Abstract
Background/Aims
We investigated whether sodium picosulfate with magnesium citrate (SPMC) plus bisacodyl compares favorably with conventional polyethylene glycol (PEG) with respect to bowel cleansing adequacy, compliance, and safety.
Methods
We performed a multicenter, prospective, single-blinded study in outpatients undergoing daytime colonoscopies. Patients were randomized into a split preparation SPMC/bisacodyl group and a conventional split PEG group. We compared preparation adequacy using the Boston bowel preparation scale (BBPS), ease of use using a modified Likert scale (LS), compliance/satisfaction level using a visual analogue scale (VAS), and safety by monitoring adverse events during the colonoscopy between the two groups.
Results
A total of 365 patients were evaluated by intention to treat (ITT) analysis, and 319 were evaluated by per protocol (PP) population analysis (153 for SPMC/bisacodyl, 166 for PEG). The mean total BBPS score was not different between the two groups in both the ITT and PP analyses (p>0.05). The mean VAS score for satisfaction and LS score for the ease of use were higher in the SPMC/bisacodyl group (p<0.001). The adverse event rate was lower in the SPMC/bisacodyl group than in the PEG group (p<0.05).
Conclusions
The SPMC/bisacodyl treatment was comparable to conventional PEG with respect to bowel preparation adequacy and superior with respect to compliance, satisfaction, and safety.
Keywords: Colonoscopy, Bowel preparation, Sodium picosulfate with magnesium citrate, Polyethylene glycols
INTRODUCTION
Colonoscopy is considered the most effective colorectal cancer screening procedure.1 The maximal preventative effect of colonoscopy requires adequate visualization of the entire colon to facilitate detection and removal of all adenomas, which involves detailed observations associated with cecal intubation, longer withdrawal time, and adequate preparation. Bowel preparation is the most important colonoscopy quality indicator because it affects both the cecal intubation and adenoma detection rates.
The ideal preparation procedure should clean the colon without mucosal damage, electrolyte imbalance, or patient discomfort. The most commonly used preparation regimens are based on polyethylene glycol (PEG). PEG is a safe and highly effective colon cleansing agent that is widely accepted as a standard bowel preparation regimen.2,3 However, ingestion of a large volume of PEG, as well as its unpleasant taste, results in low compliance rates and unsatisfactory cleansing quality.4–6 The adequate cleansing rate by conventional PEG bowel preparation has been reported to be 70% to 77% by meta-analyses,2,5–7 which is similar to that in Korea (47% to 76%).8–11
Sodium picosulfate with magnesium citrate (SPMC) is a low-volume, dual-action bowel-cleansing agent used mostly in the United Kingdom and Canada as a tolerable preparation agent.12–15 Recently, SPMC was approved in the United States for bowel preparation before colonoscopy and it is also available in Korea as Picolight powder® (Phambio Korea Co., Ltd., Seoul, Korea).16,17 Each sachet of Picolight powder® contains 12-g citrate, 3.5-g magnesium oxide, an osmotic laxative, and 10-mg sodium picosulfate hydrate, a stimulant laxative. These ingredients are identical to those in Picolax® (Ferring, West Drayton, UK) and Pico-Salax® (Ferring, North York, Canada). SPMC acts locally in the colon as both a stimulant laxative by increasing the frequency and the force of peristalsis and promoting electrolyte and water retention in the colon, and an osmotic laxative by retaining fluids in the colon.18 In previous meta-analyses, SPMC was well tolerated by patients but may be less efficacious than other agents.14,19–21 Recently, several studies have investigated various methods of improving the cleansing efficacy of SPMC, including the addition of a laxative with SPMC, consuming fluids with SPMC, and the three-sachet-split method.22,23 However, these studies were conducted largely in Western populations12–15 and SPMC preparation has not been studied in an Asian population.
We conducted this study to investigate the cleansing efficacy, safety and tolerability of SPMC with bisacodyl as a substitute bowel preparation method to conventional large volume PEG in Koreans.
MATERIALS AND METHODS
1. Study design and subjects
This was a multicenter, randomized, single blind, noninferiority trial conducted at 13 institutions belonging to the intestinal tumor research group of the Korean Association for the Study of Intestinal Disease (KASID) from June 2012 to January 2013. All participating endoscopists were faculty of a university hospital with colonoscopy expertise. Bias in endoscopist evaluation of colon cleansing was minimized by use of a single investigator blind design.
