Abstract
Background/Aims
Uninsured individuals have lower rates of screening colonoscopy (SC), and little is known regarding the pathology results obtained when they undergo colonoscopies. Since 2004, we have participated in a program that offers SC to uninsured New Yorkers; herein, we report our findings.
Methods
Uninsured, average-risk patients who were at least 50 years of age underwent SC at our institution between April 2004 and June 2011. We analyzed polyp pathology, location, size, incidence of adenomas, and incidence of adenomas with advanced pathology (AAP) with respect to ethnicity, gender, and age.
Results
Out of 493 referrals, 222 patients completed the colonoscopies. Polyps were identified in 21.2% of all patients; 14% had adenomas, and 4.5% had AAP. The rates of adenomas among African-Americans, Hispanics, and Whites were 24.3%, 12.1%, and 11.6%, respectively, and the corresponding rates of AAP were 10.8%, 3.5%, and 2.3%. Differences in the polyp type, location, and AAP did not reach statistical significance with respect to ethnicity or gender. Patients aged 60 and older were found to have a higher rate of advanced adenomas compared with younger patients (8.6% vs 2.6%, p=0.047).
Conclusions
Further efforts to fund screening colonoscopies for uninsured individuals will likely result in the identification of advanced lesions of the colon before they progress to colorectal cancer.
Keywords: Colonoscopy, Adenoma, Medically uninsured, Patient navigator, Minorities
INTRODUCTION
Colonoscopy is an effective, and often preferred, method of colorectal cancer (CRC) screening that is widely endorsed by most major medical societies.1–4 Removal of adenomatous polyps during colonoscopy can reduce the incidence of, and prevent death from, CRC.5 Data from the Centers for Disease Control and Prevention show that CRC screening rates are increasing among persons aged 50 to 75 years, from 52.3% in 2002 to 65.4% in 2010.6 Despite this important improvement, disparities in screening rates still exist. In 2012, 69.6% of Whites compared to 66.1% of African Americans age 50 and older ever had a screening colonoscopy (SC) or sigmoidoscopy. Rates were lower among Hispanics (56.8%) and persons with income less than US $15,000 per year (53.8%).7 For uninsured individuals, screening rates are particularly low. In one study, rates of 35.6% were reported,8 and another found that only 33% of uninsured patients had undergone any test for CRC screening compared to 77% of insured patients.9 Lack of health insurance is associated with worse outcomes with regard to cancer screening and treatment.10–13 In part, this is due to the fact that uninsured and Medicaid patients may present with later stage disease or complications of CRC.14,15 Thus, increasing screening rates among the uninsured, minorities, and persons from lower socioeconomic status (SES) remains an important goal.
In recent years, two general interventions were developed to increase screening colonoscopy rates: direct-access endoscopies (DAE) and Patient Navigator (PN) programs. Under a DAE system, primary care physicians refer patients directly for colonoscopy (based on specific clinical guidelines) without a preprocedure visit to the gastroenterologist or anesthesiologist.16 Potential benefits of DAE include: saving time and cost, and minimizing time taken off from work.17 PNs are healthcare professionals trained to guide a patient through the process of obtaining colonoscopy after the initial physician referral by assisting with scheduling, transportation, answering questions, and providing appointment reminders.18 For the past decade, at our institution, we have implemented a program designed to increase SC rates among our urban minority population. Using DAE with PN, we demonstrated increased SC, fecal occult blood testing, and flexible sigmoidoscopy rates among African American and Hispanic patients.19–21 In those studies, most patients had some form of health insurance, mainly public insurance (Medicare or Medicaid).
In 2004, the New York City Council authorized funding for SC for uninsured and underinsured New Yorkers over age 50.22 These funds, administered through the American Cancer Society, were made available in the form of grants to hospitals in the New York City area interested in performing SC for a discounted fee. We have been participating in this program for the past 8 years. The present report describes the results of our institution’s efforts to provide SC to a group of predominately minority, uninsured and underinsured patients through this program.
