Abstract
Background
Ciliary neurotrophic factor (CNTF) has been shown to protect retinal ganglion cells (RGCs) in traumatic optic nerve injury. We sought to evaluate this neuroprotective effect of CNTF after an ischaemic event using rodent anterior ischaemic optic neuropathy (rAION), a mouse model of non-arteritic anterior ischaemic optic neuropathy (NAION).
Methods
We induced rAION in Thy1-cyan fluorescent protein (CFP) transgenic mice by exposing the optic nerve to frequency doubled neodymium yttrium aluminium garnet laser pulses following intravenous rose bengal injection. One day after rAION induction, an intravitreal injection of 0.75 μg CNTF or vehicle (sham injection) was given. Animals were euthanised on day 15 after induction, tissues isolated and CFP cells in the RGC layer were counted using stereology in flat-mounted retina. The average number of CFP-positive (CFP+) cells was determined for each study group and the percentages of RGC loss were compared between the different groups.
Results
Two weeks after rAION induction, significantly more (CFP+) cells were preserved in CNTF-treated eyes than in sham-injected controls. Sham-treated animals showed a 58% loss of CFP+ cells. In contrast, CFP+ cell density in CNTF-treated eyes decreased by only 10%, when compared with untreated control eyes. This increased survival was statistically significant (p<0.05).
Conclusions
CNTF exerts a neuroprotective effect in ischaemic optic nerve injury and promotes RGC survival, suggesting that CNTF may be effective in the clinical treatment of human NAION.
INTRODUCTION
Ciliary-derived neurotrophic factor (CNTF), a member of the interleukin-6 family of proteins, is expressed from a multiple sources in the eye, including retinal pigment epithelium, photoreceptors, horizontal, amacrine, Müller and retinal ganglion cells (RGCs).1–3 CNTF is upregulated in response to stress factors, such as mechanical injury,4 pressure3 or bright light exposure.5 Extrinsically administered CNTF has been shown to be neuroprotective in global retinal ischaemia6 and optic nerve trauma.7,8 Virally mediated overexpression of CNTF in the eye is also neuroprotective, but very high doses of CNTF can impair photoreceptor function.9
Non-arteritic anterior ischaemic optic neuropathy (NAION) is a common cause of sudden vision loss due to optic nerve dysfunction in adults over 55 years of age.10 Unlike arteritic anterior ischaemic optic neuropathy, a manifestation of the systemic vasculitis syndrome giant cell arteritis, NAION is considered a localised ischaemic event. Potential systemic and local risk factors for NAION include hyperlipidaemia, sleep apnoea syndrome, dehydration, diabetes and a characteristic ‘crowded’ configuration of the optic nerve head with a small central optic cup. Human NAION presents with acute loss of vision, accompanied by optic nerve oedema. This is followed by optic nerve atrophy and a measurable loss of RGCs.11,12
Among the plethora of optic nerve damage models some have been used to demonstrate that CNTF administration prior to inducing injury has a beneficial effect.6,7 However, the introduction of a therapeutic agent prior to the injury and the method of optic nerve injury, such as crush or dissection of the nerve, limit the applicability of these models to clinical NAION. Therefore, it has been proposed that a model replicating as many of the features of the disease as possible is necessary to investigate the pathophysiology of NAION and explore potential therapeutic interventions.13 In this study, we induced optic nerve ischaemia using the previously described rodent model of anterior ischaemic optic neuropathy (rAION).14 This method applies neodymium yttrium aluminium garnet (Nd-YAG) laser light to the capillary supply of the optic nerve head after intravascular injection of rose bengal dye. The photochemical reaction results in superoxide radicals damaging the capillary endothelium and selectively occluding the laser-irradiated capillary supply of the optic nerve head. The central retinal artery and all capillaries outside the area of laser application remain patent, as can be proven by fluorescein angiography. Similar to human NAION, rAION results in optic nerve oedema, with blurring of the disc margin and obscuration of retinal vessels, which can be observed 1 day after treatment. This is followed by optic atrophy with visible pallor of the optic nerve 2 weeks after rAION induction. Histological features of rAION are also similar to human disease and include vacuolisation and necrotic lesions within the optic nerve. The use of Thy1 (cyan fluorescent protein (CFP)) transgenic mice for this model allows direct visualisation of fluorescent cells in the RGC layer of flat-mounted, unstained retina.15
In this study, we sought to determine the effect of CNTF treatment on the RGC response to sudden isolated optic nerve ischaemia. We modelled the natural sequence of events by administering an intra-vitreal injection of CNTF 1 day after induction of rAION to mimic the potential therapeutic effect of a single CNTF injection after NAION.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee at University of MD, Baltimore, approved all animal protocols. Animals were kept and handled according to the Declaration of Helsinki and the European Union Directive 2010/63/EU for animal experiments. Animals were given food and water ad libitum. Prior to each procedure, animals were anaesthetised using a mixture of ketamine and xylazine (80 mg/ 5 mg/kg) intraperitoneally and one drop of topical proparacaine 0.5% for ocular surface anaesthesia. The procedures were performed under aseptic conditions. After completion of each procedure, animals were allowed to recover from anaesthesia and monitored for signs of pain and distress.