All subjects 20 to 75 years of age undergoing elective daytime outpatient colonoscopy at each institution were enrolled in this study. Exclusion criteria included previous colorectal surgery, ileus or bowel obstruction, significant constipation defined as <3 bowel movements per week with or without regular laxatives, ascites, heart failure, ischemic heart disease or coronary vessel disease within the last 6 month, inflammatory bowel disease, pregnancy, cognitive impairment, and renal impairment. This study was approved by the Institutional Review Board of each participating institution and registered with the open registry of the Clinical Research Information Service (http://cris.nih.go.kr) under the identifier KCT0000580. After informed consent, the patients were randomly assigned to one of two fixed-dose treatment arms, either split-dose SPMC with bisacodyl or split-dose PEG. Randomization was conducted using a computer-generated table prepared by an independent biostatistician.
2. Patient preparation instruction
All enrolled patients were instructed to consume a low-fiber diet 3 days before colonoscopy and fast in the evening of the day before colonoscopy. Participants in the SPMC group were given Picolight powder® (Phambio Korea Co., Ltd.) and instructed to ingest one sachet with 150-mL water followed by 2-L of water at 7 PM the day before the procedure and ingest another sachet with 150-mL water followed by 2-L water 4 hours before colonoscopy, based on previous reports.23 Participants in the SPMC group were further instructed to ingest two 5-mg bisacodyl tablets the night before colonoscopy. Those patients receiving standard PEG were instructed to use the split-dose method, whereby 2-L PEG for ingestion at 6 PM the day before colonoscopy and another 2-L PEG 4 hours before colonoscopy. All colonoscopies were scheduled to be performed before 13:00 hours.
3. Outcome measurements
1) Bowel cleansing
The primary outcome was comparison of the colon-cleansing efficacy between the two preparation groups. Bowel cleansing was assessed by endoscopists who were blinded to the preparation method during the withdrawal phase of colonoscopy using the Boston bowel preparation scale (BBPS), a validated tool for assessment of the quality of colon cleansing.24 Each of the three segments of the colon (right, including the cecum and ascending colon; transverse, including the hepatic and splenic flexures; and left, including the descending colon, sigmoid, and rectum) was given a score from 0 to 3, defined as follows: 0=unprepared colon segment with mucosa not visible due to remnants of solid stool that cannot be cleared; 1=portion of mucosa of the colon segment seen, but other areas of the colon segment not as visible because of staining, residual stool, and/or opaque liquid; 2=minor amount of residual staining, small fragments of stool, and/or opaque liquid, but mucosa of colon segment visible; 3=entire mucosa of colon segment visible, with no residual staining, small fragments of stool, or opaque liquid.24 Adequate bowel preparation was defined a score ≥2 for all segments. Inadequate preparation was defined as a summed BBPS score <6 or a score in any segment <2.
2) Satisfaction and tolerability
Satisfaction with the preparation agent was assessed using a visual analogue scale (VAS) as 0 (worst) to 10 (best) on a questionnaire to patients. The tolerability of the bowel preparation was assessed via a standardized questionnaire administered to patients on the day of the colonoscopy, before receiving any preliminary sedation for the procedure. The questionnaires for the assessment of tolerability were composed of four sections describing the completeness of agent consumption, the ease, the taste and the intention to reuse the preparation. Patient could choose only one item for their responses.
The questionnaire for the completeness of agent consumption was as follows for the SPMC/bisacodyl group: (1) ‘2 sachets completely taken,’ or ‘not’; (2) additional water consumption completeness, ‘2-L for each sachet,’ ‘less than 2-L for each sachet,’ ‘1-L for each sachet,’ ‘less than 1-L for each sachet’; (3) overnight two tablets of bisacodyl, ‘completely taken,’ or ‘not.’
The questionnaire for the completeness of agent consumption was as follows for the conventional PEG group: ‘4-L completely taken,’ ‘over 75% taken,’ ‘over 50% taken,’ ‘less than 50% taken.’ The questionnaire regarding ease of use and taste of the bowel preparation was based on a modified five-point Likert scale (LS): ‘very easy (5 points),’ ‘easy (4 points),’ tolerable (3 points),’ ‘difficult (2 points),’ very difficult (1 point)’ and for taste as five points: ‘very good (5 points),’ ‘good (4 points),’ fair (3 points),’ ‘bad (2 points),’ ‘worst (1 point).’22 We determined ‘the tolerable group’ as those participants who selected three points or more in each of the assessments of ease of use and taste.
3) Safety
Safety was assessed by the incidence of adverse events reported as a ‘yes’ or ‘no’ response in a questionnaire and colonic mucosal change. Adverse events were defined as nausea, vomiting, abdominal pain, bloating, thirst, dizziness, or paresthesia during consumption of the preparation agents. The presence of mucosal changes including erythema or aphthous lesions, were recorded by endoscopists after colonoscopy. Blood chemistry and electrolyte level were not essential tests and were evaluated only when the patients had severe side effects, as determined by the endoscopist.