MATERIALS AND METHODS
1. Patient eligibility
Patients eligible for enrollment in our screening program were men and women age ≥50 who had a referral for a SC from their primary care physician, and who met clinical criteria for SC (e.g., had no gastrointestinal symptoms). Eligible patients also met the following criteria: they could not (1) have fecal occult blood test within the past year or (2) have flexible sigmoidoscopy within the past 5 years, (3) have colonoscopy within the past 10 years, (4) have a history of inflammatory bowel disease, (5) have personal history of CRC, (6) speak languages other than English or Spanish, (7) be at greater than average CRC risk, and (8) have insurance or sufficient funds to cover the cost of the SC.
2. Patient recruitment
Eligible patients were identified by their provider from primary care clinics at The Mount Sinai Hospital (Internal Medicine Associates, Med-Peds Clinic, Ob/Gyn clinic, East Harlem Health Outreach Partnership) and other local community health centers based on review of their chart and the above criteria. The providers provided information on the patient’s past medical history, whether the patient was taking aspirin, nonsteroidal anti-inflammatory medications, or anticoagulants. This information was forwarded to a gastrointestinal nurse or gastroenterologist who reviewed the information and determined whether the patient was an appropriate candidate for DAE and if so, selected an appropriate bowel preparation. This information was then given to a PN to begin scheduling the patient for SC.
3. PN
All patients in our study received the assistance of a PN. The PNs were bilingual (Spanish-English) health educators who guided the patient through the process of getting their SC from the time of their referral through completion. Upon receiving the accepted referral, the PN contacted the patient by phone to review their medical history and current medications. The PN educated the patient on the process of the SC and CRC risk factors and prevention, answered questions, addressed fears about the procedure, and scheduled the procedure. If necessary, the PN also helped arrange for transportation. Written instructions in the patient’s preferred language (English or Spanish) for bowel preparation and procedure date were mailed to the patient’s home. This was followed by a reminder postcard with the time and location of their appointment. The PN contacted patients again by phone both 2-weeks and 3-days prior to their colonoscopy, at which time they would: provide appointment reminders, review written instructions and bowel preparation, provide details on what would happen on the day of the procedure, and to answer questions.
4. Data analysis
A secure database of all patients was created, including name, self-described ethnicity, date of first contact by the PN, date of scheduled SC, and SC outcome (completion and findings). Ethnicity was categorized as Hispanic, African American, White, or Other. Following completion of the colonoscopy, each colonoscopy report was reviewed to determine completeness of the exam, any abnormal findings, and quality of the bowel preparation. Pathology reports were then reviewed (by T.H.C.) and analyzed for histological type, size, and location of any polyps that were found. A second reviewer (S.H.I.) confirmed proper classification of polyps. Data was analyzed using SPSS software version 20 for Windows (SPSS Inc., Chicago, IL, USA). We used descriptive statistics to tabulate the demographics of the study population, polyp type, and location. Adenomatous polyps were further described in terms of adenoma size, histology, dysplasia, and multiplicity. Chi-square tests were used to compare ethnicity with the colonoscopy findings. All tests of significance were two-sided with a p<0.05.
RESULTS
1. Patient selection
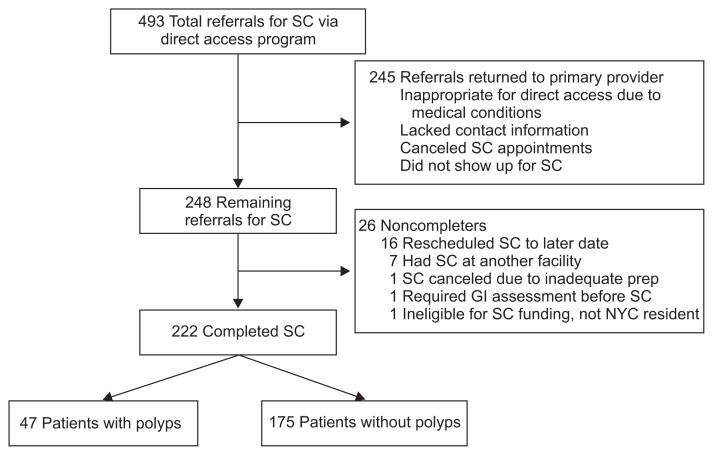
Between April 2004 and June 2011, a total of 493 patients were referred for SC via our DAE program and a total of 222 patients completed screening colonoscopies at our facility (Fig. 1).
