Summary
Triggering receptor expressed on myeloid cells 2 (TREM2) is a microglia surface receptor that triggers intracellular protein tyrosine phosphorylation. Recent genome-wide association studies have shown that a rare R47H mutation of TREM2 correlates with a substantial increase in the risk of developing Alzheimer's disease (AD). To address the basis for this genetic association, we studied TREM2 deficiency in the 5XFAD mouse model of AD. We found that TREM2 deficiency and haploinsufficiency augment β-amyloid (Aβ) accumulation due to dysfunctional response of microglia, which become apoptotic and fail to cluster around Aβ plaques. We further demonstrate that TREM2 senses a broad array of anionic and zwitterionic lipids known to associate with fibrillar Aβ in lipid membranes and to be exposed on the surface of damaged neurons. Remarkably, the R47H mutation impairs TREM2 detection of lipid ligands. Thus, TREM2 detects damage-associated lipid patterns associated with neurodegeneration, sustaining microglia response to Aβ accumulation.
Introduction
Alzheimer disease (AD) is a progressive neurodegenerative disorder with histopathological hallmarks of β-amyloid (Aβ) deposits and neurofibrillary tangles in the brain (Huang and Mucke, 2012; Tanzi, 2013). Although disease etiology is incompletely understood, families with inherited early-onset AD have mutations in three proteins directly involved in the Aβ processing pathway, suggesting a key role for Aβ in disease pathogenesis. Early studies have shown that brain microglia accumulate around Aβ deposits and occasionally contain Aβ in AD patients (D'Andrea et al., 2004; McGeer et al., 1987; Perlmutter et al., 1990) and transgenic mouse models of AD (Dickson, 1999; Frautschy et al., 1998; Stalder et al., 1999). Microglia contribute to Aβ clearance, at least in the early phases of neurodegeneration (El Khoury et al., 2007); however, the ability of microglia to clear Aβ may wane with age (Streit et al., 2004; Streit and Xue, 2009). At late stages of AD, microglia may paradoxically contribute to the disease by releasing pro-inflammatory cytokines in response to Aβ deposition (El Khoury et al., 2007; Hickman et al., 2008).
Recent genome-wide association studies (GWAS) have shown that a rare Arginine-47-Histidine (R47H) mutation of the triggering receptor expressed on myeloid cells 2 (TREM2) is associated with a substantial increase in the risk of developing AD (Guerreiro et al., 2013b; Jonsson et al., 2013). TREM2 is a cell surface receptor of the Ig-superfamily that is expressed by microglia and osteoclasts in vivo (Kiialainen et al., 2005; Paloneva et al., 2002; Schmid et al., 2002; Thrash et al., 2009) as well as monocyte-derived DC, bone marrow-derived macrophages and macrophage cell lines in vitro (Bouchon et al., 2001; Daws et al., 2001). Although TREM2 was detected in other cells of the central nervous system (CNS) (Guerreiro et al., 2013b; Sessa et al., 2004), these observations have not been confirmed (Jiang et al., 2014). TREM2 binds anionic carbohydrates, anionic bacterial products and various phospholipids (Cannon et al., 2012; Daws et al., 2003). It transmits intracellular signals through the associated transmembrane adapter DAP12, which recruits the protein tyrosine kinase Syk, leading to phosphorylation of many downstream mediators, such as PLC-γ, PI-3K and Vav2/3 (Ford and McVicar, 2009; Peng et al., 2010). Individuals homozygous for rare mutations that impair expression of either TREM2 or DAP12, develop lethal forms of progressive dementias such as Nasu- Hakola disease (NHD) and frontotemporal dementia (FTD) (Guerreiro et al., 2013a; Guerreiro et al., 2013c; Kleinberger et al., 2014; Paloneva et al., 2002).
The association between the R47H mutation of TREM2 and the increased risk for late onset AD suggests that microglia may require TREM2 to respond to Aβ deposition and to limit neuronal degeneration. Consistent with this hypothesis, we recently showed that APPPS1–21 transgenic mice, an AD model with rapid deposition of Aβ, have a marked decrease in the number and size of Aβ-associated microglia when they lack one copy of the Trem2 gene, although this defect did not increase Aβ accumulation (Ulrich et al., 2014). The mechanisms underlying this altered microglia response and its impact on Aβ deposition have not been delineated. To address these questions, we studied TREM2 deficiency in the 5XFAD mouse model of AD, in which Aβ deposition develops less rapidly than in APPPS1–21 mice (Oakley et al., 2006). We find that both TREM2 deficiency and haploinsufficiency augment Aβ accumulation due to a dysfunctional response of microglia, which become apoptotic rather than undergoing activation and proliferation. We further show that TREM2 sustains microglia survival by synergizing with colony stimulating factor-1 receptor (CSF-1R) signaling. Finally, we demonstrate that TREM2 binds to a broad array of anionic lipids, which were found in association with fibrillar Aβ and are also exposed during neuronal and glial cell death. Remarkably, the R47H mutation associated with AD impairs TREM2 binding to anionic lipids. We conclude that TREM2 is a receptor that detects damage-associated lipids, thereby enabling microglia to sense Aβ accumulation and cell damage, as well as supporting microglia survival and Aβ reactive microgliosis.
