOI] [PubMed] [Google Scholar]
- 6. Birkenmeier G, Kampfer I, Kratzsch J, Schellenberger W. Human leptin forms complexes with alpha 2-macroglobulin which are recognized by the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Eur J Endocrinol. 1998;139(2):224–30. Epub 1998/09/02. . [DOI] [PubMed] [Google Scholar]
- 7. Crookston KP, Webb DJ, Lamarre J, Gonias SL. Binding of platelet-derived growth factor-BB and transforming growth factor-beta 1 to alpha 2-macroglobulin in vitro and in vivo: comparison of receptor-recognized and non-recognized alpha 2-macroglobulin conformations. Biochem J. 1993;293 (Pt 2):443–50. Epub 1993/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LaMarre J, Wollenberg GK, Gonias SL, Hayes MA. Cytokine binding and clearance properties of proteinase-activated alpha 2-macroglobulins. Lab Invest. 1991;65(1):3–14. Epub 1991/07/01. . [PubMed] [Google Scholar]
- 9.Misra UK, Pizzo SV. Activated alpha2-Macroglobulin Binding to Human Prostate Cancer Cells Triggers Insulin-like Responses. J Biol Chem. 2015. 10.1074/jbc.M114.617837 . [DOI] [PMC free article] [PubMed]
- 10. Sunderic M, Malenkovic V, Nedic O. Complexes between insulin-like growth factor binding proteins and alpha-2-macroglobulin in patients with tumor. Experimental and molecular pathology. 2015;98(2):173–7. 10.1016/j.yexmp.2015.03.003 . [DOI] [PubMed] [Google Scholar]
- 11. Adler V, Kryukov V. Serum macroglobulin induces prion protein transition. Neurochemical Journal. 2007;1:43–52. [Google Scholar]
- 12. Flory ED, Clarris BJ, Muirden KD. Deposits of alpha 2M in the rheumatoid synovial membrane. Ann Rheum Dis. 1982;41(5):520–6. Epub 1982/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. French K, Yerbury JJ, Wilson MR. Protease activation of alpha2-macroglobulin modulates a chaperone-like action with broad specificity. Biochemistry. 2008;47(4):1176–85. Epub 2008/01/04. 10.1021/bi701976f . [DOI] [PubMed] [Google Scholar]
- 14. Hollander W, Colombo MA, Kirkpatrick B, Paddock J. Soluble proteins in the human atherosclerotic plaque. With spectral reference to immunoglobulins, C3-complement component, alpha 1-antitrypsin and alpha 2-macroglobulin. Atherosclerosis. 1979;34(4):391–405. Epub 1979/12/01. . [DOI] [PubMed] [Google Scholar]
- 15. Thal DR, Schober R, Birkenmeier G. The subunits of alpha2-macroglobulin receptor/low density lipoprotein receptor-related protein, native and transformed alpha2-macroglobulin and interleukin 6 in Alzheimer's disease. Brain Res. 1997;777(1–2):223–7. Epub 1998/02/04. doi: S0006-8993(97)01021-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 16. Wyatt AR, Constantinescu P, Ecroyd H, Dobson CM, Wilson MR, Kumita JR, et al. Protease-activated alpha-2-macroglobulin can inhibit amyloid formation via two distinct mechanisms. FEBS Lett. 2013;587(5):398–403. Epub 2013/01/29. doi: S0014-5793(13)00051-3 [pii] 10.1016/j.febslet.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yerbury JJ, Kumita JR, Meehan S, Dobson CM, Wilson MR. alpha2-Macroglobulin and haptoglobin suppress amyloid formation by interacting with prefibrillar protein species. J Biol Chem. 2009;284(7):4246–54. Epub 2008/12/17. doi: M807242200 [pii] 10.1074/jbc.M807242200 . [DOI] [PubMed] [Google Scholar]
- 18.Scopus. Elsevier B.V., 2014.