Fig. 1.
Diagnostic algorithm of patients referred for screening colonoscopy.
SC, screening colonoscopy; GI, gastrointestinal; NYC, New York City.
2. Patient demographics
Table 1 shows the demographic breakdown of the study patients by gender, ethnicity, and insurance status. Of the 222 uninsured or underinsured patients, the vast majority were uninsured (n=209, 94.1%); 13 underinsured patients had public health insurance (Medicaid or Medicare HMO) but were eligible for our program because their insurance was inactive, limited, or the patient could not afford their copayment.
Table 1.
Patient Demographics (N=222)
| Demographics | Value |
|---|---|
| Age, yr | 57.0±6.4 |
| Gender | |
| Female | 122 (55.0) |
| Male | 100 (45.0) |
| Ethnicity | |
| African American | 37 (16.6) |
| Hispanic | 141 (63.5) |
| White | 43 (19.4) |
| Other | 1 (0.5) |
| Insurance status | |
| Uninsured | 209 (94.1) |
| Underinsured | 13 (5.9) |
Data are presented as mean±SD or number (%).
A comparison of the demographics of patients who had polyps on SC (n=47) versus those without any polyps (n=175) revealed no difference in mean age or ethnicity between the two groups (Table 2).
Table 2.
Demographics of Patients with and without Polyps
| With polyps (n=47) | Without polyps (n=175) | p-value | |
|---|---|---|---|
| Age, yr | 57.6±6.0 | 57.2±6.2 | NS* |
| Gender | |||
| Female | 20 (43) | 102 (58) | 0.054† |
| Male | 27 (57) | 73 (42) | |
| Ethnicity | |||
| African American | 14 (30) | 23 (13.1) | 0.053‡ |
| Hispanic | 26 (55) | 115 (65.7) | |
| White | 7 (15) | 36 (20.6) | |
| Other | 0 | 1 (0.6) | |
Data are presented as mean±SD or number (%).
NS=not significant.
Analysis of variance test;
Comparison of gender between patients with polyps and patients without polyps (χ2=3.704);
Comparison of ethnicity between patients with polyps and patients without polyps (χ2=7.674).
Information on quality of bowel preparation was available for 207 patients. Of those, bowel preparation was rated excellent in 49 (23.7%), very good in 27 (13.0%), good in 115 (55.6%), fair in 12 (5.7%), and poor in 4 (2.0%).
3. Colonoscopy findings
Colonoscopy findings by gender, ethnicity, and age are summarized in Table 3. There were no sessile serrated polyps detected in this cohort. Adenoma location was categorized as either proximal or distal to the splenic flexure. There was no statistically significant difference in polyp type or location in terms of ethnicity.
Table 3.