Results
TREM2 modulates Aβ accumulation
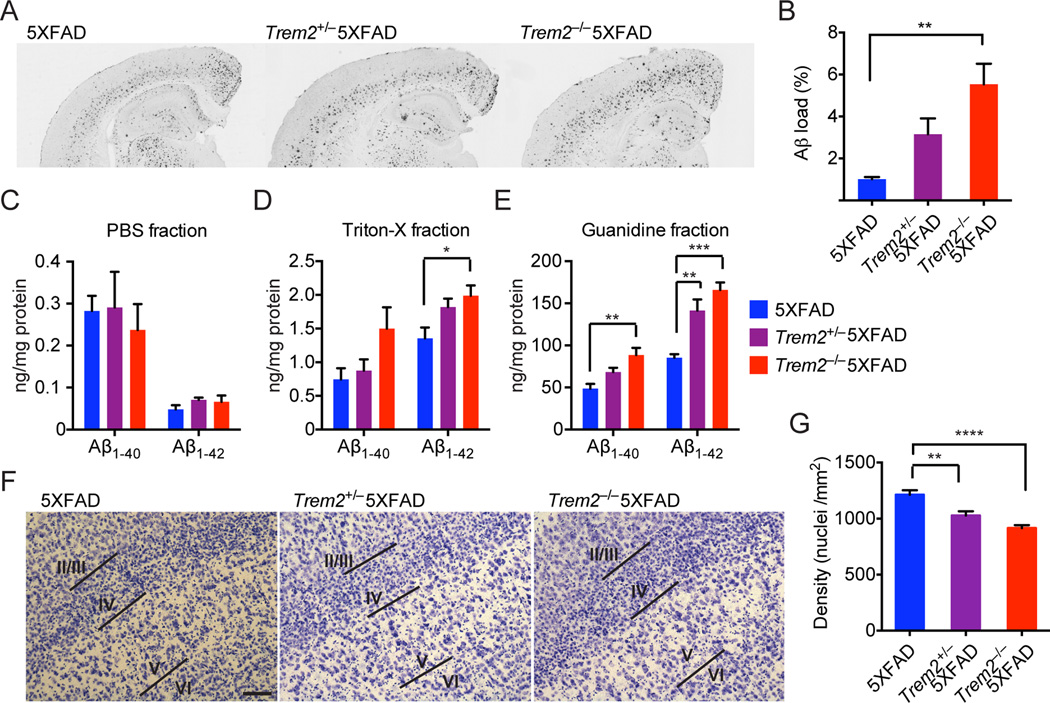
We examined the deposition of Aβ aggregates in Trem2−/− mice bred to 5XFAD transgenic mice (APPSwFlLon, PSEN1*M146L*L286V), an accelerated mouse model of AD (Oakley et al., 2006). Staining of matched coronal brain sections from Trem2−/−5XFAD mice and control 5XFAD mice at 8.5 months of age with a monoclonal antibody (mAb) against Aβ revealed significantly increased Aβ accumulation in the hippocampal but not cortical regions of Trem2−/−5XFAD mice (Fig. 1A, B, Fig. S1A). Trem2+/− 5XFAD mice had an intermediate phenotype, although it was not statistically significant (p= 0.104). We also determined levels of Aβ40 and Aβ42 in the hippocampus and cortex of these mice by ELISA. While levels of soluble Aβ40 and Aβ42 were similar (Fig. 1C, Fig S1B), we detected a significant increase in insoluble, guanidine-extracted Aβ40 and Aβ42 in the hippocampal regions of Trem2−/−5XFAD mice compared to 5XFAD mice (Fig. 1D, E). Moreover, there was a significant effect of Trem2 gene copy number on insoluble Aβ protein levels in the hippocampi, whereas levels of insoluble Aβ40 and Aβ42 in the cortex were equivalent across all three genotypes (Fig. S1C, D). We also found that the loss of layer-V neurons, a feature of 5XFAD mice (Eimer and Vassar, 2013; Oakley et al., 2006), was more prominent in Trem2−/−5XFAD mice (Fig. 1F, G). Trem2+/− 5XFAD mice presented an intermediate phenotype. Collectively, these data suggest that TREM2 modulates Aβ accumulation, limiting neuronal loss. The lack of a significant difference in Aβ accumulation in the cortices of Trem2−/−5XFAD mice and 5XFAD mice may be the result of the fast kinetics of Aβ deposition in 5XFAD mice, such that the potential cortical differences are no longer detectable at 8.5 month of age.
Figure 1. TREM2 deficient 5XFAD mice have increased hippocampal Aβ burden and accelerated loss of layer-V cortical neurons.
Aβ burden in 8.5 month-old Trem2−/−5XFAD, Trem2+/−5XFAD, and 5XFAD mice. (A) Matching coronal hippocampus and cortex sections were stained with an Aβ-specific antibody mHJ3.4. (B) Amounts of Aβ loads in hippocampi. (C–E) Soluble and insoluble Aβ1–40 and Aβ1–42 levels in hippocampi as detected by ELISA. (F–G) Densities of layer-V neurons in 8.5 month-old Trem2−/−5XFAD, Trem2+/−5XFAD and 5XFAD mice. (F) Matching coronal sections stained with cresyl violet. (G) Summary of densities of layer-V neurons. Original magnification: 10×; scale bar= 100µm. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, one-way ANOVA. Data represent analyses total of 8–10 5XFADmice, 8–12 Trem2+/− 5XFAD mice, and 8–16 Trem2−/−5XFAD mice (B, C–E, G). Bars represent mean±SEM. See also Figure S1.
TREM2 is required for reactive microgliosis
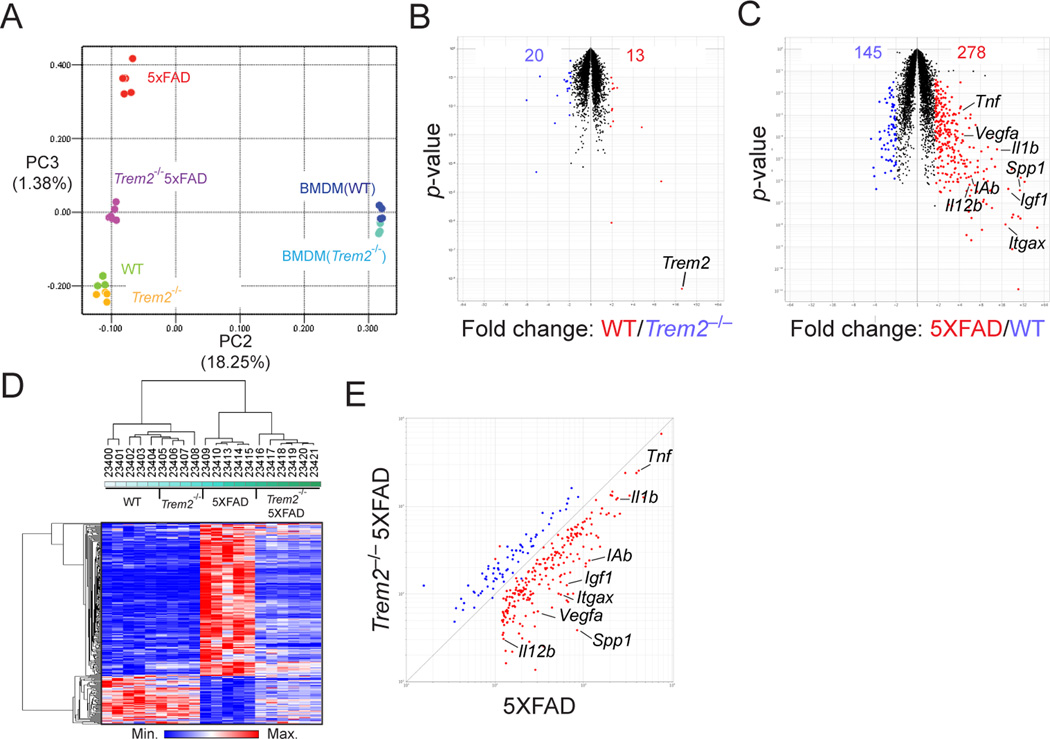
How does lack of TREM2 impact Aβ accumulation? Although TREM2 expression has been reported in CNS cells other than microglia (Guerreiro et al., 2013b; Sessa et al., 2004), this finding is controversial (Jiang et al., 2014). Indeed, a recently published RNA-seq dataset demonstrated that Trem2 is specifically expressed in microglia, but not other cells in the CNS under steady state conditions (Butovsky et al., 2014). We also found that Trem2 expression is further upregulated in microglia isolated from 5XFAD mice during Aβ deposition (Fig. S2A, B). Thus, we focused our studies on microglia. One of the many effects of Aβ deposition is the induction of reactive microgliosis, which involves the expansion of microglia and conversion to an activated state (Ransohoff and Cardona, 2010). Microgliosis predominantly involves the proliferation of brain-resident microglia, with some contribution from blood-borne monocytes and microglia migrating from adjacent non-damaged brain areas (El Khoury et al., 2007; Grathwohl et al., 2009; Malm et al., 2005; Mildner et al., 2011; Simard et al., 2006; Stalder et al., 2005). To evaluate the impact of TREM2 deficiency on Aβ-induced microglia responses in 5XFAD mice, we examined transcriptional profiles of microglia purified from 5XFAD and Trem2−/−5XFAD mice as well as transgene negative WT and Trem2−/− littermates (Fig. S2A). To evaluate changes in global transcriptomes we first performed principle component analysis (PCA) of the top 15% most variable transcripts. We noticed that WT and Trem2−/− replicates clustered closely, suggesting a limited impact of TREM2 deficiency in the steady state, which was confirmed by a volcano plot comparing the two groups (Fig. 2A, B). In contrast, 5XFAD microglia replicates were dramatically different from WT replicates (Fig. 2A), and a volcano plot revealed that 5XFAD microglia expressed many more transcripts including those associated with microglia activation (MHC-II, CD11c), production of inflammatory cytokines (IL-1β, TNF-α, IL-12 and SPP1) and neurotrophic factors (IGF1 and VEGFA) (Fig. 2C). Trem2−/−5XFAD microglia had an intermediate behavior on the principle component analysis compared to 5XFAD and WT microglia. To further interrogate how TREM2 deficiency affected microglia response to Aβ deposition, we selected the transcripts upregulated two-fold between 5XFAD and WT microglia (Fig. 2C) and compared the expression of these transcripts among the entire dataset. We found that Trem2−/−5XFAD microglia failed to upregulate these transcripts and behaved more similarly to WT microglia, as shown by hierarchical clustering and expression-by-expression plots (Fig. 2D, E). Flow cytometric analysis of isolated microglia confirmed phenotypic changes in 5XFAD microglia consistent with increased activation, including a marked increase in cell size and strong upregulation of MHC-II, CD11c and CD11b (Fig. S2C–G). We also confirmed increased expression of inflammatory cytokine transcripts by qPCR in whole brain lysates of 5XFAD mice (Fig. S2I–L). However, in Trem2−/− 5XFAD mice, these changes were markedly attenuated (Fig. 2D, E and Fig. S2C–L). In fact, Trem2−/−5XFAD microglia were phenotypically more similar to WT microglia in steady state than 5XFAD microglia. Overall, these results implied that TREM2 is required for reactive microgliosis.