4. Statistical analysis
In calculating the sample size, we assumed a successful cleansing rate of 85% for the conventional PEG preparation based on previous data.5,13 We specified a noninferiority margin of −15% for the difference in adequate preparation rates defined as the rate in the SPMC/bisacodyl group minus the rate in the PEG group, and a one-sided significance level of 0.025. Assuming a 10% dropout rate, we calculated that we would need to enroll 186 patients in each group for an 80% statistical power to demonstrate noninferiority.
The intent to treat (ITT) population included all randomized participants and the patient who underwent colonoscopy. The per protocol (PP) population was determined as the enrolled patient who completed bowel preparation according to the preparation instructions for each and underwent colonoscopy. The outcome measurement and statistical analyses of cleansing efficacy, tolerability, satisfaction, and safety were performed for both the ITT and PP populations. The safety analysis was performed for the ITT population.
We assessed noninferiority by comparing the lower bound of the one-sided 97.5% confidence interval (CI) for the rate difference to −15% and testing the corresponding hypothesis that the difference would be >−15%.
Total BBPS, VAS, and the ease of use and taste scores were analyzed as a continuous variable, after confirmation of normal distribution, by Student t-test. We compared percentages of the BBPS score of each segment of colon, the completeness of preparation agents, the tolerability, ease of use, and taste based on a standardized patient questionnaire, and the incidence of both side effects and mucosal change between the groups using a Pearson chi-square test of Fisher exact test for pooled responses and records. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as p<0.05.
RESULTS
A total of 387 patients were randomized to receive split dose SPMC/bisacodyl or split dose PEG. Twenty-two patients (12 for the SPMC/bisacodyl, 10 for the PEG group) did not undergo colonoscopy after providing informed consent and were excluded from outcome measurements. Thus, a total of 365 patients (181 for the SPMC/bisacodyl, 184 for the PEG group) in the ITT population underwent colonoscopy and cleansing efficacy, tolerability and satisfaction evaluated. There were no significant differences in baseline characteristics including age, sex, and indication of colonoscopy between the groups (Table 1). The PP population analysis is described below.
Table 1.
Demographics and Baseline Characteristics of the Intention to Treat Population
| Parameter | SPMC with bisacodyl (n=181) | Conventional 4-L PEG (n=184) | p-value* |
|---|---|---|---|
| Male sex | 94 (51.9) | 100 (54.4) | 0.644 |
| Age, yr | 53.5±10.7 | 53.8±10.4 | 0.788 |
| 20–64 | 154 (85.1) | 154 (83.7) | 0.715 |
| ≥65 | 27 (14.9) | 30 (16.3) | |
| Indication for colonoscopy | 0.195 | ||
| Symptom evaluation | 38 (21) | 37 (20.1) | |
| Screening | 68 (37.6) | 79 (42.9) | |
| Surveillance | 50 (27.6) | 34 (18.5) | |
| For endoscopic treatment | 25 (13.8) | 34 (18.5) |
Data are presented as mean±SD or number (%).
SPMC, sodium picosulfate with magnesium citrate; PEG, polyethylene glycol.
p-values were calculated using Student t-test or Pearson chi-square test, as appropriate.
1. Completeness
Most of the participants that received SPMC/bisacodyl were able to consume the split dose of SPMC both on the day before (100%) and the day of (99.7%) the colonoscopy. The ratio of completeness of agent consumption was markedly higher in the SPMC/bisacodyl (99.4%) than conventional PEG (90.2%) groups (p<0.001). However, 28 patients in the SPMC/bisacodyl group and 18 in the PEG group did not complete the entire preparation process. Excluding these 46 patients, 319 patients (153 in the SPMC/bisacodyl, 166 in the PEG group) completed the entire preparation process and were analyzed as the PP population.
During the detailed preparation process for the SPMC/bisacodyl group, two tablets of 5-mg bisacodyl were consumed by 97.8% (n=179) of patients. However, 2-L of water was consumed after each sachet of SPMC by only 74% (n=134) of patients. Overall, 86% (n=156) of participants that received SPMC with bisacodyl consumed >75% of the total volume of water (3-L) after SPMC sachet consumption.