CFP mice
Male CD1 mice with a Thy1 promoter transgene insert linked to CFP (Thy1 (CFP) mice; n=9) were obtained from Jackson Laboratory, Bar Harbor, Maine. These mice express CFP at high levels in RGC bodies and their axons resulting in blue-green fluorescence of RGCs as previously reported.16
rAION model
Under general anaesthesia, rAION was induced as previously described,14 by injecting 2.5 mM rose bengal dye (0.1 cc/kg) into a tail vein, immediately followed by exposing the optic nerve head to 12 1-s applications of 535 nm laser light (300 μ spot size, 50 mW energy) using a frequency doubled Nd-YAG laser (Iridex OcuLight GL, Iris Medical Systems, Mountainview, California, USA).
On day 1 after rAION induction, all eyes were examined and photographed under anaesthesia. All laser-treated eyes showed optic nerve oedema, indicating successful rAION induction. No complications of laser treatment, such as retinal artery or vein occlusion, were observed on day 1.
Intravitreal injection/study groups
One half of study animals received laser treatment in both eyes followed by CNTF treatment in one eye and sham injection in the fellow eye. The other half of animals received laser treatment in one eye. The treated eyes were then randomised to either CNTF or sham injection. The fellow eyes were used as untreated controls.
This resulted in 3 study groups: laser-treated eyes with CNTF injection (n=6), laser-treated eyes with sham injection (n=6) and untreated control eyes (n=6).
Intravitreal injection was performed 1 day after laser treatment using a Nano-Fil injection system (World Precision Instruments, Sarasota, Florida, USA). Eyes in the CNTF treatment group received one intravitreal injection of 0.75 μg recombinant rat CNTF (R&D Systems, Minneapolis, Minnesota, USA) diluted in 0.002 mL phosphate-buffered saline (PBS). This dosage was chosen with data obtained from previous studies.6,8,17 Eyes in the sham treatment group received an intravitreal injection of 0.002 mL PBS.
Tissue collection, imaging and stereology
Animals were euthanised on day 15 after induction by CO2-anaesthesia followed by cervical dislocation. Eyes were enucleated and fixated in 4%-paraformaldehyde-PBS (PF-PBS). Anterior chambers were removed; fixated retinae were dissected out of the eye cup, flat-mounted and examined with a 4-channel confocal microscope (Fluoview 400; Olympus, Lake Success, New York, USA) with excitation at 405 nm/visualisation at 450 nm.
Stereology was performed on 12 fields in each retina as follows: a pseudorandom number generator was used to determine the coordinates of 12 points in each retina, using the radial distance from the optic nerve (between 1 and 1200 μm) and degree of central angle (between 1° and 360°) as coordinates. The coordinates were located using low magnification and then imaged at the level of the RGC layer at 400× magnification, resulting in 12 counting fields per retina, each sized 200 μm2. Then two observers, who were masked to the study groups, manually counted CFP+ cells in each field.
Statistical analysis
Average CFP+ cell density and SDs were calculated. Comparisons of mean cell counts (mean of the 12 fields per 6 eyes) for each of the three groups (CNTF treated, sham and control) were based on a mixed effects linear model. This multilevel linear model accounted for within eye correlation, and correlation between eyes within mouse, providing appropriate estimates of variance. Analyses were implemented using the Mixed Procedure in SAS (Cary, North Carolina, USA). The percentages of lost CFP+ cells, as compared with those of untreated controls, were calculated for each treatment group. A p value of <0.05 was considered statistically significant.
