Abstract
Longer time from previous perioperative chemotherapy (TFPC) ≥ 78 weeks and Eastern Cooperative Oncology Group (ECOG) performance status (PS) = 0 were independently prognostic for better survival with cisplatin-based first-line chemotherapy for advanced urothelial carcinoma (UC) after previous perioperative cisplatin-based chemotherapy. Because of particularly poor outcomes in those with TFPC < 52 weeks, the data support using TFPC ≥ 52 weeks to rechallenge with cisplatin-based first-line chemotherapy for metastatic disease.
Background
Outcomes with cisplatin-based first-line therapy for advanced UC after previous perioperative cisplatin-based chemotherapy are unclear. In this study we evaluated outcomes with a focus on the effect of time from previous cisplatin-based perioperative chemotherapy.
Patients and Methods
Data were collected for patients who received cisplatin-based first-line therapy for advanced UC after previous perioperative cisplatin-based therapy. Cox proportional hazards models were used to investigate the prognostic ability of visceral metastasis, ECOG PS, TFPC, anemia, leukocytosis, and albumin on overall survival (OS).
Results
Data were available for 41 patients from 8 institutions including 31 men (75.6%). The median age was 61 (range, 41–77) years, most received gemcitabine plus cisplatin (n = 26; 63.4%), and the median number of cycles was 4 (range, 1–8). The median OS was 68 weeks (95% confidence interval [CI], 48.0–81.0). Multivariable Cox regression analysis results showed an independent prognostic effect on OS for PS > 0 versus 0 (hazard ratio [HR], 4.56 [95% CI, 1.66–12.52]; P = .003) and TFPC ≥ 78 weeks versus < 78 weeks (HR, 0.48 [95% CI, 0.21–1.07]; P = .072). The prognostic model for OS was internally validated with c-index = 0.68. Patients with TFPC < 52 weeks, 52 to 104 weeks, and ≥ 104 weeks had median survival of 42, 70, and 162 weeks, respectively.
Conclusion
Longer TFPC ≥ 78 weeks and ECOG PS = 0 were independently prognostic for better survival with cisplatin-based first-line chemotherapy for advanced UC after previous perioperative cisplatin-based chemotherapy. The data support using TFPC ≥ 52 weeks to rechallenge with cisplatin-based first-line chemotherapy for metastatic disease.
Keywords: Cisplatin, First-line, Peri-operative, Urothelial carcinoma
Introduction
Cisplatin-based chemotherapy is established as a standard first-line therapy for metastatic urothelial carcinoma (UC).1–4 Gemcitabine with cisplatin (GC), MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), or dose-dense (DD) MVAC are all acceptable regimens and yield a median overall survival (OS) of 12 to 15 months. However, these trials were conducted in an era before definitive evidence supporting the role of neoadjuvant cisplatin-based chemotherapy was adopted in the community.5–7 Indeed, the phase III trials that established cisplatin-based chemotherapy as first-line therapy did not enroll patients who had received previous perioperative chemotherapy. Although data supporting the role of adjuvant cisplatin-based chemotherapy for high-risk disease after radical cystectomy are still controversial, adjuvant chemotherapy is often more frequently used.8–14
The effect of previous perioperative cisplatin-based chemotherapy on outcomes when repeating cisplatin-based chemotherapy as first-line therapy for subsequent recurrent or metastatic disease is unclear. Major known prognostic factors across different studies in the setting of first-line chemotherapy include visceral metastasis, performance status (PS), hemoglobin (Hb), leukocyte count, and albumin.15–17 We hypothesized that longer time from previous perioperative cisplatin-based chemotherapy might be an additional prognostic factor, which concurs with recently reported first-line phase II trials and an ongoing important phase III trial (Cancer and Leukemia Group B [CALGB]-90601 trial), which restricted inclusion of patients to those with > 52 weeks from previous perioperative cisplatin-based chemotherapy. Hence, we conducted a retrospective analysis of patients with advanced UC who received first-line cisplatin-based chemotherapy after previous perioperative cisplatin-based chemotherapy to study outcomes and evaluate the effect of time from perioperative cisplatin-based chemotherapy after controlling for known prognostic factors.
Patients and Methods
Patient Population
Data were requested from 10 collaborating institutions regarding patients who received cisplatin-based first-line therapy for advanced UC after previous perioperative cisplatin-based therapy. Data were requested for baseline visceral metastasis, Eastern Cooperative Oncology Group (ECOG) PS, time from previous perioperative chemotherapy (TFPC), Hb, leukocyte count, and albumin. Patient outcomes, specifically best response, progression-free survival (PFS), and OS, from first-line therapy were also requested. The data were deidentified and provided in an Excel spreadsheet by all investigators. The study was conducted after institutional review board approval at the University of Alabama, Birmingham for retrospective analyses of such patients.