- 19. Marrero A, Duquerroy S, Trapani S, Goulas T, Guevara T, Andersen GR, et al. The crystal structure of human alpha2-macroglobulin reveals a unique molecular cage. Angew Chem Int Ed Engl. 2012;51(14):3340–4. Epub 2012/02/01. 10.1002/anie.201108015 . [DOI] [PubMed] [Google Scholar]
- 20. Petersen CM, Ejlersen E, Moestrup SK, Jensen PH, Sand O, Sottrup-Jensen L. Immunosuppressive properties of electrophoretically "slow" and "fast" form alpha 2-macroglobulin. Effects on cell-mediated cytotoxicity and (allo-) antigen-induced T cell proliferation. J Immunol. 1989;142(2):629–35. Epub 1989/01/15. . [PubMed] [Google Scholar]
- 21. Salvesen GS, Sayers CA, Barrett AJ. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J. 1981;195(2):453–61. Epub 1981/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larsson LJ, Lindahl P, Hallen-Sandgren C, Bjork I. The conformational changes of alpha 2-macroglobulin induced by methylamine or trypsin. Characterization by extrinsic and intrinsic spectroscopic probes. Biochem J. 1987;243(1):47–54. Epub 1987/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boisset N, Taveau JC, Pochon F, Lamy J. Similar architectures of native and transformed human alpha2-macroglobulin suggest the transformation mechanism. J Biol Chem. 1996;271(42):25762–9. Epub 1996/10/18. . [DOI] [PubMed] [Google Scholar]
- 24. Gunnarsson M, Sundstrom P, Stigbrand T, Jensen PE. Native and transformed alpha2-macroglobulin in plasma from patients with multiple sclerosis. Acta Neurol Scand. 2003;108(1):16–21. Epub 2003/06/17. doi: 079 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25. Panyutich A, Ganz T. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5(2):101–6. Epub 1991/08/01. 10.1165/ajrcmb/5.2.101 . [DOI] [PubMed] [Google Scholar]
- 26. Abbink JJ, Nuijens JH, Eerenberg AJ, Huijbregts CC, Strack van Schijndel RJ, Thijs LG, et al. Quantification of functional and inactivated alpha 2-macroglobulin in sepsis. Thromb Haemost. 1991;65(1):32–9. Epub 1991/01/23. . [PubMed] [Google Scholar]
- 27. Ignjatovic V, Greenway A, Summerhayes R, Monagle P. Thrombin generation: the functional role of alpha-2-macroglobulin and influence of developmental haemostasis. Br J Haematol. 2007;138(3):366–8. Epub 2007/06/19. doi: BJH6663 [pii] 10.1111/j.1365-2141.2007.06663.x . [DOI] [PubMed] [Google Scholar]
- 28. Donnelly PK, Trotter P, White M, Shenton BK. Immunoelectrophoretic pattern of alpha 2-macroglobulin and alpha 2-macroglobulin-protease complexes. Clin Chim Acta. 1990;195(1–2):87–92. Epub 1990/12/31. . [DOI] [PubMed] [Google Scholar]
- 29. Banks RE, Evans SW, Van Leuven F, Alexander D, McMahon MJ, Whicher JT. Measurement of the 'fast' or complexed form of alpha 2 macroglobulin in biological fluids using a sandwich enzyme immunoassay. J Immunol Methods. 1990;126(1):13–20. Epub 1990/01/24. doi: 0022-1759(90)90006-H [pii]. . [DOI] [PubMed] [Google Scholar]
- 30. Feldman SR, Ney KA, Gonias SL, Pizzo SV. In vitro binding and in vivo clearance of human alpha 2-macroglobulin after reaction with endoproteases from four different classes. Biochem Biophys Res Commun. 1983;114(2):757–62. Epub 1983/07/29. doi: 0006-291X(83)90845-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31. Reddy VY, Pizzo SV, Weiss SJ. Functional inactivation and structural disruption of human alpha 2-macroglobulin by neutrophils and eosinophils. J Biol Chem. 1989;264(23):13801–9. Epub 1989/08/15. . [PubMed] [Google Scholar]
- 32. Wyatt AR, Kumita JR, Mifsud RW, Gooden CA, Wilson MR, Dobson CM. Hypochlorite-induced structural modifications enhance the chaperone activity of human alpha2-macroglobulin. Proc Natl Acad Sci U S A. 2014;111(20):E2081–90. Epub 2014/05/07. doi: 1403379111 [pii] 10.1073/pnas.1403379111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barrett AJ. Alpha 2-macroglobulin. Methods in Enzymology. 1981;80 Pt C:737–54. [DOI] [PubMed] [Google Scholar]
- 34. Barrett AJ, Brown MA, Sayers CA. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979;181(2):401–18. Epub 1979/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6(15):3345–53. Epub 1992/12/01. . [DOI] [PubMed] [Google Scholar]