Interobserver variability for the manual cell counts was calculated. There was a high degree of concurrence with a variance of 0.1%
RESULTS
The average CFP+ cell density in all twelve 200 μ2 fields/eye ranged from 37 to 76 cells (mean=60.1 cells, SE=5.4) in the control group, 43 to 85 cells (mean=54.1 cells, SE=5.4) in the CNTF treatment group and 12 to 39 cells (mean=25.5 cells, SE=5.4) in the sham injection group. There was a statistically significant difference in cell density between the two treatment groups (p=0.002), as well as between controls and the sham treatment group (p=0.0004). There was no statistically significant difference between the CNTF treatment group and non-laser-treated controls (p=0.44). When CFP+ cell density was compared with untreated controls, we observed a 58% loss in sham-injected eyes and a 10% loss in the CNTF treatment group (figures 1–4). Injection of PBS alone did not affect the RGC density (data not shown).
Figure 1.
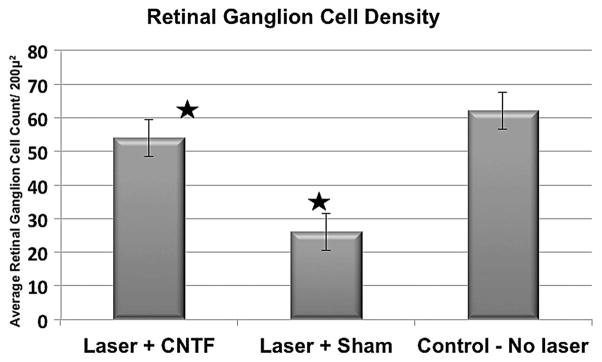
CFP+ cell density was determined in 12 randomly selected fields in each retina at the level of the retinal ganglion cell layer. The average CFP+ cell density was 60.1 (±13)/200 μ2 in the untreated control group. CFP+ cell density dropped to 25.5 (±10)/200 μ2 (−58%) after laser treatment and sham injection only. The ciliary neurotrophic factor (CNTF)-treated eyes retained a CFP+ cell density of 54.1 (±16)/ 200 μ2 (−10%). These data indicate that CNTF treatment results in a statistically significant reduction in retinal ganglion cell dropout after rodent anterior ischaemic optic neuropathy (p=0.002, asterisks). CFP, cyan fluorescent protein.
Figure 4.
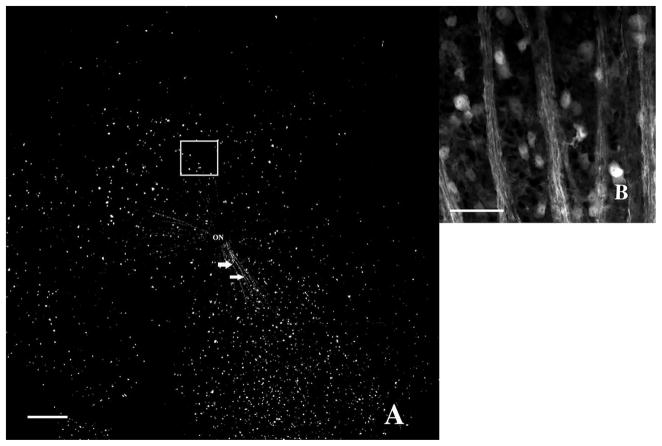
Fifteen days after rodent anterior ischemic optic neuropathy induction and subsequent ciliary neurotrophic factor injection (treatment group). (A) 40× magnification. Highly fluorescent Thy1(CFP)+ cells are distributed in a more irregular pattern than observed in controls. Overall CFP+ cell density is moderately decreased. Axonal bundles radiating from the optic nerve into all four quadrants are well preserved. Scale bar=200 μm. (B) At high magnification, even an area with apparent retinal ganglion cell (RGC) loss (white square in A) shows preservation of numerous Thy1(CFP)+ cells and RGC axons. Scale bar=50 μm. CFP, cyan fluorescent protein.
DISCUSSION
Several mechanisms of optic nerve injury lead to RGC death by apoptosis, a complex process of delayed cell death. In traumatic optic nerve injury, NAION and the rAION model alike, RGC numbers start to decline around 3 days after injury13 and peak cell death occurs after 2 weeks.18 Previous studies have suggested that CNTF administration can be neuroprotective when introduced prior to optic nerve damage.8 In this study, we investigated the effect of CNTF administration after inducing optic nerve damage in an attempt to more closely simulate the actual course of events in accidental or sudden ischaemic optic nerve damage.