Colonoscopy Findings
| Total patients (n=222) | Gender | Ethnicity | Age | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Female (n=122) | Male (n=100) | p-value | African American (n=37) | Hispanics (n=141) | White* (n=43) | p-value | <60 (n=152) | ≥60 (n=70) | p-value | ||
| Patients with polyps | 47 (21.2) | 20 (16.4) | 27 (27.0) | 0.054 | 14 (37.8) | 26 (18.4) | 7 (16.3) | 0.053 | 30 (19.7) | 17 (24.3) | 0.441 |
| Type of polyp | |||||||||||
| Adenoma | 31 (14.0) | 14 (11.5) | 17 (17.0) | 0.368 | 9 (24.3) | 17 (12.1) | 5 (11.6) | 0.734 | 19 (12.5) | 12 (17.1) | 0.714 |
| Hyperplastic | 6 (2.7) | 2 (1.6) | 4 (4.0) | 2 (5.4) | 3 (2.1) | 1 (2.3) | 5 (3.3) | 1 (1.4) | |||
| Other | 10 (4.5) | 4 (3.3) | 6 (6.0) | 3 (8.1) | 6 (4.3) | 1 (2.3) | 6 (3.9) | 4 (5.7) | |||
| Adenoma location | |||||||||||
| Proximal | 15 (6.8) | 6 (4.9) | 9 (9.0) | 0.426 | 4 (10.8) | 10 (7.1) | 1 (2.3) | 0.568 | 9 (5.9) | 6 (8.6) | 0.421 |
| Distal | 15 (6.8) | 7 (5.7) | 8 (8.0) | 5 (13.5) | 6 (4.3) | 4 (9.3) | 10 (6.6) | 5 (7.1) | |||
| Proximal and distal | 1 (0.5) | 1 (0.8) | 0 | 0 | 1 (0.7) | 0 | 0 | 1 (1.4) | |||
| Adenoma multiplicity | |||||||||||
| 1 adenoma | 28 (12.6) | 12 (9.8) | 16 (16.0) | 0.365 | 8 (21.6) | 16 (11.3) | 4 (9.3) | 0.560 | 17 (11.2) | 11 (15.7) | 0.636 |
| >1 adenoma | 3 (1.4) | 2 (1.6) | 1 (1.0) | 1 (2.7) | 1 (0.7) | 1 (2.3) | 2 (1.3) | 1 (1.4) | |||
| Adenoma histology | |||||||||||
| Tubular | 26 (11.7) | 10 (8.2) | 16 (16.0) | 0.115 | 6 (16.2) | 15 (10.6) | 5 (11.6) | 0.202 | 16 (10.5) | 10 (14.3) | 0.649 |
| Tubulovillous | 5 (2.3) | 4 (3.3) | 1 (1.0) | 3 (8.1) | 2 (1.4) | 0 | 3 (2.0) | 2 (2.9) | |||
| Adenoma degree of dysplasia | |||||||||||
| Low-grade | 29 (13.1) | 13 (10.7) | 16 (16.0) | 0.493 | 9 (24.3) | 16 (11.3) | 4 (9.3) | 0.375 | 19 (12.5) | 10 (14.3) | 0.101 |
| High-grade | 2 (0.9) | 1 (0.8) | 1 (1.0) | 0 | 1 (0.7) | 1 (2.3) | 0 | 2 (2.9) | |||
| Adenoma size, cm | |||||||||||
| ≤0.5 | 13 (5.9) | 4 (3.3) | 9 (9.0) | 0.408 | 2 (5.4) | 8 (5.7) | 3 (7.0) | 0.732 | 9 (5.9) | 4 (5.7) | 0.444 |
| 0.6–0.9 | 10 (4.5) | 6 (4.9) | 4 (4.0) | 4 (10.8) | 4 (2.8) | 2 (4.7) | 7 (4.6) | 3 (4.3) | |||
| 1.0–1.9 | 5 (2.3) | 2 (1.6) | 3 (3.0) | 2 (5.4) | 3 (2.1) | 0 | 2 (1.3) | 3 (4.3) | |||
| ≥2.0 | 3 (1.4) | 2 (1.6) | 1 (1.0) | 1 (2.7) | 2 (1.4) | 0 | 1 (0.7) | 2 (2.9) | |||
| Advanced adenoma† | 10 (4.5) | 5 (4.1) | 5 (5.0) | 0.747 | 4 (10.8) | 5 (3.5) | 1 (2.3) | 0.236 | 4 (2.6) | 6 (8.6) | 0.047 |
| Advanced adenoma location | |||||||||||
| Proximal | 4 (1.8) | 1 (0.8) | 3 (3.0) | 0.409 | 1 (2.7) | 3 (2.1) | 0 | 0.407 | 1 (0.7) | 3 (4.3) | 0.099 |
| Distal | 6 (2.7) | 4 (3.3) | 2 (2.0) | 3 (8.1) | 2 (1.4) | 1 (2.3) | 3 (2.0) | 3 (4.3) | |||
Data are presented as number (%).