Figure 2. TREM2 deficiency impairs Aβ-induced transcriptional program in microglia.
Transcriptional analysis of microglia isolated from hippocampi and cortices of 8.5 month-old Trem2−/−5XFAD, 5XFAD, Trem2−/− and WT mice. (A) Top 15% most variable transcripts were subjected to principle component analysis (PCA). Plot shows 2-dimensional (PC2 vs. PC3) comparison of transcriptional changes in all classes analyzed. WT and Trem2−/− BM-derived macrophages were used as references. (B) Volcano plot comparing microglia transcripts in Trem2−/− and WT mice. Trem2 transcript is indicated. (C) Volcano plot comparing microglia transcripts in 5XFAD and WT mice. Numbers in plots B and C indicate probes that are significantly upregulated or downregulated (±2 fold, p<0.05, Student's t-test). Representative transcripts are indicated. (D, E) Visualization of Aβ-induced changes in microglia transcripts from (C). (D) A heatmap displays hierarchical clustering of all samples analyzed. (E) A scatter plot compares these transcriptional changes in Trem2−/−5XFAD and 5XFAD microglia. Representative transcripts are shown. See also Figure S2.
Microglia fail to colocalize with Aβ plaques in Trem2−/− mice
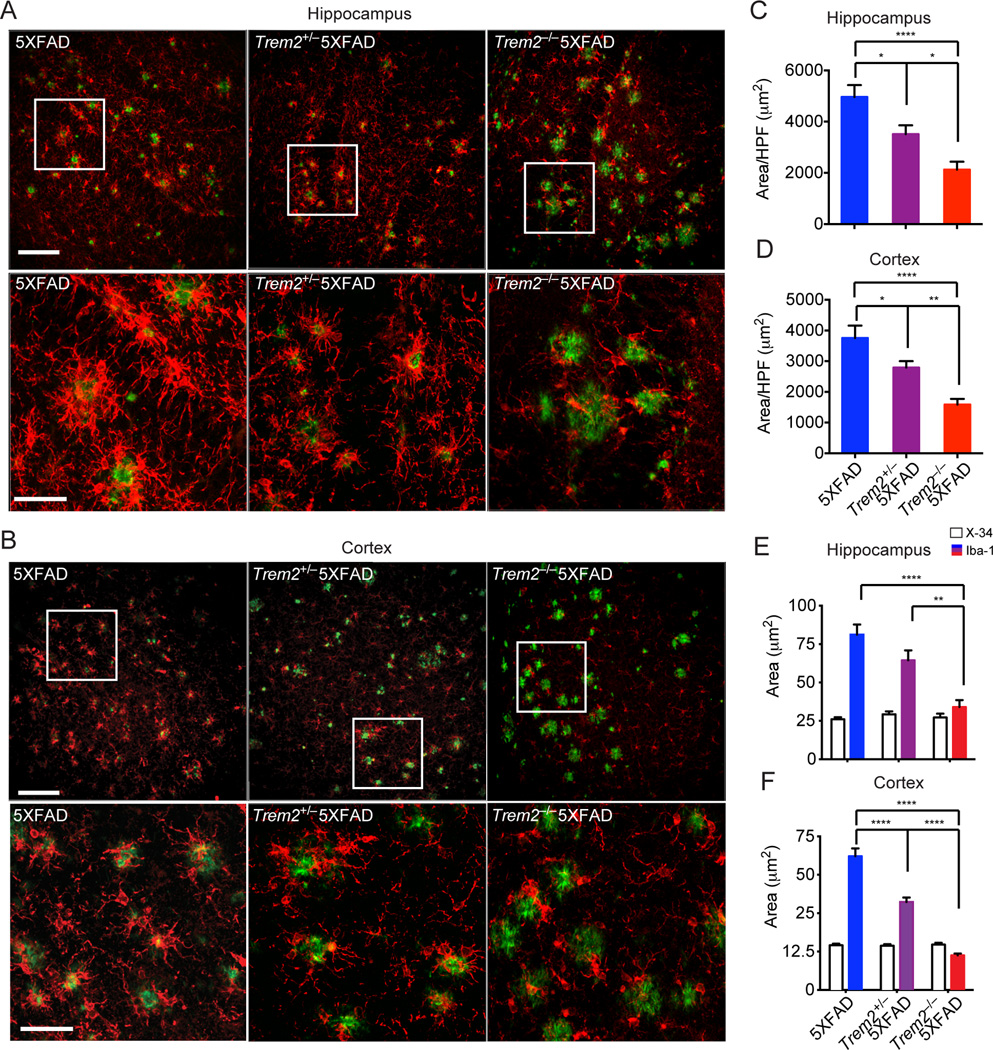
Initial staining of microglia in coronal brain sections with Iba-1 revealed very similar distribution of microglia in Trem2−/−, Trem2+/− and WT adult mice (Fig. S3A–C). However, co-staining coronal brain sections of Trem2−/−5XFAD and 5XFAD mice with Iba-1 and X-34 to visualize microglia and amyloid plaques respectively, showed remarkable differences. We found that Trem2−/−5XFAD mice had reduced Iba-1 reactivity both in the hippocampi and cortices compared to 5XFAD mice (Fig. 3A–D). This was particularly evident in the areas surrounding amyloid plaques (Fig. 3E, F, Movie S1–3), suggesting a preferential reduction of microgliosis near amyloid deposits. Trem2+/− 5XFAD mice also had a partial reduction of amyloid-associated Iba-1 reactivity.
Figure 3. TREM2 deficiency leads to reduced microgliosis in 5XFAD mice.