The Thy1 (CFP) transgenic mouse strain is highly useful for studies requiring the identification of RGCs19 by allowing direct imaging of RGCs with minimal tissue processing. The baseline number of CFP+ cells, as measured in untreated control eyes (3100 (±650)/mm2) concurred with previously reported RGC numbers in mice (1500–3200/mm2).19,20 Our numbers being near the upper end of the previously reported range probably results from counting all CFP+ cells within the RGC layer and thus likely including a small percentage of CFP+ displaced amacrine cells. Previous studies on CFP expression in the Thy1 (CFP) transgenic mouse, using retrograde labelling and immunohistochemical staining, have shown that cholinergic amacrine and displaced amacrine cells may also be weakly CFP+. The same studies also demonstrated that 93%–97% of CFP+ cells in the RGC layer are RGCs,19,20 and that most of the amacrine cell’s small somata are located in the proximal inner nuclear layer, which we did not include in our confocal images. However, induction of rAION does not significantly affect the number of amacrine cells.21 Therefore, we may have slightly underestimated the amount of cell loss in all study groups after rAION.
In our study, rAION induction resulted in a 58% loss of CFP+ cells. This number aligns closely with previous reports.15 It has been postulated that RGC density in the mouse retina varies by quadrant and by distance from the optic nerve,19 although other RGC density analyses have found no regional differences.15 We noticed an irregular distribution of CFP+ cells in the RGC layer after rAION induction, which did not follow a regional pattern. To account for this irregular distribution and for possible innate regional cell density variations, we used a random grid to most accurately estimate the average CFP+ cell density in each retina.
We observed that CFP+ cell density in the PBS-injected eyes 2 weeks after rAION induction was only 42% of untreated control. In comparison, 90% of CFP+ cells were preserved when rAION induction was followed by intravitreal treatment with CNTF, indicating a statistically significant rate of RGC survival 2 weeks after rAION induction. In fact, the neuroprotective effect of CNTF was so pronounced that, 2 weeks after injection, the amount of cell loss when compared with naive control eyes was not significant. Thus our data suggest a substantial neuroprotective effect of CNTF treatment applied 24 h after ischaemic optic nerve damage. Although the demonstrated effect on RGC survival may be temporary and the acme of cell death may only be delayed by CNTF administration,22 this opens a neuroprotective window of opportunity.
The intracellular pathways of CNTF action are complex and incompletely understood. RGC survival and regrowth of axons, both stimulated by CNTF, appear to depend on different intraocular kinase pathways, involving among others, the antiapoptotic Janus kinase/signal transducer and activator of transcription, protein kinase A and mitogen-activated protein kinase pathways.17 Some studies have found that activation of these pathways can also have negative effects on survival. The presence or absence of cyclic adenosine monophosphate23 and the administered dosage of CNTF may be an important determinant of the end result. CNTF administration after direct laser trauma to the retina has also been shown to suppress the c-fos gene, which is an early indicator of programmed cell death in retina and optic nerve.14
To successfully translate these findings to the bedside, an effective method for delivering neuroprotective substances to the eye needs to be found. One challenge is to overcome or bypass the blood-retinal barrier, which prohibits most molecules from passing through Bruch’s membrane. Topically applied agents usually do not achieve a sufficiently high concentration within the eye to result in a therapeutic effect. In contrast, the intravitreal injection route that we chose is widely used in multiple ophthalmologic applications. Even frequent intravitreal injections have been shown to be safe and effective. Previous studies have explored a wide CNTF dosage range: As little as 0.01 μg/mL have been reported to promote retinal cell survival24 and up to 60 μg/μL have been used without significant ocular complications.25 Intravitreal implants are a novel method of drug delivery. Currently, therapeutic trials to evaluate CNTF encapsulated cell devices in the treatment of retinal degeneration, such as retinitis pigmentosa, are underway.22
In summary, using the rAION model, we were able to show that CNTF promotes RGC survival in optic nerve ischaemia. This may be a temporary phenomenon, but prolonging RGC survival may open a window of opportunity to apply novel therapeutic strategies for preservation or restoration of optic nerve function.
Figure 2.