Statistical Methods
Descriptive statistics was used to summarize patient and treatment characteristics and outcomes. The primary clinical end point of interest was OS from the date of beginning first-line chemotherapy. The Kaplan–Meier method was used to estimate time to event outcomes. OS was defined according to those alive with or without disease, and PFS was defined according to those alive and free from disease progression, from the first date the patient received first-line therapy. Univariable Cox proportional hazards models were used to investigate the prognostic ability of age, sex, number of cycles of chemotherapy, dose of cisplatin per 3- to 4-week cycle, calculated creatinine clearance, setting of previous perioperative chemotherapy (neoadjuvant or adjuvant), first-line regimen (GC or other), visceral metastasis, ECOG-PS, Hb, leukocyte count, albumin, and TFPC to initiating first-line chemotherapy on OS and PFS. Predefined cutoff points of TFPC were assessed for prognostic effect including 52 weeks (approximately 1 year), 78 weeks (approximately 1.5 years), and 104 weeks (approximately 2 years). Anemia was defined as Hb < the lower limit of normal according to sex as recorded by the local laboratory. Leukocytosis was defined as a white blood cell count > the upper limit of normal (ULN) based on the local laboratory. Albumin was evaluated on a continuous scale. A forward stepwise selection method was used to create an optimal multivariable model of prognostic factors. Albumin was excluded from the multivariable model selection because of a large number of missing data, but all other factors were included. Because stepwise selection requires complete case data, results for the final multivariable model were calculated based on data from all patients with complete data on the selected factors. All tests and confidence intervals (CIs) were 2-sided and set at P = .05 level of significance. Internal validation of the final multivariate models were performed by performing 2000 bootstrap replications and calculating the estimated median and 95% bias-corrected and accelerated (BCa) CIs for the hazard ratio (HR) estimates of each factor, and for the concordance-statistic (c-statistic).
Results
Patient Characteristics
Individual level data for 41 patients from 8 institutions who were treated between the years 1999 and 2013 were obtained (Table 1). Two institutions could not identify any eligible patients. The evaluable patients came from Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy (n = 16), University Federico II Napoli, Italy (n = 13), British Columbia Cancer Agency, Vancouver, Canada (n = 3), City of Hope, CA (n = 3), University of Utah, Salt Lake City, UT (n = 3), Wayne State University Cancer Center, Detroit, MI, Heinrich Heine University, Dusseldorf, Germany, University of Liverpool, Liverpool, United Kingdom (n = 1 each). The cohort included 31 men (75.6%), the median age was 61 (range, 41 to 77) years, and 46.3% had visceral disease. Most had an ECOG-PS of 0 (n = 32; 78.1%) and only 1 patient had an ECOG-PS of 2. Most received first-line GC (n = 28; 68.3%), the median number of cycles was 4 (range, 2–8), and the median time from previous perioperative cisplatin-based chemotherapy to first-line therapy was 68 weeks. The previous perioperative cisplatin-based chemotherapy was administered in the adjuvant setting in most patients (63.4%). Pathologic T0 (pT0) disease was observed in 1 of the 30 patients for whom pathologic staging at the time of radical cystectomy was available. This patient had received neoadjuvant chemotherapy.
Table 1.
Patient Characteristics (n = 41)
| Characteristic | Value |
|---|---|
| Male Sex, n (%) | 31 (75.6) |
| Type of Perioperative Chemotherapy, n (%) | |
| Adjuvant; cisplatin-based | 26 (63.4) |
| GC | 28 (68.3) |
| MVAC | 12 (29.3) |
| Other cisplatin-based | 1 (2.4)a |
| Number of Cycles | |
| Median (range) | 4 (2–8) |
| ≥4, n (%) | 26 (63.4) |
| ≥6, n (%) | 11 (26.8) |
| Visceral Metastases at Recurrence, n (%) | 19 (46.3) |
| First-Line Chemotherapy | |
| Weeks From Last Perioperative Chemotherapy to First-Line Chemotherapy | |