- 36. Sottrup-Jensen L, Hansen HF, Pedersen HS, Kristensen L. Localization of epsilon-lysyl-gamma-glutamyl cross-links in five human alpha 2-macroglobulin-proteinase complexes. Nature of the high molecular weight cross-linked products. J Biol Chem. 1990;265(29):17727–37. Epub 1990/10/15. . [PubMed] [Google Scholar]
- 37. Van Leuven F, Cassiman JJ, Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. I. Characterization of alpha 2 M after derivatization by methylamine and by factor XIII. J Biol Chem. 1981;256(17):9016–22. . [PubMed] [Google Scholar]
- 38. Van Leuven F, Cassiman JJ, Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. Kinetics of inactivation of alpha 2-macroglobulin by methylamine, and formation of anomalous complexes with trypsin. Biochem J. 1982;201(1):119–28. Epub 1982/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonner JC, Goodell AL, Lasky JA, Hoffman MR. Reversible binding of platelet-derived growth factor-AA,-AB, and-BB isoforms to a similar site on the "slow" and "fast" conformations of alpha 2-macroglobulin. J Biol Chem. 1992;267(18):12837–44. Epub 1992/06/25. . [PubMed] [Google Scholar]
- 40. Crookston KP, Webb DJ, Wolf BB, Gonias SL. Classification of alpha 2-macroglobulin-cytokine interactions based on affinity of noncovalent association in solution under apparent equilibrium conditions. J Biol Chem. 1994;269(2):1533–40. Epub 1994/01/14. . [PubMed] [Google Scholar]
- 41. Dennis PA, Saksela O, Harpel P, Rifkin DB. Alpha 2-macroglobulin is a binding protein for basic fibroblast growth factor. J Biol Chem. 1989;264(13):7210–6. Epub 1989/05/05. . [PubMed] [Google Scholar]
- 42. Du Y, Ni B, Glinn M, Dodel RC, Bales KR, Zhang Z, et al. alpha2-Macroglobulin as a beta-amyloid peptide-binding plasma protein. J Neurochem. 1997;69(1):299–305. Epub 1997/07/01. . [PubMed] [Google Scholar]
- 43. Hughes SR, Khorkova O, Goyal S, Knaeblein J, Heroux J, Riedel NG, et al. Alpha2-macroglobulin associates with beta-amyloid peptide and prevents fibril formation. Proc Natl Acad Sci U S A. 1998;95(6):3275–80. Epub 1998/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lauer D, Reichenbach A, Birkenmeier G. Alpha 2-macroglobulin-mediated degradation of amyloid beta 1–42: a mechanism to enhance amyloid beta catabolism. Exp Neurol. 2001;167(2):385–92. Epub 2001/02/13. 10.1006/exnr.2000.7569 S0014-4886(00)97569-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 45. Mettenburg JM, Webb DJ, Gonias SL. Distinct binding sites in the structure of alpha 2-macroglobulin mediate the interaction with beta-amyloid peptide and growth factors. J Biol Chem. 2002;277(15):13338–45. Epub 2002/02/02. 10.1074/jbc.M106792200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 46. Kurecki T, Kress LF, Laskowski M, Sr. Purification of human plasma alpha 2 macroglobulin and alpha 1 proteinase inhibitor using zinc chelate chromatography. Analytical biochemistry. 1979;99(2):415–20. . [DOI] [PubMed] [Google Scholar]
- 47. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Analytical biochemistry. 1985;150(1):76–85. . [DOI] [PubMed] [Google Scholar]
- 48. Erlanger BF, Kokowsky N, Cohen W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961;95:271–8. . [DOI] [PubMed] [Google Scholar]
- 49. Moestrup SK, Gliemann J. Analysis of ligand recognition by the purified alpha 2-macroglobulin receptor (low density lipoprotein receptor-related protein). Evidence that high affinity of alpha 2-macroglobulin-proteinase complex is achieved by binding to adjacent receptors. J Biol Chem. 1991;266(21):14011–7. . [PubMed] [Google Scholar]
- 50. Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266(31):21232–8. Epub 1991/11/05. . [PubMed] [Google Scholar]
- 51.Carpenter JF, Manning MC, Randolph TW. Long-term storage of proteins. Current protocols in protein science / editorial board, John E Coligan [et al]. 2002;Chapter 4:Unit 4 6. 10.1002/0471140864.ps0406s27 . [DOI] [PubMed]
- 52. Pikal-Cleland KA, Cleland JL, Anchordoquy TJ, Carpenter JF. Effect of glycine on pH changes and protein stability during freeze-thawing in phosphate buffer systems. J Pharm Sci. 2002;91(9):1969–79. Epub 2002/09/05. 10.1002/jps.10184 . [DOI] [PubMed] [Google Scholar]
