Abstract
Purpose
Little is known about the impact of alcohol consumption on warfarin safety, or whether demographic, clinical, or genetic factors modify risk of adverse events. We conducted a case–control study to assess the association between screening positive for moderate/severe alcohol misuse and the risk of major bleeding in a community sample of patients using warfarin.
Methods
The study sample consisted of 570 adult patients continuously enrolled in Group Heath for at least 2 years and receiving warfarin. The main outcome was major bleeding validated through medical record review. Cases experienced major bleeding, and controls did not experience major bleeding. Exposures were Alcohol Use Disorders Identification Test Consumption Questionnaire (AUDIT-C) scores and report of heavy episodic drinking (≥5 drinks on an occasion). The odds of major bleeding were estimated with multivariate logistic regression models. The overall sample was 55% male, 94% Caucasian, and had a mean age of 70 years.
Results
Among 265 cases and 305 controls, AUDIT-C scores indicative of moderate/severe alcohol misuse and heavy episodic drinking were associated with increased risk of major bleeding (OR = 2.10, 95% CI = 1.08–4.07; and OR = 2.36, 95% CI = 1.24–4.50, respectively). Stratified analyses demonstrated increased alcohol-related major bleeding risk in patients on warfarin for ≥1 year and in those with a low-dose genotype (CYP2C9*2/*3, VKORC1(1173G>A), CYP4F2*1), but not in other sub-groups evaluated.
Conclusions
Alcohol screening questionnaires, potentially coupled with genetic testing, could have clinical utility in selecting patients for warfarin therapy, as well as refining dosing and monitoring practices.
Keywords: warfarin, AUDIT-C, alcohol, CYP2C9, VKORC1, CYP4F2, major bleeding, pharmacoepidemiology
INTRODUCTION
Each year, more than 1 million U.S. patients are prescribed warfarin to reduce their risk of thrombotic events.1 Most experience the well-documented benefits of warfarin, but 3–6% of warfarin users experience major bleeding each year.2 Newer direct thrombin and factor Xa inhibitors are alternatives for some patients,3–9 but their use is limited because of a lack of experience in some patient subgroups, a lack of reversibility, and relatively high cost.10–12 Therefore, understanding risk factors for major bleeding on warfarin remains critical, so that clinicians can select appropriate patients for warfarin anticoagulation as well as mitigate preventable bleeding risks in patients prescribed warfarin.
Among the many risk factors for major bleeding on warfarin, alcohol use is among the strongest13–15 but least well studied in practice.16 For example, the American Heart Association (AHA) cautions patients taking warfarin to use alcohol infrequently and limit to one to two drinks, but specific evidence is not cited.17 Further, it is plausible that patients initiating chronic warfarin therapy may adhere to clinical recommendations about alcohol use, but later increase their level and/or frequency of alcohol consumption, and thus increase their risk of major bleeding. Last, alcohol use could have different implications for warfarin safety in patients with genetic variants that impact warfarin metabolism and the anticoagulation cascade, and therefore warfarin dose requirements.18–21 Specifically, patients with genotypes that confer lower warfarin dose requirements to achieve therapeutic anticoagulation levels might be at particularly high bleeding risk if genetic status is unknown, a standard warfarin dose is prescribed, and there is subsequent alcohol misuse. No studies, to our knowledge, have evaluated whether patients who screen positive for moderate/severe alcohol misuse or heavy episodic drinking are at increased risk of major bleeding when taking warfarin, and whether demographic and clinical characteristics, duration of anticoagulation, or genetic variants modify that risk.
The objective of this study was to evaluate the association between screening positive for alcohol misuse with the Alcohol Use Disorders Identification Test Consumption Questionnaire (AUDIT-C), a widely used validated alcohol screening instrument,22 and the risk of major bleeding in a community setting. We also evaluated the effect of modification by age group, obesity status, duration of warfarin use, and genetic variants of the cytochrome P450 enzymes (CYP2C9*2/*3 and CYP4F2*3) and vitamin K epoxide reductase complex (VKORC1 1173G>A), biochemical systems that have been demonstrated to influence warfarin outcomes.18,19,21 If such associations exist, it would suggest that routine alcohol screening, perhaps coupled with genetic testing, could have clinical utility in informing decisions about the safety of initiating warfarin, as well as dosing and monitoring practices.
METHODS
Overview
We conducted secondary analyses using data from an existing case–control study among patients receiving warfarin therapy. The parent study (Warfarin Investigation for Safety and Health, or WISH) was designed to evaluate the association between genetic variants that influence warfarin dose requirements and major bleeding risk.21 A detailed description of the parent study was previously reported.21
Setting and population
The study participants were selected from patients enrolled in Group Health (GH), a non-profit integrated health care system that covers approximately 620 000 patients in Washington and Idaho.23 GH maintains automated records of patient demographics, enrollment, inpatient/outpatient encounters, laboratory results, and pharmacy dispensing. A unique enrollee number links all records.
We determined warfarin use from automated pharmacy records. A prior study demonstrated excellent warfarin use agreement between GH automated pharmacy data and medical charts.24 Additionally, results from several studies show that enrollees obtain about 97% of their medications at GH owned or contracted pharmacies.23,25 We calculated warfarin treatment duration based on the “current episode” of warfarin use, defined as continuous supply of warfarin with no more than 90 gap days.