| Median (range) | 68 (3–451) |
| ≥52 | 25 (61.0) |
| ≥78 | 17 (41.5) |
| ≥104 | 9 (22.0) |
| Median age (range), years | 61 (41–77) |
| ECOG Performance Status | |
| 0 | 32 (78.1) |
| 1 | 8 (19.5) |
| 2 | 1 (2.4) |
| Hemoglobin, n (%) | |
| In normal range | 19 (46.3) |
| <Lower limit of normal (anemia) | 21 (51.2) |
| Unknown | 1 (2.4) |
| Leukocyte count | |
| >ULN | 29 (70.7) |
| ≤ULN | 10 (24.4) |
| Unknown | 2 (4.9) |
| Creatinine clearance, n (%) | |
| <60 mL/min | 8 (19.5) |
| ≥60 mL/min | 31 (75.6) |
| Unknown | 2 (4.9) |
| Albumin | |
| Median (range) | 4.0 (2.9–5.1) |
| Unknown, n (%) | 7 (17.1) |
| Type of first-line chemotherapy | |
| GC | 26 (63.4) |
| MVAC | 5 (12.2) |
| Other cisplatin-based | 10 (24.4) |
| Number of Cycles | |
| Median (range) | 4 (1, 8) |
| ≥4, n (%) | 30 (73.2) |
| ≥6, n (%) | 17 (41.5) |
| Dose of cisplatin ≥70 mg/m2 per cycle, n (%) | 38 (92.7) |
| Removed because of toxicity, n (%) | 8 (19.5) |
| Outcomes | |
| Best response to first-line chemotherapy | |
| CR | 3 (7.3) |
| PR | 18 (43.9) |
| SD | 13 (31.7) |
| PD | 6 (14.6) |
| Not available | 1 (2.4) |
| Progression-free survivala | |
| Events, n (%) | 38 (92.7) |
| Median weeks (95% CI) | 30.4 (24.0–40.0) |
| 26 Weeks (95% CI), % | 60.4 (43.7–73.6) |
| 52 Weeks (95% CI), % | 25.2 (13.1–39.2) |
| Overall survivala | |
| Events, n (%) | 30 (73.2) |
| Median weeks (95% CI) | 68.0 (48.0–81.0) |
| 26 Weeks (95% CI), % | 63.7 (46.4–76.7) |
| 52 Weeks (95% CI), % | 26.1 (13.1–41.1) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; GC = gemcitabine and cisplatin; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; ULN = upper limit of normal.
One patient (City of Hope) is alive after 421 weeks (8.1 years).
Effect of Potential Prognostic Factors on OS
Of the 41 patients, 30 (73.2%) were known to have died at a median of 68.0 (95% CI, 48.0–81.0) weeks. In univariate analyses (Table 2), ECOG-PS > 0 (HR, 4.96 [95% CI, 1.80–13.68]; P = .002), leukocytosis (HR, 2.82 [95% CI, 1.27–6.23]; P = .011), and TFPC ≥ 78 weeks (HR, 0.45 [95% CI, 0.20–0.99]; P = .048) were significantly prognostic of OS. The effect of comprehensive (tumor, node, metastases) pathologic staging at the time of cystectomy was unavailable in all patients and was not examined. The single patient with pT0 disease had received neoadjuvant chemotherapy and exhibited a survival of 177 weeks.
Table 2.
Prognostic Factors for Overall Survival
| Factor | n | Hazard Ratio (95% CI) | P |
|---|---|---|---|
| Sex, Male versus Female | 41 | 1.31 (0.54–3.17) | .55 |
| Type of Perioperative Chemotherapy | |||
| Adjuvant versus neoadjuvant | 41 | 0.85 (0.38–1.88) | .68 |
| GC versus others | 41 | 1.21 (0.56–2.63) | .62 |
| Number of Cycles of Perioperative Chemotherapy, Continuous | 41 | 1.20 (0.94–1.53) | .15 |
| Metastases at Recurrence, Visceral versus Nonvisceral | 41 | 1.55 (0.74–3.27) | .25 |
| Time From Last Perioperative Chemotherapy to First-Line Chemotherapy | |||
| ≥52 Weeks versus <52 weeks | 41 | 0.58 (0.28–1.24) | .16 |
| ≥78 Weeks versus <78 weeks | 41 | 0.45 (0.20–0.99) | .048 |
| ≥104 Weeks versus <104 weeks | 41 | 0.44 (0.17–1.16) | .097 |
| Log-transformation | 41 | 0.75 (0.51–1.09) | .13 |
| Age, Years | 41 | 0.96 (0.91–1.01) | .14 |
| ECOG Performance Status, 1–2 versus 0 | 41 | 4.96 (1.80–13.68) | .002 |
| Hemoglobin, Anemic (<LLN) versus Nonanemic | 40 | 1.61 (0.78–3.30) | .20 |
| Leukocyte Count, ≥ULN versus <ULN | 39 | 2.82 (1.27–6.23) | .011 |
| Creatinine Clearance, ≥60 versus <60 ml/minute | 39 | 1.49 (0.59–3.75) | .39 |
| Albumin per Unit | 34 | 0.55 (0.26–1.16) | .12 |
| Type of First-Line Chemotherapy, GC versus Other | 41 | 0.81 (0.39–1.67) | .57 |
| Dose of Cisplatin, ≥70 versus <70 mg/m2 per Cycle | 41 | 0.86 (0.26–2.87) | .81 |
| Multivariate Model | |||
| ECOG performance status, 1–2 versus 0 | 41 | 4.56 (1.66–12.52) | .003 |
| Time from last perioperative chemotherapy to first-line chemotherapy, ≥78 weeks versus <78 weeks | 41 | 0.48 (0.21–1.07) | .072 |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; GC = gemcitabine and cisplatin; LLN = lower limit of normal; ULN = upper limit of normal.