Microgliosis in 8.5 month-old Trem2−/−5XFAD, Trem2+/−5XFAD and 5XFAD mice. (A, B) Matching coronal sections were stained with Iba-1 (red) for microglia and X-34 (green) for amyloid plaques. Representative Z-stack images with maximum projection are shown. (C–D) Quantification of total Iba-1 reactivity per high power field (HPF) in hippocampi and cortices. (E, F) Quantification of microgliosis associated with plaques of similar sizes in hippocampi and cortices. Original magnification 20× (A, B, upper panels), 40× (A, B, lower panels); Scale bar= 10µm (A, B, upper panels), 50 µm (A, B, lower panels). *p<0.05, **p<0.01, ****p<0.0001, one-way ANOVA. Data represent analyses of a total of 8–10 5XFAD, 8–12 Trem2+/− 5XFAD mice, and 8–16 Trem2−/−5XFAD mice. Bars represent mean±SEM. See also Figure S3 and Movie S1–3.
Examination of a second model of AD, APPPS1–21 mice that have been bred to Cx3cr1GFP/+ mice in order to visualize endogenous microglia, confirmed that complete TREM2 deficiency results in a marked reduction of GFP+ microglia clusters around amyloid plaques (Fig. S3D–F). This corroborates our previous observation that TREM2 haploinsufficiency correlates with fewer amyloid-associated microglia in APPPS1–21xCx3cr1GFP/+ mice (Ulrich et al., 2014). Moreover, since CX3CR1 marks brain-resident microglia (Ransohoff and Cardona, 2010), these results also suggest that TREM2 deficiency primarily affects the response of brain-resident microglia to Aβ.
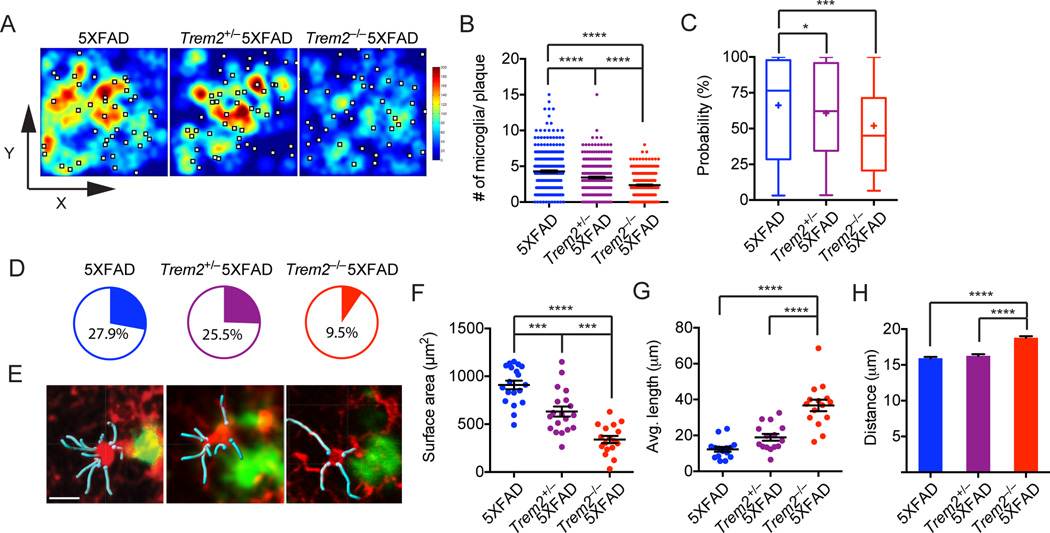
To further quantify the number of microglia around amyloid plaques, we recorded the coordinates (x, y, z) of all visible microglial cell bodies and the location of amyloid plaques in each Z-stack confocal image and calculated the number of microglia within 30µm radius of the plaques (defined as plaque-associated microglia) and non-plaque-associated microglia. While no statistical significant difference was observed among non-plaque-associated microglia (Fig. S4A), we noted a high degree of microglia clustering around amyloid plaques in 5XFAD mice (average 4.28 microglia per plaque), which gradually decreased in Trem2+/− 5XFAD mice (average 3.42 microglia per plaque) and Trem2−/−5XFAD mice (average 2.36 microglia per plaque) (Fig. 4A, B). To confirm the “casualness” of microglia responses to Aβ in the absence of TREM2, we compared the actual frequency of microglia per plaque to that obtained by Monte Carlo simulations where the same numbers of microglia and plaques observed in Z-stack images were positioned by chance in each genotype (Fig. S4B). The probability that observed microglia frequencies per plaque fell outside of simulated random frequencies was inversely proportional to Trem2 gene copy number (Fig. 4C). Moreover, while 27.9% of microglia distribution in 5XFAD mice with respect to amyloid plaques was not explained statistically by chance, the frequency of nonrandom microglia distribution was reduced to 9.5% in Trem2−/− 5XFAD mice (Fig. 4D).
Figure 4. TREM2 deficiency diminishes the capacity of microglia to cluster around amyloid plaques.
Frequencies of plaque-associated microglia in 8.5 month-old Trem2−/−5XFAD, Trem2+/−5XFAD and 5XFAD mice were determined. (A) Heatmap shows frequencies of microglia in relation to amyloid plaques shown as white squares. (B) Summary of frequencies of plaque-associated microglia in all analyzed genotypes. (C, D) Microglia clustering around plaques in 5XFAD, Trem2+/−5XFAD and Trem2−/−5XFAD mice were compared to Monte Carlo simulations that assume total randomness between plaques and microglia. Probabilities that any given microglia-plaque cluster are non-random are shown in (C). Pie charts show frequencies of microglia-plaque clusters that cannot be statistically explained as random (p<0.05) (D). (E) Morphology of plaque-associated microglia highlighting the shape of cell bodies (red) and primary processes (cyan). (F–H) Plaque-associated microglia are analyzed for their surface area (cell body only), average length of primary processes and distance from the center of adjacent amyloid plaque. Original magnification: 20×; scale bar= 15µm. *p<0.05, ***p<0.001, ****p<0.0001, one-way ANOVA. Data represent analyses of a total 7 mice per group (A–D) and a total of 5 mice per group (E–G). Bars represent mean±SEM. See also Figure S4.
Another feature of reactive microgliosis is their morphological transformation. In 5XFAD mice, plaque-associated microglia showed morphological changes associated with microglia activation including a partial retraction and a slight hypertrophy of the microglial cell processes as well as an increase in size (Fig. 4E–G). These changes in microglia morphology were significantly attenuated in Trem2+/−5XFAD and Trem2−/−5XFAD mice (Fig. 4E–G) and were paralleled by an increased distance between microglia and the center of their associated plaques (Fig. 4H). Collectively, these data indicate that TREM2 is essential for microglia response to Aβ plaques.