2. Cleansing efficacy
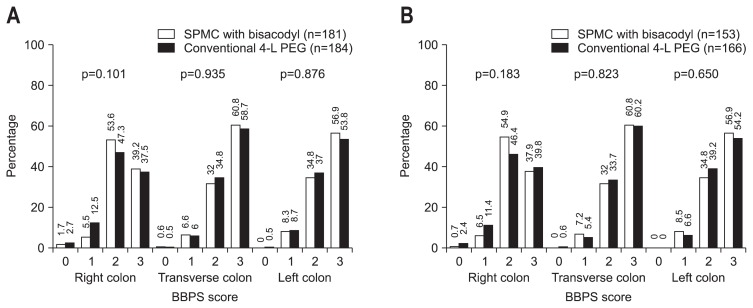
The SPMC/bisacodyl group achieved better overall colon cleansing than the PEG group, as measured by the ratio of adequate preparation based on BBPS. In the ITT analysis, the adequate preparation rate was 87.9% in the SPMC/bisacodyl group and 81.0% in the PEG group, similar to the results of the PP analysis (86.9% SPMC/bisacodyl vs 83.7% in the PEG group). The rate difference, defined as the rate of the SPMC/bisacodyl group—the rate in the PEG group, was 6.9% in the ITT analysis and 3.2% in the PP analysis. The lower bound of the one-sided 97.5% CI for rate difference rate was greater than the noninferiority margin of −15%, demonstrating noninferiority of the SPMC with bisacodyl group to the PEG group in both the ITT (one-sided 97.5% CI, −0.5% to 14.3%; p<0.001) and PP (one-sided 97.5% CI, −4.6% to 10.9%; p<0.001) analyses. However, there was no significant difference in mean BBPS when comparing the cleansing effect between the two preparation methods (Table 2). The mean BBPS of all colon segments for each group was higher than 2 points. The percentage of each BPSS score in all segments was also similar between the two preparation methods (Fig. 1).
Table 2.
Efficacy of Bowel Cleansing in Patients Undergoing Colonoscopy Using the Boston Bowel Preparation Scale
| Colon segment | ITT population (n=365) | PP population (n=319) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SPMC with bisacodyl (n=181) | Conventional 4-L PEG (n=184) | p-value* | SPMC with bisacodyl (n=153) | Conventional 4-L PEG (n=166) | p-value* | |
| Right colon | 2.3±0.7 | 2.2±0.8 | 0.144 | 2.3±0.6 | 2.2±0.7 | 0.391 |
| Transverse colon | 2.5±0.6 | 2.5±0.6 | 0.834 | 2.5±0.6 | 2.5±0.6 | 0.998 |
| Left colon | 2.5±0.6 | 2.4±0.7 | 0.507 | 2.5±0.6 | 2.3±0.6 | 0.913 |
| Total colon | 7.3±1.6 | 7.2±1.7 | 0.329 | 7.3±1.6 | 7.2±1.6 | 0.680 |
Data are presented as mean±SD.
ITT, intention to treat; PP, per protocol; SPMC, sodium picosulfate with magnesium citrate; PEG, polyethylene glycol.
p-values were calculated using Student t-test.
Fig. 1.
The Boston bowel preparation scale (BBPS) score percentage of each colon segment with sodium picosulfate with magnesium citrate (SPMC)/bisacodyl and conventional polyethylene glycol (PEG). (A) Intention to treat analysis. (B) Per protocol analysis.
3. Satisfaction and tolerability
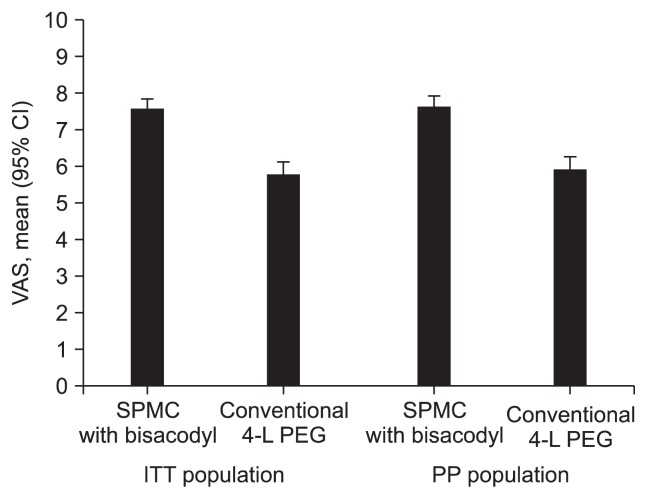
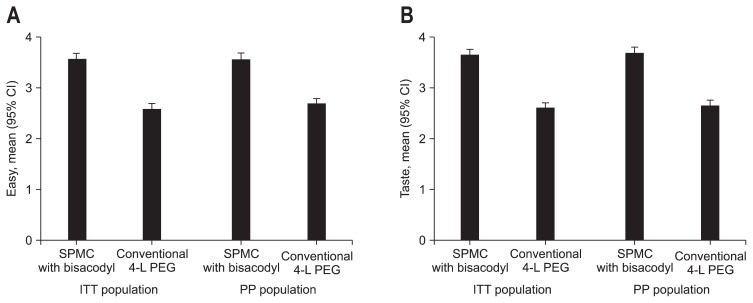
The mean VAS scale of the SPMC/bisacodyl group was significantly superior to the PEG group, in both ITT (7.58±1.94 vs 5.79±2.43, p<0.001) and PP (7.62±1.95 vs 5.92±2.35, p<0.001) (Fig. 2). SPMC with bisacodyl was better tolerated than PEG with respect to ease of use and taste (Fig. 3). Using a modified five-point LS, the mean score for ease of use of SPMC/bisacodyl was higher than that of PEG (ITT, 3.57±0.82 vs 2.59±0.79, p<0.001; PP, 3.56±0.81 vs 2.63±0.78, p<0.001). The mean score for taste