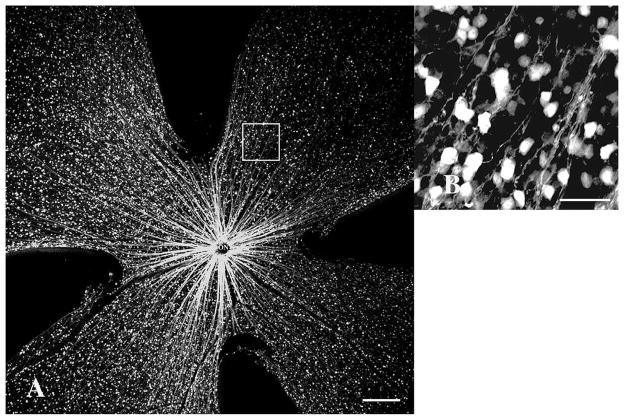
Thy1(CFP)-fluorescence of cells and axons in a control mouse retina. (A) 40× magnification. Highly fluorescent retinal ganglion cells (RGCs) are evenly distributed throughout the RGC layer. Lightly fluorescent axon bundles (horizontal arrows) radiate from the optic nerve. Retinal vessels (vertical arrows) are non-fluorescent, contrasting with fluorescent cells and axons, scale bar=200 μm. (B) High-magnification (400×) view of the retinal mid-periphery (white square in A). Intensely CFP+ RGCs populating the RGC layer are interspersed with smaller, weakly fluorescent displaced amacrine cells, scale bar=50 μm. CFP, cyan fluorescent protein.
Figure 3.
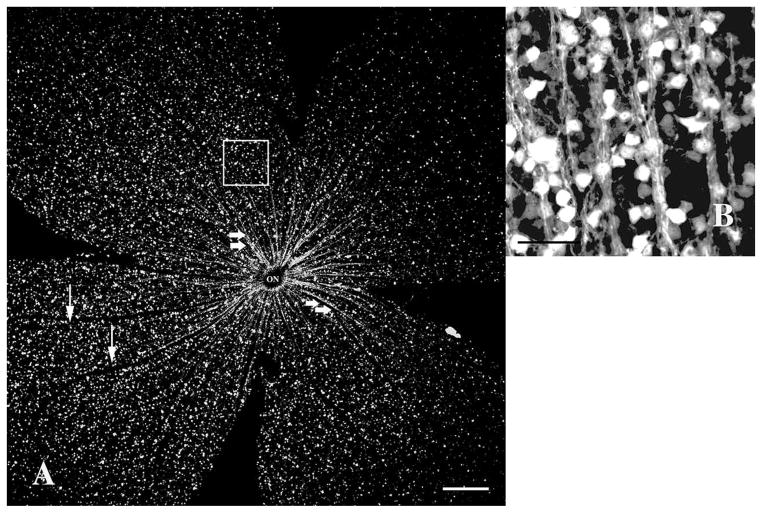
Fifteen days after rodent anterior ischemic optic neuropathy induction without subsequent ciliary neurotrophic factor injection (sham group). (A) Highly fluorescent CFP+ retinal ganglion cells can still be seen, but in an irregular distribution pattern. Overall CFP+ cell density is significantly decreased. Few axonal bundles (arrows) remain in retinal sectors with relatively higher cell density, radiating from the optic nerve. 40× magnification, scale bar=200 μm. (B) At high magnification (400×, white square in A), the axons, though less fluorescent, can still be identified. Severely reduced CFP+ cell density in the retinal ganglion cell layer is confirmed. Scale bar=50 μm. CFP, cyan fluorescent protein.
Acknowledgments
The authors wish to thank Allan Hunter, MD for his assistance with stereology.
Funding This study was supported in part by NIH grant 5K08EY016357-05 (National Eye Institute, MKM).
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Full record of methods, cell counts and data analysis is available from the corresponding author on request.
Contributors Writing the manuscript: MKM, YG, PL, SLB; research design: MKM, SLB; funding: MKM; experiments and imaging: MKM, YG; data collection: MKM, YG; and data analysis: PL.