TREM2 deficiency affects microglia survival in 5XFAD mice
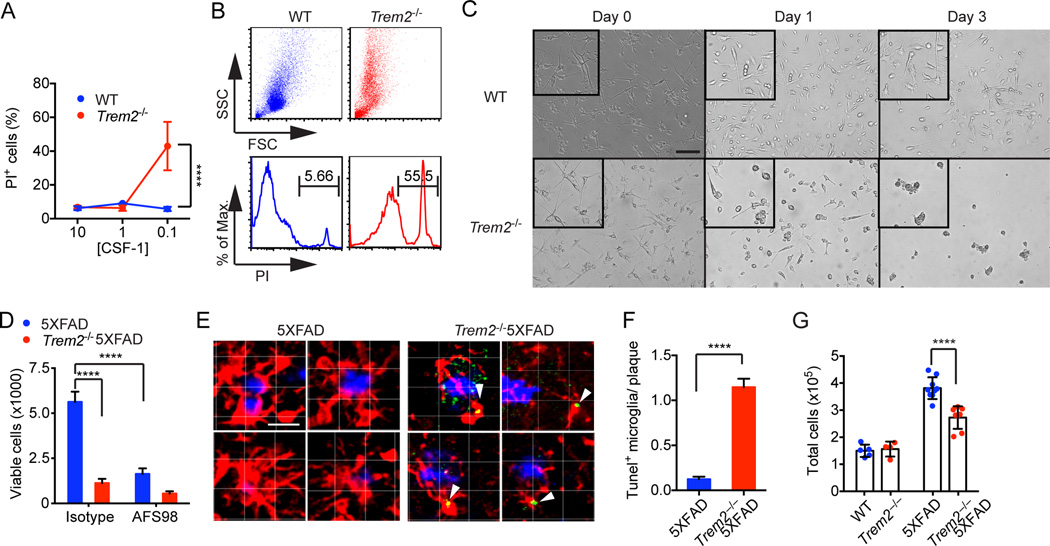
Why is TREM2 required for Aβreactive microgliosis? We first hypothesized that TREM2 may be necessary for Aβ uptake and microglia activation. We initially investigated the impact of TREM2 deficiency on microglia activation in vitro. For this analysis, we used primary microglia isolated from adult mice and expanded in the presence of optimal amounts of CSF-1 and TGF-β (Fig. S5A), as they closely resemble microglia in vivo (Butovsky et al., 2014). TREM2 deficiency did not affect microglia expansion, migration or TNF-α secretion in response to Aβ (Fig. S5B–D). In contrast, Trem2−/− microglia produced significant more TNF-α than WT microglia in response to LPS, consistent with previous demonstrations that TREM2 attenuates cytokine responses to certain TLR ligands (Hamerman et al., 2006; Turnbull et al., 2006). Moreover, TREM2 deficiency had very little impact on microglia uptake of Aβ aggregates (Fig. S5E, Movie S4) or their subsequent proteolytic processing, as demonstrated by similar degradation of the intracellular concentration of Aβ after initial loading (Fig. S5F). Thus, TREM2 deficiency does not engender a direct defect in phagocytosis of Aβ.
Previous studies have suggested that the CSF-1-CSF-1R pathway promotes reactive microgliosis (Chitu and Stanley, 2006) and Aβ clearance (Mitrasinovic et al., 2003); consistent with this, CSF1-deficient osteopetrotic (op/op) mice are characterized by increased deposition of Aβ,scarcity of microgliosis and neuronal loss (Kaku et al., 2003). We had previously demonstrated that TREM2 signaling via its associated adaptor DAP12 synergizes with CSF-1R signaling to promote survival of macrophages (Otero et al., 2012; Otero et al., 2009). Specifically, TREM2/DAP12 were required to induce activation of the Syk tyrosine kinase pathway downstream of CSF-1R (Otero et al., 2009; Zou et al., 2008). Thus, we hypothesized that TREM2 may synergize with CSF-1-CSF-1R signaling to sustain reactive microgliosis during Aβ deposition. We initially tested this hypothesis in vitro by measuring the survival of adult primary microglia cultures from WT and Trem2−/− mice in the presence of graded concentrations of CSF-1 (10%, 1% and 0.1% L-cell conditioned medium (LCM)). While TREM2 deficiency did not affect viability at high concentrations of CSF-1 (10% and 1%), Trem2−/− microglia were markedly less viable than WT microglia in 0.1% CSF-1 (Fig. 5AC). We next purified microglia from Trem2−/−5XFAD and 5XFAD mice and cultured them in medium containing low levels of CSF-1 (0.1% LCM) for 5 days. Trem2−/−5XFAD microglia were significantly less viable than 5XFAD microglia (Fig. 5D). Since CSF-1R captures CSF-1 and targets it for degradation (Stanley and Chitu, 2014), the reduced survival of Trem2−/− microglia at low CSF-1 concentrations may reflect a marked susceptibility of these cells to CSF-1 deprivation that occurs when microglia consume a limited supply of CSF-1. Indeed, CSF-1R blockade reduced viability of 5XFAD microglia, confirming that TREM2 pro-survival effect cannot replace that of CSF-1R, but only synergize with it (Fig. 5D).
Figure 5. TREM2 promotes microglia survival ex vivo and in vivo.
(A–C) Adult primary microglia were cultured with various concentration of CSF-1-containing L-cell medium (LCM). Viability of microglia by PI staining (A–B) and morphology (C) were assessed on day 3. (D) Microglia were purified ex vivo from 5XFAD mice and cultured in 0.1% LCM with or without CSF-1R blocking antibody AFS98. Viability was determined on day 5. (E, F) Apoptosis of plaque-associated microglia cells (Iba-1, red) in 5XFAD and Trem2−/−5XFAD mice was determined by TUNEL staining (green). Plaques were identified by X-34 (blue). Representative single-stack images of 5XFAD and Trem2−/−5XFAD microglia (E) and summary of frequencies of TUNEL+ microglia associated with plaques (F) are shown. Original magnification: 20×; scale bar= 10µm (C), 15µm (E). (G) Total numbers of live microglia cells in cortices and hippocampi of 5XFAD, Trem2−/− 5XFAD, Trem2−/− and WT mice. ****p<0.0001, two-way ANOVA (A, D, G), student's t-test (F). Data represent a total of three independent experiments (A–D) and a total of 5–8 mice per group (E–G). Bars represent mean±SEM. See also Figure S5 and Movie S4.
To evaluate the impact of TREM2 deficiency on microglia apoptosis in vivo we analyzed coronal sections of Trem2−/−5XFAD and 5XFAD mice by TUNEL staining. Markedly more TUNEL+ microglia were evident in Trem2−/−5XFAD mice than the very few observed in control 5XFAD mice (Fig. 5E, F), corroborating a role for TREM2 in maintaining microglia survival during reactive microgliosis. Consistent with this, significantly fewer microglia were recovered from the cortices and hippocampi of Trem2−/−5XFAD mice than from 5XFAD mice (Fig. 5G). We postulate that reactive microgliosis is associated with increased CSF-1 uptake by CSF-1R and degradation restricting CSF-1 range of action, such that microglia in close proximity must compete for CSF-1. Because of their inability to survive CSF-1 limitation, TREM2-deficient microglia are incapable of sustaining reactive microgliosis and undergo apoptosis rather than becoming activated and expanding.