The one patient with ethnicity listed as “Other” had a normal colonoscopy and is not listed separately in this table;
Advanced adenoma defined as an adenoma ≥1 cm in diameter or any adenoma (regardless of size) with villous histology, high-grade dysplasia, or cancer.
Among the 31 patients who were found to have adenomas, the majority had single (90%), tubular adenomas (84%) with low grade dysplasia (94%). Adenoma rates were highest among African Americans (24.3%) compared to Hispanics (12.1%) and Whites (11.6%). Overall, 10 of 222 (4.5%) patients screened were found to have adenomas with advanced pathology (AAP) defined as an adenoma ≥1 cm in diameter, villous/tubulovillous histology, high-grade dysplasia, or cancer (regardless of size). Half of all AAP were tubulovillous, four of 10 (40%) were located in the proximal colon. No cancers were detected, but two AAP had high grade dysplasia. There were no statistically significant differences among the three ethnic groups in terms of rate of adenoma, location, size, number, degree of dysplasia, or presence of AAP.
In terms of age, patients ≥60 years old had significantly more AAP than patients <60 (8.6% vs 2.6%, p=0.047). Age was not a significant predictor of adenoma prevalence, degree of dysplasia, or size. No significant differences in polyp type, location, adenoma or AAP rate, were found based on gender.
DISCUSSION
Our study focused on the SC findings of a group of primarily uninsured, low SES urban minority patients. Among 493 patients referred for screening colonoscopy, fully half (n=245, 50%) were either inappropriate referrals, canceled or did not show up for their appointment and therefore did not receive SC. Although we do not have exact data on how many of these 245 patients were inappropriate referrals, we suspect that many were not screened because either the referrals were incomplete, or the patients did not meet criteria for SC. One of the drawbacks of a DAE system may be an increase in inappropriate referrals for SC.23 However, of the remaining 248 patients, 222 patients (90%) completed their colonoscopy, indicating that SC can be successfully accomplished in the context of an organized, programmatic effort.
Our screening program detected clinically significant pathology. Specifically, 24.3% of African Americans, 12.1% of Hispanics, and 11.6% of Whites had adenomas detected and removed. Moreover, 10.8% of African Americans, 3.5% of Hispanics, and 2.3% of Whites had adenomas with advanced pathology. In our earlier DAE/PN study consisting of mostly minority patients with health insurance, we found a relatively lower adenoma rate in African Americans and a higher rate among Hispanics. In that study, 12% African Americans, 19% Hispanics, and 11% other patients had adenomas and rates of AAP were similar in each group (approximately 2%).21 In a more recent, larger prospective cohort trial of over 500 Black and Hispanic individuals, we found that the overall prevalence of adenomas and proximal adenomas were 26.4% and 20%, respectively. Advanced adenomas occurred in 12.2% Blacks and 10.8% Hispanics.24
Table 4 provides a comparison of our data with other studies in minority individuals. The combined data indicate that ethnic minorities have premalignant colorectal pathology at rates that are at least as high as that of Whites.21,24–33 Our findings among African American patients differ from some of the earlier, larger studies, that suggests a higher prevalence of adenomas, AAP, and proximal polyps than was found in our group of African Americans.27–29 We suspect that these differences may be partially explained by our smaller sample size.
Table 4.