References
- 1.Beltran WA, Zhang Q, Kijas JW, et al. Cloning, mapping, and retinal expression of the canine ciliary neurotrophic factor receptor alpha (CNTFRalpha) Invest Ophthalmol Vis Sci. 2003;44:3642–9. doi: 10.1167/iovs.02-0763. [DOI] [PubMed] [Google Scholar]
- 2.Kirsch M, Lee MY, Meyer V, et al. Evidence for multiple, local functions of ciliary neurotrophic factor (CNTF) in retinal development: expression of CNTF and its receptors and in vitro effects on target cells. J Neurochem. 1997;68:979–90. doi: 10.1046/j.1471-4159.1997.68030979.x. [DOI] [PubMed] [Google Scholar]
- 3.Ju WK, Lee MY, Hofmann HD, et al. Expression of CNTF in Muller cells of the rat retina after pressure-induced ischemia. Neuroreport. 1999;10:419–22. doi: 10.1097/00001756-199902050-00038. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Wen R, Li F, et al. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp Eye Res. 1997;65:241–8. doi: 10.1006/exer.1997.0328. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Peng M, Laties AM, et al. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–44. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unoki K, LaVail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994;35:907–15. [PubMed] [Google Scholar]
- 7.Hellström M, Pollett MA, Harvey AR. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J Neurotrauma. 2011;28:2475–83. doi: 10.1089/neu.2011.1928. [DOI] [PubMed] [Google Scholar]
- 8.Zhang CW, Lu Q, You SW, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005;46:1497–503. doi: 10.1167/iovs.04-0664. [DOI] [PubMed] [Google Scholar]
- 9.McGill TJ, Prusky GT, Douglas RM, et al. Intraocular CNTF reduces vision in normal rats in a dose-dependent manner. Invest Ophthalmol Vis Sci. 2007;48:5756–66. doi: 10.1167/iovs.07-0054. [DOI] [PubMed] [Google Scholar]
- 10.Kerr NM, Chew SS, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci. 2009;16:994–1000. doi: 10.1016/j.jocn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Gonul S, Koktekir BE, Bakbak B, et al. Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2013;97:1045–50. doi: 10.1136/bjophthalmol-2013-303438. [DOI] [PubMed] [Google Scholar]
- 12.Mathews MK. Nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol. 2005;16:341–5. doi: 10.1097/01.icu.0000188361.52166.93. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein SL, Johnson MA, Miller NR. Nonarteritic anterior ischemic optic neuropathy (NAION) and its experimental models. Prog Retin Eye Res. 2011;30:167–87. doi: 10.1016/j.preteyeres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg-Cohen N, Guo Y, Margolis F, et al. Oligodendrocyte dysfunction after induction of experimental anterior optic nerve ischemia. Invest Ophthalmol Vis Sci. 2005;46:2716–25. doi: 10.1167/iovs.04-0547. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein SL, Guo Y, Slater BJ, et al. Neuron stress and loss following rodent anterior ischemic optic neuropathy in double-reporter transgenic mice. Invest Ophthalmol Vis Sci. 2007;48:2304–10. doi: 10.1167/iovs.06-0486. [DOI] [PubMed] [Google Scholar]
- 16.Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 17.Ji J, Elyaman W, Yip HK, et al. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–72. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 18.Berkelaar M, Clarke DB, Wang YC, et al. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–74. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond ID, Vila A, Huynh UC, et al. Cyan fluorescent protein expression in ganglion and amacrine cells in a thy1-CFP transgenic mouse retina. Mol Vis. 2008;14:1559–74. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Archibald ML, Stevens K, et al. Cyan fluorescent protein (CFP) expressing cells in the retina of Thy1-CFP transgenic mice before and after optic nerve injury. Neurosci Lett. 2010;468:110–14. doi: 10.1016/j.neulet.2009.10.077. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein SL, Guo Y. Changes in cholinergic amacrine cells after rodent anterior ischemic optic neuropathy (rAION) Invest Ophthalmol Vis Sci. 2011;52:904–10. doi: 10.1167/iovs.10-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park K, Luo JM, Hisheh S, et al. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci. 2004;24:10806–15. doi: 10.1523/JNEUROSCI.3532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caffe AR, Soderpalm AK, Holmqvist I, et al. A combination of CNTF and BDNF rescues rd photoreceptors but changes rod differentiation in the presence of RPE in retinal explants. Invest Ophthalmol Vis Sci. 2001;42:275–82. [PubMed] [Google Scholar]
- 25.Chong NH, Alexander RA, Waters L, et al. Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest Ophthalmol Vis Sci. 1999;40:1298–305. [PubMed] [Google Scholar]