of SPMC/bisacodyl was also superior to that of PEG (ITT, 3.65±0.73 vs 2.60±0.79, p<0.001; PP, 3.69±0.71 vs 2.64±0.77, p<0.001). Furthermore, the ‘tolerable group’ ratio was a significantly higher proportion of the SPMC/bisacodyl than PEG with respect to ease of use (ITT, 94.5% vs 63.0%, p<0.001; PP, 94.8% vs 65.7%, p<0.001) and taste (ITT, 97.8% vs 60.3%, p<0.001; PP, 98.0% vs 61.4%, p<0.001). Finally, a positive response regarding the intention to reuse the received agent was higher in the SPMC/bisacodyl group than the PEG group (ITT, 83.2% vs 48.5%, p<0.001; PP, 80.5% vs 49.5%, p<0.001).
Fig. 2.
Error bar plot of the visual analogue scale (VAS) score for each preparation group in the intention to treat (ITT) and per protocol (PP) populations. The mean VAS score of the sodium picosulfate with magnesium citrate (SPMC)/bisacodyl group was significantly greater than that of the conventional polyethylene glycol (PEG) group in both the ITT and PP analyses.
CI, confidence interval.
Fig. 3.
Error bar plot of the modified Likert scale between the two preparation groups within the intention to treat (ITT) and per protocol (PP) populations. (A) Ease of use. The mean modified Likert scale score of the sodium picosulfate with magnesium citrate (SPMC)/bisacodyl group was significantly greater than that of the conventional polyethylene glycol (PEG) group in both the ITT and PP analyses. (B) Taste. The mean modified Likert scale score of the SPMC/bisacodyl group was significantly greater than that of the conventional PEG group in both the ITT and PP analyses. CI, confidence interval.
4. Safety
The overall prevalence of adverse effects related to preparation was lower in the SPMC/bisacodyl group than the PEG group (65.2% vs 77.7%, p=0.011). Symptoms of vomiting, abdominal pain, and paresthesia occurred at a similar rate in both groups, while nausea, bloating, thirst were more common in the conventional PEG group and dizziness was more common in the SPMC/bisacodyl group (Table 3). Mucosal changes including erythema (5.5% of the SPMC/bisacodyl group vs 4.9% of the PEG group by PP analysis, p=0.139) and aphthous lesion (2.2% in the SPMC/bisacodyl group only) were observed in only a small number of patients in each group.
Table 3.
Comparison of Preparation-Related Adverse Events Using Intention to Treat Analysis
| Adverse effect | SPMC with bisacodyl (n=181) | Conventional 4-L PEG (n=184) | p-value* |
|---|---|---|---|
| Nausea | 52 (28.7) | 78 (42.4) | 0.006 |
| Vomiting | 23 (12.7) | 31 (16.9) | 0.265 |
| Abdominal pain | 48 (26.5) | 44 (23.9) | 0.566 |
| Bloating | 70 (38.7) | 103 (56.0) | 0.001 |
| Thirst | 19 (10.5) | 39 (21.2) | 0.005 |
| Dizziness | 44 (24.3) | 27 (14.7) | 0.020 |
| Parasthesia | 3 (1.7) | 6 (3.3) | 0.503 |
Data are presented as number (%).
SPMC, sodium picosulfate with magnesium citrate; PEG, polyethylene glycol.
p-values were calculated using Student t-test or Pearson chi-square test, as appropriate.