Colonoscopy Findings from Earlier Studies
| Study | Study type | Adenoma or polyp prevalence by ethnicity, % | Polyp distribution, % | AAP prevalence, % | Study population demographics | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Overall | African American | Hispanic | White/Other | Proximal | Distal | ||||
| Current study | Prospective | 14.0 | 24.3 | 12.1 | 11.6 | 7.7* | 9.9* | 4.5 | Uninsured; African American (37), Hispanic (141), White (43) |
| Chen et al.21 | Prospective | 16.4 | 12.0 | 19.0 | 11.0 | 8.8* | 20.3* | 2.0 | African American (98), Hispanic (211), Others (44) |
| Lee et al.24 | Prospective | 26.4 | 24.8 | 27.7 | NA | 20.2 | NA | 11.5 | African American (270), Hispanic (314) |
| Kanna et al.25 | Retrospective | 14.5 | NA | NA | NA | 53.2† | 46.8† | 4.3 | African American (485), Hispanic (3,475) |
| Lebwohl et al.26 | Retrospective | 20.3 | 26.0 | 22.0 | 19.0 | 9.2* | NA | 4.4 | African American (591), Hispanic (942), White (3,542) |
| Rex et al.27 | Prospective | 35.0 | 35.0 | NA | NA | 65.3† | 34.7† | 2.5 | African Americans (121) |
| Thornton et al.28,‡ | Prospective | 37.8 | 35.0 | NA | 38.0 | 20.1* | 18.7* | NA | African Americans (3,195) |
| Nouraie et al.29 | Retrospective | 83.9† | NA | NA | NA | 36.1† | 34† | NA | 90% African American (review of 5,013 colorectal polyps) |
| Thoma et al.30 | Retrospective | 47.0 | NA | 45.0 | 48.0 | 19* | NA | 4.0 | Hispanic (607), White (1,251) |
| Zheng et al.33 | Retrospective | 19.0 | 21.9 | 14.8 | 24.0 | 45† | 42† | NA | African American (635), Hispanic (738), White (283) |
AAP, adenomas with advanced pathology; NA, not applicable.
As percentage of total patients;
As percentage of total polyps;
Data for polyp histological subtype not available.
We found similar prevalence of polyps and adenomas between Hispanic and White patients, which is consistent with other studies showing similar or lower risk of adenomas in Hispanics.30–33
When examining the groups by age, we found that patients age 60 and older had a higher rate of AAP compared to patients younger than 60. This is consistent with data suggesting that adenoma detection and probability of developing advanced colorectal neoplasia increases with age.34,35
Because of the barriers related to SC, it has been assumed that it would be difficult to execute a SC program among uninsured patients. Over the past decade, we have had considerable success getting a similar population of minority individuals of low SES who had mostly public insurance to complete colonoscopy. We showed that PNs increased SC compliance, which was associated with higher patient satisfaction.19–21,36 Moreover, a cost analysis of our program found that use of PNs among our patients with mostly public health insurance generated a net increase in income, mainly by increasing colonoscopy completion rates.37 These results were in keeping with other trials that showed that use of PNs was associated with higher SC completion rates and better preparation quality among low income minority patients with health insurance.38–42 Indeed, we observed very low fair or poor bowel preparation rates in the present study. This implies that use of PNs can help circumvent at least some of the barriers to obtaining SC in patients with health insurance, and our findings herein extend this observation to the uninsured.
Patients in this study had their SC funded through the New York City Council, partnered with the American Cancer Society. This funding was part of a larger campaign launched in 2004 by New York City Department of Health and Mental Hygiene, together with the Citywide Colon Cancer Control Coalition (C5 Coalition) to increase SC in underserved populations. A recent study looking at rates of SC among New Yorkers during the first 5 years of this campaign found that timely colonoscopy screening increased from 41.7% to 61.7% overall.43 Furthermore, racial and ethnic disparities improved to the point where rates of SC became similar among Whites, African Americans, Hispanics, men, and women. However, whereas screening rates among the uninsured also improved (from 15% to 43.3%), they continue to lag behind patients with private insurance, Medicare, and Medicaid who had screening rates of 66.8%, 61.4%, and 60.4%, respectively. Thus, uninsured patients are still lagging behind insured patients when it comes to CRC screening via colonoscopy.