TREM2 is a sensor for anionic and zwitterionic lipids that accumulate in the CNS during Aβ deposition
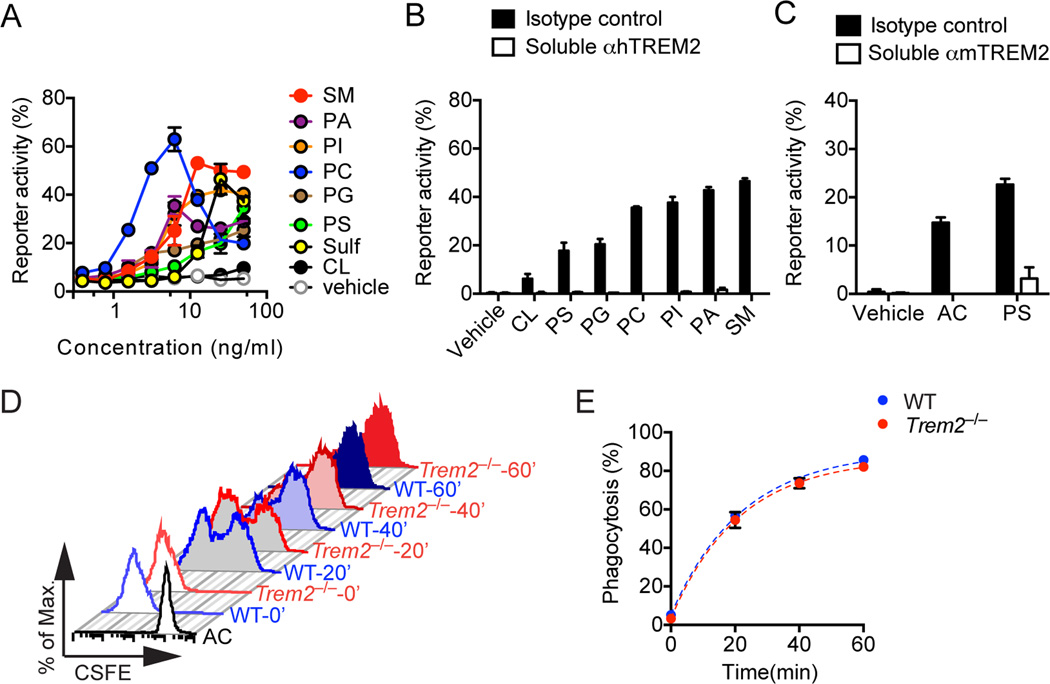
We next sought to identify the ligand(s) that trigger TREM2 signaling during Aβ deposition. Since TREM2 binds anionic carbohydrates, anionic bacterial products and phospholipids (Cannon et al., 2012; Daws et al., 2003), we focused on lipids that have been shown to accumulate during Aβ deposition and might stimulate microglia. These included: negatively charged phospholipids, which have been shown to associate with Aβ in lipid membranes (Ahyayauch et al., 2012; Nagarathinam et al., 2013); membrane phospholipids, such as phosphatidylserine, which are exposed by damaged neurons and glial cells; and anionic and zwitterionic non-phosphate lipids, such as sulfatides and sphingomyelin, which are released by damaged myelin. We transfected human TREM2 in reporter cells that express GFP under the control of NFAT, such that Ca2+ mobilization turns on GFP expression when TREM2 is engaged. Incubation of TREM2 reporter cells with many of these lipids activated reporter activity, although to differing extents, with phosphatidylcholine (PC) and sphingomyelin (SM) performing best in these assays (Fig. 6A, B). Similar results were obtained with a mouse TREM2 reporter (data not shown). Addition of a blocking TREM2 antibody abolished reporter activation by all ligands, demonstrating specificity (Fig. 6B). Interestingly, other potential candidates, such as cardiolipin, which is released by damaged mitochondria, did not significantly activate the TREM2 reporter despite its phospholipid structure. This suggests that the ability to engage TREM2 may only partially depend on the presence of negatively charged moieties like phosphatidic acid (Fig. 6A, B). Furthermore, TREM2 reporter activation was not detected with plate-bound synthetic or extracted Aβ (data not shown). In agreement with the ability of phosphotidylserine (PS) to activate TREM2 reporter cells, apoptotic cells, which expose PS on the cell surface, also activated TREM2 reporter cells (Fig. 6C). However, microglia isolated from Trem2−/−5XFAD and 5xFAD mice engulfed apoptotic cells equally well (Fig. 6D, E). Thus, TREM2 is not directly involved in phagocytosis of apoptotic cells. We conclude that TREM2 is a sensor for several anionic and zwitterionic lipids that are exposed during Aβ deposition as well as during neuronal and glial cell death.
Figure 6. TREM2 is a receptor for lipid patterns associated with Aβ.
(A, B) Human TREM2 reporter cells were stimulated with various phospholipids, anionic and zwitterionic lipids at the indicated concentrations. Reporter activation (GFP expression) was assessed after overnight incubation by flow cytometry. Kinetics of TREM2 reporter cells responding to lipids at various concentrations are shown in (A). Blockade of reporter activation by a soluble anti-hTREM2 mAb is shown in (B). (C) mTREM2 reporter cells were cultured with either apoptotic cells (AC) or phosphatidylserine (PS) in the presence of soluble anti-TREM2 mAb or isotype control. (D, E) Adult primary microglia from Trem2−/−5XFAD and 5XFAD mice were pulsed with CSFE-labeled AC. (D) Phagocytosis of AC was determined 20, 40 and 60 min post co-culturing by flow cytometry. (E) Summary of AC uptake by WT and Trem2−/− microglia. Data represent a total of three (A–C) and two (D, E) independent experiments.
R47H mutation impairs Trem2 recognition of lipid ligands
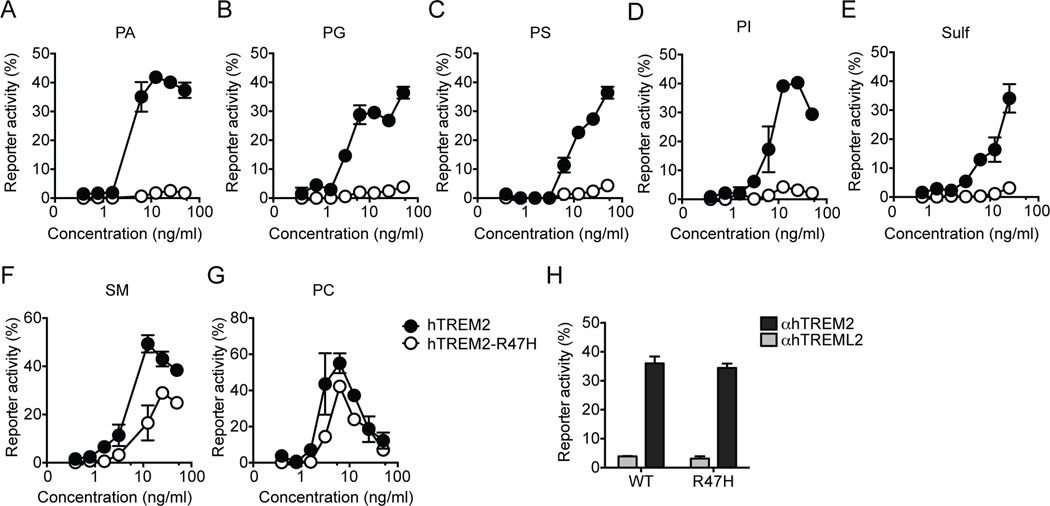
What is the impact of the R47H mutation on TREM2 ligand recognition? We generated TREM2 R47H reporter cells and compared their response to identified ligands to that of TREM2 reporter cells. The R47H mutation considerably reduced reporter activation in response to many ligands, including phosphatidic acid (PA), phosphatidylglycerol (PG), PS, phosphatidylinositol (PI) and sulfatides (Fig. 7A–G). The R47H mutation had less impact on SM recognition and very little influence on PC-mediated activation. Importantly, the R47H mutation did not detectably affect cell surface expression or signaling of TREM2, as assessed by stimulating the R47H reporter cells with a plate-bound anti-TREM2 antibody (Fig. 7H). Thus, these data suggest that the R47H may reduce the overall capacity of TREM2 to bind anionic ligands.