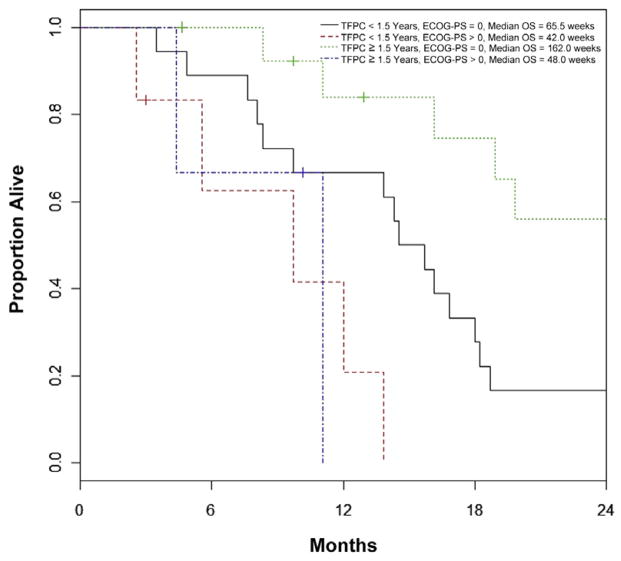
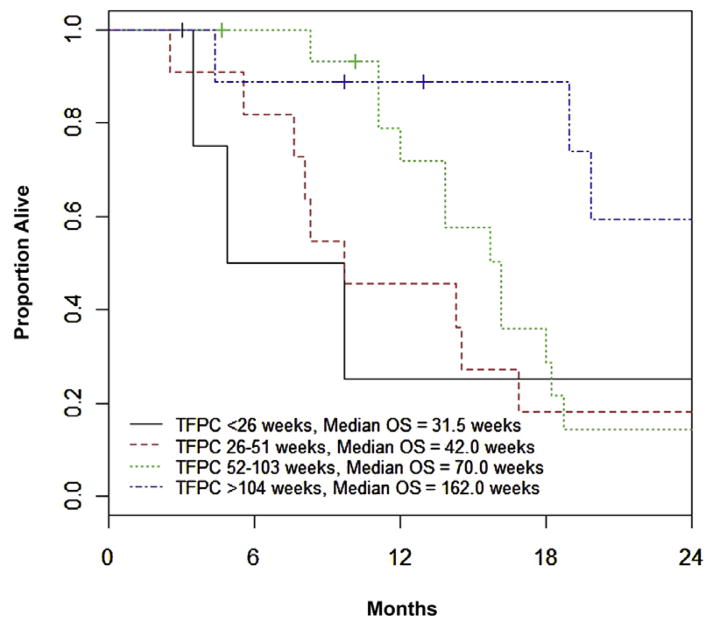
The initial multivariate model was constructed using stepwise selection on the 38 patients with complete data, and ECOG-PS > 0 and TFPC ≥ 78 weeks were identified as factors significant for OS. The final multivariate model included data on all 41 patients, and showed ECOG-PS > 0 (HR, 4.56 [95% CI, 1.66–12.52]; P = .003), and TFPC ≥ 78 weeks (HR, 0.48 [95% CI, 0.21–1.07]; P = .072) as prognostic for OS (Figure 1). Notably, other cutoff values for TFPC (52 or 104 weeks), and log-transformed TFPC as a continuous variable, were not statistically significant after adjusting for ECOG PS (P values of .096, .20, and .15, respectively); however, the estimated HR trended toward a favorable effect for longer TFPC (HRs of 0.52, 0.52, and 0.75). Of 5, 11, 16, and 9 patients with TFPC < 26 weeks, 26 to 51 weeks, 52 to 103 weeks, and ≥ 104 weeks, median survival was 31.5 (range, 15-not reached), 42 (range, 24–73), 70 (range, 48–79), and 162 (range, 19–185) weeks, respectively (Figure 2). If the same cisplatin-based combination regimen was administered as perioperative and first-line therapy (eg, GC and GC or MVAC and MVAC), there was no independent effect on OS (HR, 1.03; 95% CI, 0.48–2.22; P = .94) or PFS (HR, 1.62; 95% CI, 0.82–3.19; P = .17).
Figure 1. Overall Survival Based on Major Prognostic Factors.
Abbreviations: ECOG-PS = Eastern Cooperative Oncology Group Performance Status; OS = Overall Survival; TFPC = Time from Previous Perioperative Cisplatin-Based Chemotherapy.
Figure 2. Overall Survival Based on Time From Previous Perioperative Cisplatin-Based Chemotherapy.
Abbreviation: TFPC = Time from Previous Perioperative Cisplatin-Based Chemotherapy.