Blood chemistry tests for serum electrolytes and renal function (blood urea nitrogen and creatinine) were performed in 58 participants. There was no difference in the blood examination rate between the groups (14.9% of SPMC/bisacodyl vs 16.8% of PEG group). While there were no abnormal blood chemistry findings in the SPMC/bisacodyl group, one hyponatremia and two hypochloridemia cases were identified in the patients who received conventional PEG preparation in the ITT population.
DISCUSSION
Although the small volume of SPMC is well tolerated and results in improved preparation compliance, its cleansing effect remains uncertain. Some studies in the United Kingdom reported that SPMC was less effective than conventional PEG in bowel preparation before colonoscopy.12–14 To improve the cleansing effect of SPMC, a split method using two sachets taken the evening the day before colonoscopy and 4 to 5 hours before colonoscopy, combined with a laxative, has been proposed.22,23,25 Additionally, sufficient fluid intake is critical to the quality of preparation with SPMC; 1.5- to 2-L are recommended for each sachet.23,26
In the current study, we based the SPMC and bisacodyl combination method used on previous comparisons of cleansing efficacy with that of conventional PEG.16,17,22 We designed the split SPMC method to comprise ingestion of two sachets of SPMC with 2-L of water per sachet, in combination with 10-mg bisacodyl. We also educated all participants to partake in a low-fiber diet 3 days before colonoscopy.27 With this preparation protocol, the adequate preparation rate of SPMC/bisacodyl was comparable to that of conventional PEG in both the ITT and PP analyses. In the noninferiority analysis, the lower bound of CI for the difference in the adequate preparation rate was greater than the noninferiority margin hypothesized in this study, and fulfilled the noninferiority condition. There were no differences in the mean BBPS scores and BBPS percentage scores in the right, transverse and left colon. Favorable comparisons between SPMC and PEG were likely due to the additional bisacodyl given to the SPMC group. Bisacodyl is a stimulant laxative that works directly on the colon to increase the frequency and force of peristalsis and enhance bowel movement. Therefore the use of a stimulant laxative in this study might have overcome the reported weaknesses of a SPMC-only preparation, especially in the right colon cleansing.12–14,22
In addition to addition of a stimulant laxative to the SPMC preparation regimen, sufficient fluid intake following each sachet of SPMC is also important. Good quality cleansing with at least 3-L of total fluid intake in SPMC preparations has been reported.16,17,22 Interestingly, SPMC/bisacodyl was significantly superior to conventional PEG with respect to satisfaction and tolerability, even though the patients were instructed to consume 2-L of water following ingestion of 150 mL with the SPMC sachet and only 74% of patients consumed the recommended fluid volume, lower than that for conventional PEG (90.2%) in the current study. Sufficient fluid intake is likely the most difficult obstacle to overcome in SPMC preparation. Although, we designed 2-L of fluid intake following ingestion of each SPMC sachet in this study, 1.5-L of fluid intake per sachet may be appropriate based on the excellent results reported previously.16,17,23
In our study, PEG produced more symptoms of nausea, bloating and thirst, which may be related to the taste and large volume. SPMC produced more dizziness, which may be a result of biochemical derangements and dehydration. Reports of the biochemical and hemodynamic effects of SPMC are limited. Given that SPMC acts in a similar fashion to sodium phosphate, SPMC is likely to cause similar fluid and electrolyte shifts. In one study, PEG was associated with a significant decrease in potassium, while SPMC was associated with significant decreases in chloride and significant increases in magnesium.14 In 2002, the Australian Adverse Drug Reactions Advisory Committee described 16 cases of serious adverse events with SPMC with no fatalities, eight reports of adverse events associated with hyponatremia, and four reports of syncope and dehydration without documented electrolyte abnormalities.28 Electrolyte abnormalities resulting in seizures were reported in a patient using SPMC.29 In the current study, blood chemistry and electrolyte tests were performed only when patients developed severe side effects, a total of 15.9% of the participants, with no abnormal findings. These safety results may be related to our exclusion of patients with serious comorbidities, such as renal impairment and cardiovascular concerns, and those older than 75 years. Although the normal blood test results in the SPMC/bisacodyl group may reflect the safety of SPMC to the healthy group, further evaluation regarding the safety of SPMC/bisacodyl is necessary.
Mucosal changes, including inflammation and ulceration, were reported in 3.5% of patients who received the SPMC preparation,30 which is similar to the SPMC/bisacodyl in the current study. Although a previous study reported that the rate of acute mucosal inflammation was 10-fold higher in patients receiving SPMC than PEG,30 our data suggest no difference in mucosal inflammation, such as erythematous changes or aphthous lesions.