Targeted funding for SC (for example, in the form of grants) may be one way to help address this continued disparity. Elmunzer et al.44 recently reported on a flexible sigmoidoscopy-based CRC screening “health fair” for uninsured patients. Using a standard health clinic converted into an endoscopy suite, they were able to screen 52 patients at a cost of US $126 per patient.44 Dimase et al.45 reported on early efforts to provide no-cost SC and PN to underserved patients in Rhode Island using a coalition of participating endoscopists. This provides examples of how medical philanthropy may help address the low rates of CRC screening among the uninsured. A more broad overview of CRC screening approaches and issues in underserved populations has been recently published.46
Our study has a number of limitations. Our patient sample size is relatively small, potentially limiting the power to find pathological differences that might have been detected with a larger sample. Our cohort consisted of mostly uninsured minority patients from a relatively narrow geographic area in New York City, so results may not generalize to other populations. Recruitment of patients took place over several years, and we do not have exact figures on which patients did not complete SC because they either did not show up for, or canceled their appointment, lacked essential data, or had a contraindication. Despite these limitations, we found significant pathology among our patient population. Further research is needed to better understand the distribution and pathology of colorectal polyps in uninsured patients.
ACKNOWLEDGEMENTS
This study was funded by a grant from the American Cancer Society.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Qaseem A, Denberg TD, Hopkins RH, Jr, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 2.Burt RW, Barthel JS, Dunn KB, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010;8:8–61. doi: 10.6004/jnccn.2010.0003. [DOI] [PubMed] [Google Scholar]
- 3.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 5.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening, incidence, and mortality: United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–889. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Behavioral risk factor surveillance system [Internet] Atlanta: Centers for Disease Control and Prevention; 2013. [cited 2013 Aug 21]. Available from: http://www.cdc.gov/brfss. [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening among adults aged 50–75 years: United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:808–812. [PubMed] [Google Scholar]
- 9.Matthews BA, Anderson RC, Nattinger AB. Colorectal cancer screening behavior and health insurance status (United States) Cancer Causes Control. 2005;16:735–742. doi: 10.1007/s10552-005-1228-z. [DOI] [PubMed] [Google Scholar]
- 10.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27:3627–3633. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 11.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 12.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010;16:614–621. doi: 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 13.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Van Durme DJ, Krischer JP. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90:1746–1754. doi: 10.2105/AJPH.90.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diggs JC, Xu F, Diaz M, Cooper GS, Koroukian SM. Failure to screen: predictors and burden of emergency colorectal cancer resection. Am J Manag Care. 2007;13:157–164. [PubMed] [Google Scholar]
- 15.Kelz RR, Gimotty PA, Polsky D, Norman S, Fraker D, DeMichele A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004;101:2187–2194. doi: 10.1002/cncr.20624. [DOI] [PubMed] [Google Scholar]
- 16.Charles RJ, Cooper GS, Wong RC, Sivak MV, Jr, Chak A. Effectiveness of open-access endoscopy in routine primary-care practice. Gastrointest Endosc. 2003;57:183–186. doi: 10.1067/mge.2003.55. [DOI] [PubMed] [Google Scholar]
- 17.Eisen GM, Baron TH, Dominitz JA, et al. Open access endoscopy. Gastrointest Endosc. 2002;56:793–795. doi: 10.1016/S0016-5107(02)70349-0. [DOI] [PubMed] [Google Scholar]
- 18.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21:1614–1617. doi: 10.1158/1055-9965.EPI-12-0982. [DOI] [PubMed] [Google Scholar]
- 19.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82:216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Roth D, Cohen L, Frieden TR. Take care New York: third year progress report [Internet] New York: New York City Department of Health and Mental Hygiene; 2007. [cited 2007 Aug 1]. Available from: http://www.nyc.gov/html/doh/downloads/pdf/tcny/tcny-report-2007.pdf. [Google Scholar]