A supportive analysis was performed after imputing data for missing Hb, creatinine clearance < 60 mL/min, and leukocytes> ULN measurements. Because each factor was yes or no for 1 or 2 patients, all possible scenarios were tested. Results were similar for all possible imputed values of Hb and creatinine clearance; however, leukocyte count > ULN bordered on statistical significance (HR, 2.32; 95% CI, 0.98–5.47; P = .055) after adjusting for ECOG-PS status and TFPC if 1 of the 2 patients with missing data had leukocytosis and the other did not.
Effect of Potential Prognostic Factors on PFS
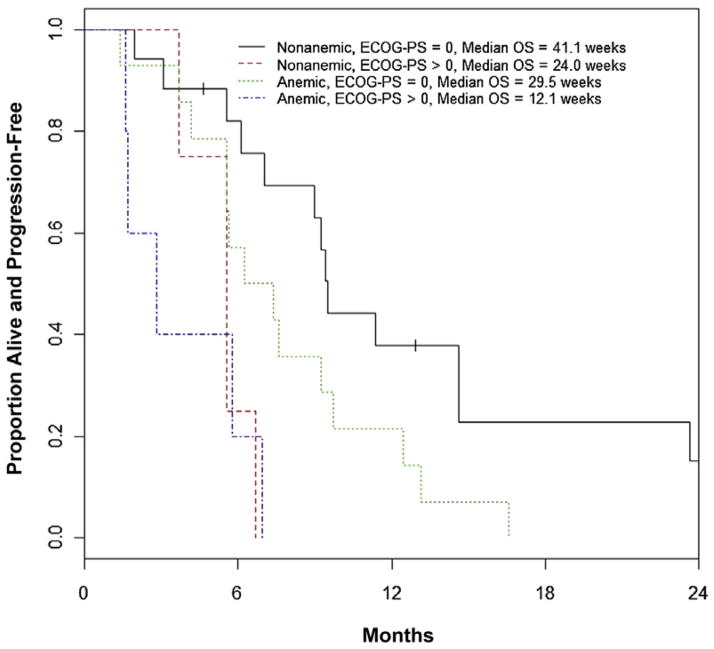
Median PFS was 30.4 (95% CI, 24.0–40.0) weeks. In univariate analyses (Table 3), ECOG-PS > 0 and anemia were the only statistically significant factors prognostic for PFS (HR, 4.78 [95% CI, 1.95–11.72]; P < .001 and HR 2.00 [95% CI, 1.02–3.92]; P = .045). The final multivariate model included ECOG PS > 0 (HR, 4.47 [95% CI, 1.83–10.93]; P = .001) and anemia (HR, 1.95 [95% CI, 0.99–3.84]; P = .053) and was based on the 40 patients with ECOG-PS and Hb data (Figure 3). Results were similar for all possible scenarios of imputing data for the patients with missing data.
Table 3.
Prognostic Factors for Progression-Free Survival
| n | Hazard Ratio (95% CI) | P | |
|---|---|---|---|
| Sex, Male versus Female | 41 | 0.86 (0.39–1.90) | .71 |
| Type of Perioperative Chemotherapy | |||
| Adjuvant versus neoadjuvant | 41 | 0.65 (0.32–1.32) | .23 |
| GC versus others | 41 | 1.72 (0.86–3.43) | .13 |
| Number of Cycles of Perioperative Chemotherapy, Continuous | 41 | 1.04 (0.81–1.32) | .77 |
| Metastases at Recurrence, Visceral versus Nonvisceral | 41 | 1.55 (0.80–2.98) | .19 |
| Time From Last Perioperative Chemotherapy to First-Line Chemotherapy | |||
| ≥52 Weeks versus <52 weeks | 41 | 0.66 (0.34–1.27) | .21 |
| ≥78 Weeks versus <78 weeks | 41 | 0.66 (0.34–1.27) | .21 |
| ≥104 Weeks versus <104 weeks | 41 | 0.57 (0.26–1.25) | .16 |
| Log-transformation | 41 | 0.83 (0.60–1.13) | .24 |
| Age, Years | 41 | 0.99 (0.94–1.03) | .54 |
| ECOG Performance Status, 1–2 versus 0 | 41 | 4.78 (1.95–11.74) | <.001 |
| Hemoglobin, Anemic (<LLN) versus Nonanemic | 40 | 2.00 (1.02–3.92) | .045 |
| Leukocyte Count, >ULN versus <ULN | 39 | 1.77 (0.83–3.74) | .14 |
| Creatinine Clearance, ≥60 versus <60 ml/minute | 39 | 1.05 (0.45–2.43) | .92 |
| Albumin per Unit | 34 | 0.84 (0.44–1.61) | .60 |
| Type of First-Line Chemotherapy, GC versus Other | 41 | 1.20 (0.62–2.33) | .59 |
| Dose of Cisplatin, ≥70 versus <70 mg/m2 per Cycle | 41 | 0.90 (0.27–2.96) | .86 |
| Multivariate Model | |||
| ECOG performance status, 1–2 versus 0 | 40 | 4.47 (1.83–10.93) | .001 |
| Hemoglobin, anemic versus nonanemic | 40 | 1.95 (0.99–3.84) | .053 |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; GC = gemcitabine and cisplatin; LLN = lower limit of normal; ULN = upper limit of normal.