Figure 7. R47H mutation attenuates TREM2 recognition of lipids.
(A–H). Reporter cells expressing either common allele or R47H variant of human TREM2 were stimulated with various species of lipids or plate-bound anti-hTREM2 mAb. A plate-bound control antibody (anti-hTREML2) was used as a negative control. Data represent a total of two independent experiments. Bars represent mean±SEM.
Discussion
This study showed that TREM2 modulates Aβ accumulation in the 5XFAD mouse model of AD, thereby reducing neuronal damage. The importance of TREM2 in Aβ clearance is underscored by the fact that even the loss of one copy of Trem2 gene is sufficient to increase Aβ accumulation. TREM2 acts in microglia by supporting Aβ-reactive microgliosis, a process of expansion and activation that leads to microglia clustering around Aβ plaques and subsequent Aβ removal (Ransohoff and Cardona, 2010). In the absence of TREM2, this microgliosis is impaired. In fact microglia from Trem2−/−5XFAD mice are unable to survive, as evidenced by the accumulation of apoptotic microglia around Aβ plaques. Cells involved in TREM2-dependent microgliosis had phenotypic features of brain resident microglia, such as expression of CX3CR1. However, it is possible that monocytes from peripheral blood contribute to microgliosis and that TREM2 supports their survival as well.
Previous studies have shown that CSF-1-CSF-1R signaling is essential for microgliosis in response to Aβ (Chitu and Stanley, 2006; Kaku et al., 2003; Mitrasinovic et al., 2003). Since CSF-1 is rapidly consumed during this process (Stanley and Chitu, 2014), there is probably a limited supply of CSF-1 surrounding the amyloid plaques. Our results demonstrate that TREM2 provides a signal that is necessary for survival of microglia at low CSF-1 concentrations. We postulate that TREM2 acts as a costimulatory molecule that sustains survival of microglia, which are activated and proliferate in the presence of Aβ. Previous studies of cultured myeloid cells indicate that TREM2 may synergize with CSF-1-CSF-1R signaling to activate the protein tyrosine kinase Syk, which in turns activates multiple downstream mediators, such as ERK, PI-3K and Akt (Zou et al., 2008). In addition, TREM2 may provide survival signals through activation of anti-apoptotic mediators such as β-catenin (Otero et al., 2009) and Mcl-1 (Peng et al., 2010). It is also possible that TREM2 is necessary to support increased microglia metabolism during activation.
Why is TREM2 activated during Aβ accumulation? Previous studies have indicated that TREM2 binds phospholipids, such as PS, and acts as a scavenger receptor for apoptotic cells that might be generated during neuronal damage (Hsieh et al., 2009; Takahashi et al., 2007; Takahashi et al., 2005). In our study we demonstrate that TREM2 is a sensor for a broad array of acidic and zwitterionic lipids, which may or may not contain a phosphatic acid moiety. Membranes containing these lipids strongly interact with Aβ, facilitating the conversion of fibrillar Aβ from Aβ peptides (Ahyayauch et al., 2012; Del Mar Martinez-Senac et al., 1999; Nagarathinam et al., 2013). Moreover, some TREM2 lipidic ligands accumulate on the cell surface of neurons and glial cells damaged by Aβ accumulation, such as PS (Eckert et al., 2005; McLaurin and Chakrabartty, 1996), or are released by damaged myelin, such as SM and sulfatides. In contrast, TREM2 did not directly bind Aβ. Consistent with its ability to bind anionic lipids, the TREM2 extracellular domain is rich in arginine residues that may form salt bridges with polyanions. Remarkably, we found that the R47H mutation associated with AD affected the binding of multiple lipid ligands, although to differing extents. Most likely, the R47H mutation is sufficient to considerably reduce the binding affinity of TREM2 extracellular domain for most anionic ligands. Structural studies will be essential to validate this model.
Our findings demonstrated that TREM2 functions as a microglial sensor that is alerted by damage-induced molecules that share a common lipidic backbone and an anionic group. In contrast with previous reports (Hsieh et al., 2009; Takahashi et al., 2007; Takahashi et al., 2005), we found that the engagement of TREM2 does not directly mediate phagocytosis of apoptotic cells. However, TREM2 signaling may indirectly supports phagocytosis by promoting survival of activated microglia. It has been shown that individuals homozygous for rare mutations that impair expression of either TREM2 or DAP12, develop lethal forms of progressive, early-onset dementia such as Nasu-Hakola disease (NHD) and frontotemporal dementia (Guerreiro et al., 2013a; Guerreiro et al., 2013c; Kleinberger et al., 2014; Paloneva et al., 2002). Although the pathology of these forms of dementia differs from that of AD and often involves demyelination, our study suggests that TREM2 may be required for microglia to sense glycolipids such as SM and sulfatides that are exposed on damaged myelin sheaths; thus, TREM2 binding to these glycolipids may trigger the microglial response to damaged myelin, which is necessary to clear myelin residues and produce trophic factors that induce repair and remyelination. While the R47H mutation associated with AD did not entirely abolish ligand binding, mutations associated with Nasu-Hakola disease result in a complete lack of TREM2 expression (Kleinberger et al., 2014), which may explain the distinct pathology and more dramatic clinical course of this disease.
Experimental Procedures
Mice
Trem2−/− mice were generated as previously described. 5XFAD mice were purchased from the Jackson Lab (MMRRC) and crossed to Trem2−/− mice to generate Trem2+/− 5XFAD and Trem2−/−5XFAD mice. All mice were bred and housed in the same animal facility. Trem2−/−Cx3cr1+/GFPAPPPS1–21 mice were generated in a similar manner, as previously described (Ulrich et al., 2014). All animal studies were approved by the Washington University Animal Studies Committee.
Preparation of brain samples
For histological analysis 5XFAD mice, APPPS1–21 and transgene negative controls were anesthetized with ketamine and perfused with ice-cold PBS. Right-brain hemispheres were fixed in 4% PFA overnight and placed in 30% sucrose before freezing and cutting on a freezing sliding microtome. Serial 40µm coronal sections of the brain were collected from the rostral anterior commissure to caudal hippocampus as landmarks. For biochemical and mRNA expression analysis, cortices and hippocampi of the left-brain hemispheres were carefully dissected out and flash froze in liquid nitrogen.
Immunohistochemistry and confocal microscopy
For detailed procedures, see the Extended Experimental Procedures.
Gene expression analysis
For frozen brain tissues, RNA was extracted using a RNeasy mini kit according to manufacture protocol (Qiagen). Microglia were FACS-sorted directly into RLT-plus lysis buffer and RNA extraction was performed using a RNeasy micro kit according to manufacture protocol (Qiagen). Primers for qPCR analysis are provided in Supplemental Table 1. For detailed procedure on microarray analysis, see the Extended Experimental procedures. All data have been deposited at GEO (GSE65067)
ELISA
Aβ levels were assessed using sandwich ELISAs as described (Kim et al., 2009). For detailed procedure, see the Extended Experimental Procedures.