- 23.Grassini M, Verna C, Niola P, Navino M, Battaglia E, Bassotti G. Appropriateness of colonoscopy: diagnostic yield and safety in guidelines. World J Gastroenterol. 2007;13:1816–1819. doi: 10.3748/wjg.v13.i12.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KK, Jandorf L, Thélèmaque L, Itzkowitz SH. Colorectal neoplasia detection among black and Latino individuals undergoing screening colonoscopy: a prospective cohort study. Gastrointest Endosc. 2014;79:466–472. doi: 10.1016/j.gie.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanna B, Schori M, Azeez S, Kumar S, Soni A. Colorectal tumors within an urban minority population in New York City. J Gen Intern Med. 2007;22:835–840. doi: 10.1007/s11606-007-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebwohl B, Capiak K, Neugut AI, Kastrinos F. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Aliment Pharmacol Ther. 2012;35:1467–1473. doi: 10.1111/j.1365-2036.2012.05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rex DK, Khan AM, Shah P, Newton J, Cummings OW. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc. 2000;51:524–527. doi: 10.1016/S0016-5107(00)70283-5. [DOI] [PubMed] [Google Scholar]
- 28.Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99:723–728. [PMC free article] [PubMed] [Google Scholar]
- 29.Nouraie M, Hosseinkhah F, Brim H, Zamanifekri B, Smoot DT, Ashktorab H. Clinicopathological features of colon polyps from African-Americans. Dig Dis Sci. 2010;55:1442–1449. doi: 10.1007/s10620-010-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thoma MN, Jimenez Cantisano BG, Hernandez AV, Perez A, Castro F. Comparison of adenoma detection rate in Hispanics and whites undergoing first screening colonoscopy: a retrospective chart review. Gastrointest Endosc. 2013;77:430–435. doi: 10.1016/j.gie.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Lee B, Holub J, Peters D, Lieberman D. Prevalence of colon polyps detected by colonoscopy screening of asymptomatic Hispanic patients. Dig Dis Sci. 2012;57:481–488. doi: 10.1007/s10620-011-1898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaib YH, Rabaa E, Qaseem T. The site distribution and characteristics of colorectal adenomas in Hispanics: a comparative study. Am J Gastroenterol. 2002;97:2100–2102. doi: 10.1111/j.1572-0241.2002.05849.x. [DOI] [PubMed] [Google Scholar]
- 33.Zheng XE, Li T, Lipka S, et al. Location-dependent ethnic differences in the risk of colorectal adenoma: a retrospective multiethnic study. J Clin Gastroenterol. 2014;48:e1–e7. doi: 10.1097/MCG.0b013e3182834989. [DOI] [PubMed] [Google Scholar]
- 34.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc. 2011;74:135–140. doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 36.Jandorf L, Cooperman JL, Stossel LM, et al. Implementation of culturally targeted patient navigation system for screening colonoscopy in a direct referral system. Health Educ Res. 2013;28:803–815. doi: 10.1093/her/cyt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jandorf L, Stossel LM, Cooperman JL, et al. Cost analysis of a patient navigation system to increase screening colonoscopy adherence among urban minorities. Cancer. 2013;119:612–620. doi: 10.1002/cncr.27759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nash D, Azeez S, Vlahov D, Schori M. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83:231–243. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers RE, Hyslop T, Sifri R, et al. Tailored navigation in colorectal cancer screening. Med Care. 2008;46(9 Suppl 1):S123–S131. doi: 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]
- 40.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24:211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebwohl B, Neugut AI, Stavsky E, et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011;45:e47–e53. doi: 10.1097/MCG.0b013e3181f595c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171:906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 43.Richards CA, Kerker BD, Thorpe L, et al. Increased screening colonoscopy rates and reduced racial disparities in the New York Citywide campaign: an urban model. Am J Gastroenterol. 2011;106:1880–1886. doi: 10.1038/ajg.2011.191. [DOI] [PubMed] [Google Scholar]
- 44.Elmunzer BJ, O’Connell MT, Prendes S, et al. Improving access to colorectal cancer screening through medical philanthropy: feasibility of a flexible sigmoidoscopy health fair for uninsured patients. Am J Gastroenterol. 2011;106:1741–1746. doi: 10.1038/ajg.2011.147. [DOI] [PubMed] [Google Scholar]
- 45.Dimase J, Pressman A, Asser S. Screening colonoscopy in the underserved population: report of the first year of a no-cost colorectal cancer screening program for the underserved in Rhode Island. Am J Gastroenterol. 2011;106:1193–1195. doi: 10.1038/ajg.2011.4. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106:dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]