- 53. Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–32. Epub 2011/07/22. doi: nature10317 [pii] 10.1038/nature10317 . [DOI] [PubMed] [Google Scholar]
- 54. Arakawa T, Tsumoto K, Kita Y, Chang B, Ejima D. Biotechnology applications of amino acids in protein purification and formulations. Amino Acids. 2007;33(4):587–605. Epub 2007/03/16. 10.1007/s00726-007-0506-3 . [DOI] [PubMed] [Google Scholar]
- 55. Birkenmeier G, Carlsson-Bostedt L, Shanbhag V, Kriegel T, Kopperschlager G, Sottrup-Jensen L, et al. Differences in hydrophobic properties for human alpha 2-macroglobulin and pregnancy zone protein as studied by affinity phase partitioning. Eur J Biochem. 1989;183(2):239–43. Epub 1989/08/01. . [DOI] [PubMed] [Google Scholar]
- 56. Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2008;25(7):1487–99. Epub 2008/01/04. 10.1007/s11095-007-9516-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gabellieri E, Strambini GB. Perturbation of protein tertiary structure in frozen solutions revealed by 1-anilino-8-naphthalene sulfonate fluorescence. Biophys J. 2003;85(5):3214–20. Epub 2003/10/29. doi: S0006-3495(03)74739-0 [pii] 10.1016/S0006-3495(03)74739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gabellieri E, Strambini GB. ANS fluorescence detects widespread perturbations of protein tertiary structure in ice. Biophys J. 2006;90(9):3239–45. Epub 2006/02/08. doi: S0006-3495(06)72506-1 [pii] 10.1529/biophysj.105.074948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hilton GR, Lioe H, Stengel F, Baldwin AJ, Benesch JL. Small heat-shock proteins: paramedics of the cell. Top Curr Chem. 2013;328:69–98. Epub 2012/05/12. 10.1007/128_2012_324 . [DOI] [PubMed] [Google Scholar]
- 60. Roman SG, Chebotareva NA, Eronina TB, Kleymenov SY, Makeeva VF, Poliansky NB, et al. Does the crowded cell-like environment reduce the chaperone-like activity of alpha-crystallin? Biochemistry. 2011;50(49):10607–23. 10.1021/bi201030y . [DOI] [PubMed] [Google Scholar]
- 61. Lale SV, Goyal M, Bansal AK. Development of lyophilization cycle and effect of excipients on the stability of catalase during lyophilization. Int J Pharm Investig. 2011;1(4):214–21. Epub 2012/10/17. 10.4103/2230-973X.93007IJPI-1-214 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem. 1981;256(14):7193–201. . [PubMed] [Google Scholar]
- 63. Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–75. 10.1007/s11095-009-0045-6 . [DOI] [PubMed] [Google Scholar]
- 64. Carpenter JF, Crowe JH. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 1988;25(3):244–55. Epub 1988/06/01. doi: 0011-2240(88)90032-6 [pii]. . [DOI] [PubMed] [Google Scholar]
- 65. Allison SD, Chang B, Randolph TW, Carpenter JF. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch Biochem Biophys. 1999;365(2):289–98. Epub 1999/05/18. doi: S0003-9861(99)91175-X [pii] 10.1006/abbi.1999.1175 . [DOI] [PubMed] [Google Scholar]
- 66. Reddy VY, Desorchers PE, Pizzo SV, Gonias SL, Sahakian JA, Levine RL, et al. Oxidative dissociation of human alpha 2-macroglobulin tetramers into dysfunctional dimers. J Biol Chem. 1994;269(6):4683–91. Epub 1994/02/11. . [PubMed] [Google Scholar]
- 67. Salvesen GS, Barrett AJ. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980;187(3):695–701. Epub 1980/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu SM, Boyer CM, Pizzo SV. The binding of receptor-recognized alpha2-macroglobulin to the low density lipoprotein receptor-related protein and the alpha2M signaling receptor is decoupled by oxidation. J Biol Chem. 1997;272(33):20627–35. Epub 1997/08/15. . [DOI] [PubMed] [Google Scholar]
- 69. Ozawa D, Hasegawa K, Lee YH, Sakurai K, Yanagi K, Ookoshi T, et al. Inhibition of beta2-microglobulin amyloid fibril formation by alpha2-macroglobulin. J Biol Chem. 2011;286(11):9668–76. Epub 2011/01/11. doi: M110.167965 [pii] 10.1074/jbc.M110.167965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu SM, Patel DD, Pizzo SV. Oxidized alpha2-macroglobulin (alpha2M) differentially regulates receptor binding by cytokines/growth factors: implications for tissue injury and repair mechanisms in inflammation. J Immunol. 1998;161(8):4356–65. Epub 1998/10/21. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