Ex vivo microglia cultures
Primary adult microglia culture was generated as previously described (Butovsky et al., 2014). Briefly, purified adult microglia were cultured in the presence of 15% LSM media (Otero et al., 2009) and 10ng/mL human TGF-β1 (PeproTech) for 7 days before experiments. For details on in vitro assays performed, see the Extended Experimental Procedures.
Reporter assay
2B4 GFP-NFAT reporter T cells were stably transfected with murine or human TREM2 cDNAs. Cells were cultured with apoptotic thymocytes in round-bottom 96-well plates or plated onto high-absorbance flat bottom plate coated with various lipids at indicated concentration. Reporter cells were assessed after overnight incubation. Reporter activity (%) is defined as %GFP+ cells subtracted from background (vehicle controls).
Statistics
Data in figures are presented as mean ± SEM. All statistical analysis was performed using Prism (GraphPad). Statistical analysis to compare the mean values for multiple groups was performed using a one-way or two-way ANOVA with correction for multiple comparisons. Comparison of two groups was performed using a two-tailed unpaired t-test (Mann Whitney). Values were accepted as significant if P≤0.05.
Supplementary Material
Acknowledgement
We thank the Genome Technology Access Center (Washington University School of Medicine) for assistance with microarray analysis. We thank S. Raju (Washington University School of Medicine) for assistance with live imaging. We thank R.B. DeMattos, J.D. Sedgewick and A.P. Martin (Eli Lilly and Company) for helpful suggestions and critical comments. Y.W. is supported by the Lilly Innovation Fellowship Award. B.H.Z. is supported by DDRCC grant P30 DK52574. M.Ce. is supported by grant RG4687A1/1 from National Multiple Sclerosis Society. M.Co. is supported by the Knight Alzheimer’s Disease Research Center pilot grant P50 AG005681-30.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
Y.W., J.D.U., M.L.R., S.S. and B.H.Z. performed 2-photon and confocal imaging analyses; Y.W., B.H.Z. and G.M.K. performed computational analyses; K.M., K.L.Y. and J.R.C. analyzed Aβ depositions; Y.W. performed ex vivo cell culture experiments; M.Ce. and Y.W. generated TREM2 reporter cells and performed reporter assays; S.G. generated and maintained Trem2+/− and Trem2−/−5XFAD mice. D.M.H. supervised research on APPPS1–21 mice and provided critical comments. M.Co. supervised the entire project; M.Co. and Y.W. wrote the manuscript.
References
- Ahyayauch H, Raab M, Busto JV, Andraka N, Arrondo JL, Masserini M, Tvaroska I, Goni FM. Binding of beta-amyloid (1–42) peptide to negatively charged phospholipid membranes in the liquid-ordered state: modeling and experimental studies. Biophys j. 2012;103:453–463. doi: 10.1016/j.bpj.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JP, O'Driscoll M, Litman GW. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics. 2012;64:39–47. doi: 10.1007/s00251-011-0562-4. [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Cole GM, Ard MD. The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol Aging. 2004;25:675–683. doi: 10.1016/j.neurobiolaging.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Daws MR, Lanier LL, Seaman WE, Ryan JC. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur J Immunol. 2001;31:783–791. doi: 10.1002/1521-4141(200103)31:3<783::aid-immu783>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- Del Mar Martinez-Senac M, Villalain J, Gomez-Fernandez JC. Structure of the Alzheimer beta-amyloid peptide (25–35) and its interaction with negatively charged phospholipid vesicles. Eur J Biochem. 1999;265:744–753. doi: 10.1046/j.1432-1327.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Microglia in Alzheimer's disease and transgenic models. How close the fit? Am J Pathol. 1999;154:1627–1631. doi: 10.1016/S0002-9440(10)65416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Wood WG, Muller WE. Membrane disordering effects of beta-amyloid peptides. Subcell Biochem. 2005;38:319–337. doi: 10.1007/0-387-23226-5_16. [DOI] [PubMed] [Google Scholar]
- Eimer WA, Vassar R. Neuron loss in the 5XFAD mouse model of Alzheimer's disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol Neurodegener. 2013;8:2. doi: 10.1186/1750-1326-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Bilgic B, Guven G, Bras J, Rohrer J, Lohmann E, Hanagasi H, Gurvit H, Emre M. Novel compound heterozygous mutation in TREM2 found in a Turkish frontotemporal dementia-like family. Neurobiol Aging. 2013a;34:2890 e2891–2890 e2895. doi: 10.1016/j.neurobiolaging.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013b;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Lohmann E, Bras JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol. 2013c;70:78–84. doi: 10.1001/jamaneurol.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. The Journal of neuroscience : J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Tan L, Zhu XC, Zhang QQ, Cao L, Tan MS, Gu LZ, Wang HF, Ding ZZ, Zhang YD, et al. Upregulation of TREM2 Ameliorates Neuropathology and Rescues Spatial Cognitive Impairment in a Transgenic Mouse Model of Alzheimer's Disease. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M, Tsutsui K, Motokawa M, Kawata T, Fujita T, Kohno S, Tohma Y, Ohtani J, Tenjoh K, Tanne K. Amyloid beta protein deposition and neuron loss in osteopetrotic (op/op) mice. Brain Res Brain Res Protoc. 2003;12:104–108. doi: 10.1016/j.brainresprot.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kiialainen A, Hovanes K, Paloneva J, Kopra O, Peltonen L. Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol Dis. 2005;18:314–322. doi: 10.1016/j.nbd.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra286. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271:26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease. The Journal of neuroscience : J Neurosci. 2011;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrasinovic OM, Vincent VA, Simsek D, Murphy GM., Jr Macrophage colony stimulating factor promotes phagocytosis by murine microglia. Neurosci Lett. 2003;344:185–188. doi: 10.1016/s0304-3940(03)00474-9. [DOI] [PubMed] [Google Scholar]
- Nagarathinam A, Hoflinger P, Buhler A, Schafer C, McGovern G, Jeffrey M, Staufenbiel M, Jucker M, Baumann F. Membrane-anchored Abeta accelerates amyloid formation and exacerbates amyloid-associated toxicity in mice. The Journal of neuroscience : J Neurosci. 2013;33:19284–19294. doi: 10.1523/JNEUROSCI.2542-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. The Journal of neuroscience : J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, Faccio R, Ross FP, Teitelbaum SL, Takayanagi H, et al. TREM2 and beta-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 2012;188:2612–2621. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer's disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P, Meldolesi J. Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur J Neurosci. 2004;20:2617–2628. doi: 10.1111/j.1460-9568.2004.03729.x. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. The Journal of neuroscience : J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE. A brief history of Alzheimer's disease gene discovery. J Alzheimers Dis. 2013;33(Suppl 1):S5–S13. doi: 10.3233/JAD-2012-129044. [DOI] [PubMed] [Google Scholar]
- Thrash JC, Torbett BE, Carson MJ. Developmental regulation of TREM2 and DAP12 expression in the murine CNS: implications for Nasu-Hakola disease. Neurochem Res. 2009;34:38–45. doi: 10.1007/s11064-008-9657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- Ulrich JD, Finn MB, Wang Y, Shen A, Mahan TE, Jiang H, Stewart FR, Piccio L, Colonna M, Holtzman DM. Altered microglial response to Abeta plaques in APPPS1–21 mice heterozygous for TREM2. Mol Neurodegener. 2014;9:20. doi: 10.1186/1750-1326-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.