Figure 3. Progression-Free Survival Based on Major Prognostic Factors. Anemia Was Defined As Hemoglobin < Lower Limit of Normal.
Abbreviation: ECOG-PS = Eastern Cooperative Oncology Group Performance Status.
Internal Validation
Bootstrapping was performed to internally validate the regression estimates of the optimal multivariate model. With OS as the outcome, the estimated median HRs for ECOG PS > 0 and TFPC ≥ 78 weeks was 5.04 [95% BCa CI, 1.95–15.83], and 0.45 [95% BCa CI, 0.19–1.20], and the c-statistic for the model was 0.68 [95% BCa CI, 0.60–0.75]. For PFS, the estimated median HRs for ECOG PS > 0 and anemia was 4.91 [95% BCa CI, 2.04–14.85], and 2.03 [95% BCa CI, 0.95–3.64] and the c-statistic for the model was 0.67 [95% BCa CI, 0.60–0.74].
Discussion
This retrospective study including 41 patients sheds light on outcomes in administration of cisplatin-based first-line chemotherapy for metastatic UC after previous perioperative cisplatin-based chemotherapy. The study assembled patients treated at multiple institutions, because of the difficulty of identifying a large number of such patients from a single institution. Indeed, 2 of the 10 collaborating institutions initially approached could not identify a single patient fulfilling all criteria. The major independent prognostic factors for OS were ECOG PS > 0 (HR, 4.56 [95% CI, 1.66–12.52]; P = .003) and TFPC ≥ 78 weeks (HR, 0.48 [95% CI, 0.21–1.07]; P = .072), and the major factors associated with PFS were ECOG PS > 0 (HR, 4.47 [95% CI, 1.83–10.93]; P = .001) and anemia (HR, 1.95 [95% CI, 0.99–3.84]; P = .053). Moreover, the prognostic models for OS and PFS were internally validated with c-indices of 0.68 and 0.67, respectively. Although the cut point of 78 weeks was data-driven and assessment of other cutoffs for TFPC were limited by sample size, a consistent trend of improved survival being associated with longer TFPC was observed across all cut points.
The patients in our study were well selected for treatment with cisplatin-based first-line chemotherapy, as suggested by the excellent ECOG PS of 0 to 1 and calculated creatinine clearance ≥ 60 mL/min in most patients. It is also likely that patients did not display comorbidities precluding cisplatin use and did not have residual prohibitive neuropathy from the previous perioperative cisplatin treatment. The patient population might also be characterized as having favorable disease, because of the relatively long median TFPC of 69 weeks, normal Hb in 45%, median albumin of 4.0 g/dL, and visceral disease in only 45% of patients. We did not study or capture toxicities because these patients were not treated in a trial and toxicities are not usually graded formally or consistently when not part of a study protocol.
Reported phase III trials of first-line cisplatin-based chemotherapy did not include patients who had received previous peri-operative chemotherapy.1–4 One study reported the activity of DD-MVAC in 45 patients with metastatic disease after previous gemcitabine with platinum (cisplatin, carboplatin, or oxaliplatin) in the perioperative (n = 18) or metastatic settings (n = 27).18 In this report, the median OS was 16.5 and 5.7 months when previous gemcitabine-platinum was administered in the perioperative and metastatic settings, respectively. Although no differences were found in the response rate with DD-MVAC according to previous platinum agent used and the interval between gemcitabine-platinum and DD-MVAC ≤ 6 months versus > 6 months, the effect of longer intervals (eg, ≥ 1 year) was not reported. In contrast, DD-MVAC for metastatic disease after previous gemcitabine-platinum in the perioperative setting appeared to yield better activity.