Our study was limited in that we did not confirm interobserver agreement in the BBPS. However, we instructed the participating endoscopists in using the BBPS, a valid and reliable instrument for assessing bowel cleanliness, in detail in the study protocol. BBPS is easier to assess than the Ottawa scale, and no difficulties were encountered because all of the endoscopists were skilled in colonoscopy. Although the current study was designed to be single-blinded due to the differences in the administration of bowel preparations, and this represents a potential source of bias, precautions were taken to ensure the endoscopists remained blinded to the bowel preparation method. In particular, our findings suggest that SPMC/bisacodyl has advantages with respect to bowel cleansing, tolerability and safety compared to conventional PEG; however, our findings should not be generalized to a high-risk population because we excluded patients with renal insufficiency and cardiovascular concerns. The broad noninferiority margin (15%) is one of the weak points of the current study. Although its range estimated based on previous studies might be wide,5,13 the cleansing effect of SPMC with bisacodyl was comparable to that of conventional PEG not only in the noninferiority analysis but also in the simple comparison using the mean BBPS score and adequate preparation rate.
In conclusion, this is the first prospective randomized controlled study of SPMC conducted at multiple institutions in Korea and the first in an Asian population. The results indicate that split dosing of SPMC with bisacodyl was comparable to conventional PEG with respect to preparation adequacy and superior to conventional PEG with respect to tolerability, satisfaction and safety. Therefore, a split dosing of SPMC with bisacodyl is a good substitute for conventional PEG preparation before colonoscopy.
ACKNOWLEDGEMENTS
This study was conducted by the intestinal tumor research group of the Korean Association for the Study of Intestinal Disease (KASID).
Footnotes
CONFLICTS OF INTEREST
Specific author contributions: All authors contributing to planning and conducting the study, collecting and interpreting the data, and drafting the manuscript.
Financial support: Financial support for data analysis and manuscript development was provided by Phambio Korea Co., Ltd.
Potential competing interests: None.
REFERENCES
- 1.Ransohoff DF. Colon cancer screening in 2005: status and challenges. Gastroenterology. 2005;128:1685–1695. doi: 10.1053/j.gastro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Belsey J, Crosta C, Epstein O, et al. Meta-analysis: the relative efficacy of oral bowel preparations for colonoscopy 1985–2010. Aliment Pharmacol Ther. 2012;35:222–237. doi: 10.1111/j.1365-2036.2011.04927.x. [DOI] [PubMed] [Google Scholar]
- 3.Hassan C, Bretthauer M, Kaminski MF, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:142–150. doi: 10.1055/s-0032-1326186. [DOI] [PubMed] [Google Scholar]
- 4.Lawrance IC, Willert RP, Murray K. A validated bowel-preparation tolerability questionnaire and assessment of three commonly used bowel-cleansing agents. Dig Dis Sci. 2013;58:926–935. doi: 10.1007/s10620-012-2449-0. [DOI] [PubMed] [Google Scholar]
- 5.Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy: a meta-analysis. Colorectal Dis. 2006;8:247–258. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 6.Juluri R, Eckert G, Imperiale TF. Meta-analysis: randomized controlled trials of 4-L polyethylene glycol and sodium phosphate solution as bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2010;32:171–181. doi: 10.1111/j.1365-2036.2010.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276–282. doi: 10.1016/S0016-5107(98)70191-9. [DOI] [PubMed] [Google Scholar]
- 8.Chung YW, Han DS, Park KH, et al. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol. 2009;43:448–452. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Kim TO, Shin BC, et al. Efficacy of prokinetics with a split-dose of polyethylene glycol in bowel preparation for morning colonoscopy: a randomized controlled trial. Digestion. 2012;86:194–200. doi: 10.1159/000339780. [DOI] [PubMed] [Google Scholar]
- 10.Seo EH, Kim TO, Park MJ, et al. Optimal preparation-to-colonoscopy interval in split-dose PEG bowel preparation determines satisfactory bowel preparation quality: an observational prospective study. Gastrointest Endosc. 2012;75:583–590. doi: 10.1016/j.gie.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Park SS, Sinn DH, Kim YH, et al. Efficacy and tolerability of split-dose magnesium citrate: low-volume (2 liters) polyethylene glycol vs. single- or split-dose polyethylene glycol bowel preparation for morning colonoscopy. Am J Gastroenterol. 2010;105:1319–1326. doi: 10.1038/ajg.2010.79. [DOI] [PubMed] [Google Scholar]