Additionally, some second-line trials allow previous perioperative chemotherapy as the only previous regimen without regard to TFPC, and others require TFPC < 52 weeks in such patients.19,20 Recently reported first-line phase II trials and an ongoing important first-line phase III trial, CALGB-90601 (comparing GC combined with either bevacizumab or placebo), allowed patients > 52 weeks from previous perioperative cisplatin-based chemotherapy.21 The findings from our study support the use of ≥ 52 weeks from perioperative cisplatin-based chemotherapy to institute cisplatin-based chemotherapy rather than a second-line regimen. In contrast, the median OS for those with TFPC < 52 weeks was < 1 year, suggesting that a tolerable second-line regimen instead of rechallenge with cisplatin-based combination therapy might be justified in such patients. Interestingly, in the setting of second-line chemotherapy, TFPC was validated for association with PFS, but not OS.22,23 TFPC was a significant prognostic factor in the context of second-line therapy on a continuous scale. However, external validation of the effect of TFPC on OS might have been compromised by use of the phase III vinflunine trial data set, which required previous chemotherapy for metastatic disease and did not exhibit a wide range of TFPC.24
Although our data suggest that first-line cisplatin-based chemotherapy might not be highly effective when administered < 52 weeks from previous perioperative cisplatin-based chemotherapy, it is unclear if a different second-line agent yields better outcomes than a repeat of cisplatin-based regimen. Nevertheless, because of the suboptimal survival and toxicities of cisplatin-based combination therapy in those with TFPC < 52 weeks, a different second-line agent might be more rational in such patients. Conversely, in those with TFPC ≥ 52 weeks, although the median OS of those receiving cisplatin-based chemotherapy appears to exceed a year, it might be suggested that a different second-line agent or noncisplatin-based combination (eg, gemcitabine with paclitaxel) might yield similar outcomes with fewer complications.25–27 However, because of its curative potential, it might be prudent to offer cisplatin-based combination chemotherapy to patients without contraindications such as poor PS, renal dysfunction, or comorbidities. Indeed, 3 patients (all with ECOG PS = 0; 2 of the 3 with Hb data available had normal Hb) in our cohort demonstrated durable PFS > 104 weeks (approximately 2 years) after starting first-line cisplatin-based chemotherapy after previous perioperative chemotherapy 90, 168, and 8 weeks earlier, respectively. Because the most recurrences occur within 2 years and 2-year PFS is robustly associated with 5-year survival, these patients might be potentially cured.22
Our study is limited by the small sample size and retrospective design. Ten academic institutions collaborated on this study and 2 institutions did not identify patients fulfilling eligibility criteria. Potentially, a larger number of institutions could have been requested to participate in our study to improve our sample size, but resource limitations did not permit a large international project. A trend was observed for the effect of TFPC in the prognostic model for OS, which probably did not attain statistical significance (ie, P < .05) because of the small sample size. Nevertheless, TFPC was significantly associated with OS on univariate analyses and the data, in aggregate, demonstrate its effect. To evaluate a homogeneous cohort of patients, we only considered patients who had received previous cisplatin-based chemotherapy in the perioperative setting and not those who had received previous cisplatin-based therapy for metastatic disease. However, patients considered for reinstitution of cisplatin-based chemotherapy (ie, exhibiting a long TFPC after previous cisplatin-based chemotherapy for metastatic disease) are much less commonly seen. We also focused exclusively on patients who received cisplatin in the perioperative and subsequent meta-static settings, did not attempt to collect data on patients who received carboplatin-based or nonplatinum-based first-line combination regimens at either time point, or those who received a second-line agent. We planned to focus on a homogeneous data set of patients who received proven and conventional perioperative and first-line cisplatin-based regimens at both time points. Data do not exist for supporting perioperative noncisplatin-based regimens. Moreover, a meta-analysis has shown that carboplatin-based first-line regimens appear to yield inferior outcomes compared with cisplatin-based regimens.28 Other studies have reported the efficacy of carboplatin with paclitaxel after previous cisplatin-based chemotherapy in perioperative or metastatic settings and demonstrated suboptimal outcomes with a median survival of only 6 to 8 months.29,30 Pathologic complete response (pCR) to neoadjuvant chemotherapy at the time of cystectomy is associated with a low risk of recurrence (approximately 10%–15%) and might be hypothesized to confer better outcomes with reinstitution of cisplatin-based first-line chemotherapy at the time of recurrence. However, only 15 patients received previous neoadjuvant chemotherapy in our data set and pathologic stage at the time of cystectomy was unavailable in all patients. Consequently, the data set was underpowered to evaluate the effect of previous pCR, because it was observed in only 1 patient after neoadjuvant chemotherapy who did exhibit a prolonged survival of 177 weeks. Indeed, previous pCR is an extremely unlikely event in our data set because we only studied patients whose disease recurred after previous perioperative cisplatin-based chemotherapy.
Conclusion
To summarize, results of this retrospective analysis suggest a favorable prognostic effect of longer time from previous perioperative cisplatin-based chemotherapy when reinstituting cisplatin-based first-line chemotherapy for metastatic UC. Despite the limitations of small sample size, the patients included reflect and validate the good clinical practice of careful selection of patients with good PS and longer TFPC for repeating cisplatin-based therapy. Our data require external validation, but strongly suggest that the current practice of offering cisplatin-based first-line therapy to those with TFPC ≥ 52 weeks is rational.
Clinical Practice Points.