- 12.Dakkak M, Aziz K, Bennett JR. Short report: comparison of two orally administered bowel preparations for colonoscopy--polyethylene glycol and sodium picosulphate. Aliment Pharmacol Ther. 1992;6:513–519. doi: 10.1111/j.1365-2036.1992.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 13.Saunders BP, Masaki T, Fukumoto M, Halligan S, Williams CB. The quest for a more acceptable bowel preparation: comparison of a polyethylene glycol/electrolyte solution and a mannitol/Picolax mixture for colonoscopy. Postgrad Med J. 1995;71:476–479. doi: 10.1136/pgmj.71.838.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton D, Mulcahy D, Walsh D, Farrelly C, Tormey WP, Watson G. Sodium picosulphate compared with polyethylene glycol solution for large bowel lavage: a prospective randomised trial. Br J Clin Pract. 1996;50:73–75. [PubMed] [Google Scholar]
- 15.Regev A, Fraser G, Delpre G, et al. Comparison of two bowel preparations for colonoscopy: sodium picosulphate with magnesium citrate versus sulphate-free polyethylene glycol lavage solution. Am J Gastroenterol. 1998;93:1478–1482. doi: 10.1111/j.1572-0241.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK, Katz PO, Bertiger G, et al. Split-dose administration of a dual-action, low-volume bowel cleanser for colonoscopy: the SEE CLEAR I study. Gastrointest Endosc. 2013;78:132–141. doi: 10.1016/j.gie.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Katz PO, Rex DK, Epstein M, et al. A dual-action, low-volume bowel cleanser administered the day before colonoscopy: results from the SEE CLEAR II study. Am J Gastroenterol. 2013;108:401–409. doi: 10.1038/ajg.2012.441. [DOI] [PubMed] [Google Scholar]
- 18.Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. 2009;69:123–136. doi: 10.2165/00003495-200969010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hookey LC, Vanner S. A review of current issues underlying colon cleansing before colonoscopy. Can J Gastroenterol. 2007;21:105–111. doi: 10.1155/2007/634125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt LM, Williams P, King D, Perera D. Picoprep-3 is a superior colonoscopy preparation to Fleet: a randomized, controlled trial comparing the two bowel preparations. Dis Colon Rectum. 2004;47:238–242. doi: 10.1007/s10350-003-0027-4. [DOI] [PubMed] [Google Scholar]
- 21.Macleod AJ, Duncan KA, Pearson RH, Bleakney RR. A comparison of Fleet Phospho-soda with Picolax in the preparation of the colon for double contrast barium enema. Clin Radiol. 1998;53:612–614. doi: 10.1016/S0009-9260(98)80156-6. [DOI] [PubMed] [Google Scholar]
- 22.Hookey LC, Vanner SJ. Pico-salax plus two-day bisacodyl is superior to pico-salax alone or oral sodium phosphate for colon cleansing before colonoscopy. Am J Gastroenterol. 2009;104:703–709. doi: 10.1038/ajg.2008.167. [DOI] [PubMed] [Google Scholar]
- 23.Flemming JA, Vanner SJ, Hookey LC. Split-dose picosulfate, magnesium oxide, and citric acid solution markedly enhances colon cleansing before colonoscopy: a randomized, controlled trial. Gastrointest Endosc. 2012;75:537–544. doi: 10.1016/j.gie.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620–625. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TK, Kim HW, Kim SJ, et al. Importance of the time interval between bowel preparation and colonoscopy in determining the quality of bowel preparation for full-dose polyethylene glycol preparation. Gut Liver. 2014;8:625–631. doi: 10.5009/gnl13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety: a Canadian Association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699–710. doi: 10.1155/2006/915368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung YS, Seok HS, Park DI, et al. A clear liquid diet is not mandatory for polyethylene glycol-based bowel preparation for afternoon colonoscopy in healthy outpatients. Gut Liver. 2013;7:681–687. doi: 10.5009/gnl.2013.7.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adverse Drug Reactions Advisory Committee. Electrolyte disturbances with sodium picosulfate bowel cleansing products. Aust Adv Drug React Bull. 2002;21:2. [Google Scholar]
- 29.Frizelle FA, Colls BM. Hyponatremia and seizures after bowel preparation: report of three cases. Dis Colon Rectum. 2005;48:393–396. doi: 10.1007/s10350-004-0778-6. [DOI] [PubMed] [Google Scholar]
- 30.Lawrance IC, Willert RP, Murray K. Bowel cleansing for colonoscopy: prospective randomized assessment of efficacy and of induced mucosal abnormality with three preparation agents. Endoscopy. 2011;43:412–418. doi: 10.1055/s-0030-1256193. [DOI] [PubMed] [Google Scholar]