Longer TFPC ≥ 78 weeks and ECOG PS = 0 were independently prognostic for better survival with cisplatin-based first-line chemotherapy for advanced UC after previous perioperative cisplatin-based chemotherapy.
Those with TFPC < 52 weeks demonstrated a particularly poor median OS, which suggests that using TFPC ≥ 52 weeks to rechallenge with cisplatin-based first-line therapy is prudent.
Patients with TFPC < 52 weeks from previous perioperative cisplatin-based chemotherapy might be better treated with a different second-line agent.
Adoption of these data in clinical trials and routine clinical practice will improve interpretability of trials and the therapeutic index of systemic therapy.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 2.Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15:2564–9. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 3.Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8:1050–5. doi: 10.1200/JCO.1990.8.6.1050. [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30:1107–13. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 8.David KA, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol. 2007;178:451–4. doi: 10.1016/j.juro.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 9.Feifer A, Taylor JM, Shouery M, et al. Multi-institutional quality-of-care initiative for nonmetastatic, muscle-invasive, transitional cell carcinoma of the bladder (abstract) J Clin Oncol. 2011;29(suppl 7):abstract 240. [Google Scholar]
- 10.Miles BJ, Fairey AS, Eliasziw M, et al. Referral and treatment rates of neoadjuvant chemotherapy in muscle-invasive bladder cancer before and after publication of a clinical practice guideline. Can Urol Assoc J. 2010;4:263–7. doi: 10.5489/cuaj09134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner DG, Daniels JR, Russell CA, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol. 1991;145:459–64. doi: 10.1016/s0022-5347(17)38368-4. discussion 64–7. [DOI] [PubMed] [Google Scholar]
- 12.Freiha F, Reese J, Torti FM. A randomized trial of radical cystectomy versus radical cystectomy plus cisplatin, vinblastine and methotrexate chemotherapy for muscle invasive bladder cancer. J Urol. 1996;155:495–9. discussion 499–500. [PubMed] [Google Scholar]
- 13.Svatek RS, Shariat SF, Lasky RE, et al. The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin Cancer Res. 2010;16:4461–7. doi: 10.1158/1078-0432.CCR-10-0457. [DOI] [PubMed] [Google Scholar]
- 14.Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–81. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 16.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. 2013;105:499–503. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galsky MD, Moshier E, Krege S, et al. Nomogram for predicting survival in patients with unresectable and/or metastatic urothelial cancer who are treated with cisplatin-based chemotherapy. Cancer. 2013;119:3012–9. doi: 10.1002/cncr.28146. [DOI] [PubMed] [Google Scholar]
- 18.Edeline J, Loriot Y, Culine S, et al. Accelerated MVAC chemotherapy in patients with advanced bladder cancer previously treated with a platinum-gemcitabine regimen. Eur J Cancer. 2012;48:1141–6. doi: 10.1016/j.ejca.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Sonpavde G, Galsky MD, Bellmunt J. A new approach to second-line therapy for urothelial cancer? Lancet Oncol. 2013;14:682–4. doi: 10.1016/S1470-2045(13)70175-X. [DOI] [PubMed] [Google Scholar]
- 20.Sonpavde G, Bellmunt J, Rosenberg JE, et al. Patient eligibility and trial design for the salvage therapy of advanced urothelial carcinoma. Clin Genitourin Cancer. doi: 10.1016/j.clgc.2014.03.016. Published online June 10, 2014. http://dx.doi.org/10.1016/j.clgc.2014.03.016. [DOI] [PubMed]
- 21.Hussain M, Daignault S, Agarwal N, et al. A randomized phase 2 trial of gem-citabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer. 2014;120:2684–93. doi: 10.1002/cncr.28767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonpavde G, Pond GR, Fougeray R, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63:717–23. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–5. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 24.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–61. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 25.Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99] Ann Oncol. 2011;22:288–94. doi: 10.1093/annonc/mdq398. [DOI] [PubMed] [Google Scholar]
- 26.Calabro F, Lorusso V, Rosati G, et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer. 2009;115:2652–9. doi: 10.1002/cncr.24313. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg CN, Calabro F, Pizzocaro G, Marini L, Schnetzer S, Sella A. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer. 2001;92:2993–8. doi: 10.1002/1097-0142(20011215)92:12<2993::aid-cncr10108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23:406–10. doi: 10.1093/annonc/mdr156. [DOI] [PubMed] [Google Scholar]
- 29.Kouno T, Ando M, Yonemori K, et al. Weekly paclitaxel and carboplatin against advanced transitional cell cancer after failure of a platinum-based regimen. Eur Urol. 2007;52:1115–22. doi: 10.1016/j.eururo.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 30.Vaishampayan UN, Faulkner JR, Small EJ, et al. Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma: a Southwest Oncology Group study. Cancer. 2005;104:1627–32. doi: 10.1002/cncr.21370. [DOI] [PubMed] [Google Scholar]