Case and control selection
We used GH automated encounter data to identify patients with major bleeding events (cases) between 1 January 2005 and 1 April 2011. To be considered a “case”, patients’ bleeding events had to occur within their warfarin supply period, and they had to meet the following criteria at the index (bleeding) date: age ≥18 years, continuously enrolled for 2+ years prior to index date, and no record of major bleeding in the year prior to the index date. We identified potential major bleeding events using an established ICD-9 algorithm26 and validated events through medical record review.21 Events were classified as “major bleeding” if they were clinically overt and resulted in hospitalization, hemoglobin dropped >2 mg/dl, and/or >2 units of packed red blood cells were transfused.26,27 This process identified 702 cases (71% identified through hospitalization), and all were approached to participate. We also classified bleeding events as occurring in the gastrointestinal system or “other” location (See Online Supplement).
To identify a comparable control group, we randomly selected one control for each case from among 27 928 patients with ≥1 outpatient pharmacy fills for warfarin and no major bleeding in the year prior to a matched index date. We used a risk set sampling approach in which we randomly assigned controls meeting all eligibility criteria above (except major bleeding) an index date and then selected those who were using warfarin at the time of each case’s major bleeding event.28
Survey
We approached potential study participants with a mailed invitation letter. After obtaining consent, trained staff from the GH survey program administered a 44-item telephone survey to participants to document clinical, demographic, and behavioral data elements not available in automated databases (including alcohol screening questions).21,29 The survey specifically asked about the 1-year period prior to the index date. Following the survey, participants were mailed buccal swabs for genotyping.21
Of 702 cases and 702 controls approached, 265 (38.0%) and 305 (43.9%) participated-response rates similar to that of prior studies involving elderly patients with multiple comorbid conditions.30,31 Participants and non-participants did not differ significantly by age (p = 0.98), gender (p = 0.82), duration of GH enrollment (p = 0.93), or Charlson comorbidity index (p = 0.88).32
Alcohol misuse screening instruments
We adapted the AUDIT-C for use in this study (Online Supplement, eTable 1). The AUDIT-C was validated as a screen for alcohol misuse in the VA and non-VA primary care settings29,33,34 and is now in wide-spread use.22,35–38 We calculated AUDIT-C scores in the typical manner with each question scored 0–4 points, with total AUDIT-C scores (0–12 points) being the sum of the individual items. We evaluated moderate/severe alcohol misuse (AUDIT-C scores of 5–7 and 8–12, respectively), because scores of 5–12 are associated with upper gastrointestinal bleeding and other complications, and AUDIT-C scores ≤4 can be obtained by drinking within AHA guidelines and are not associated with poor warfarin out-comes.16,17 Because of the limited number of patients who screened positive for severe misuse (n = 12), we analyzed a dichotomous AUDIT-C measure of alcohol misuse (no/mild or moderate/severe).
We also considered “heavy episodic drinking” as a second measure of alcohol misuse, defined as patients who reported ≥ 5 drinks on an occasion on AUDIT-C question #3. This is one of the several validated single-item alcohol screens that ask about heavy episodic drinking.29,33,34,39–42
Genotyping
Buccal swabs (Epicentre Catch-All Mailer, Madison, WI) were used to collect DNA for evaluation of three genetic variants: CYP2C9*2/*3, VKORC1 1173G>A, and CYP4F2*3. These polymorphisms were selected because of their noted impact on warfarin dose requirements and bleeding risk.18,19,43 Consistent with prior studies, for each genetic variant, heterozygous and homozygous patients were combined in a single “variant” category because of the small number of homozygous patients.18,21,44–48
Clinical, demographic, and behavioral covariates
We obtained the clinical/demographic data elements shown in Table 1 from GH automated databases and the study survey.
Table 1.
Clinical and demographic covariates by case and control status in the study sample of warfarin therapy patients. See online supplement eTables 2 and 3 for covariate comparisons by AUDIT-C score and heavy episodic drinking status
| Variable | Cases (n = 265) | Controls (n = 305) | p-Value |
|---|---|---|---|
| Automated data variables | |||
| Age in years at index date, mean (SD) | 71.1 (12.7) | 69.5 (11.2) | 0.92 |
| Duration of plan Enrollment at index date (years), mean (SD) n (column %) |
15.8 (6.0) | 14.8 (6.3) | 0.91 |
| Male | 134 (50.6%) | 176 (57.7%) | 0.09 |
| Body mass index (BMI) | 0.08 | ||
| Underweight (<18.5) | 1 (0.3%) | 1 (0.3%) | |
| Normal weight (18.5–24.9) | 48 (18.1%) | 42 (13.8%) | |
| Over weight (25.0–29.9) | 91 (34.3%) | 85 (27.9%) | |
| Obese (>30.0) | 125 (47.2%) | 177 (58.0%) | |
| Missing | 0 | 0 | |
| Duration of warfarin Therapy at index date | <0.01 | ||
| <6 months | 69 (26.0%) | 54 (17.7%) | |
| 6 months to 1 year | 26 (9.8%) | 24 (7.9%) | |
| >1 year | 170 (64.2%) | 227 (74.4%) | |
| Missing | 0 | 0 | |
| Comorbidities | |||
| Cancer | 8 (3.0%) | 14 (4.6%) | 0.32 |
| Diabetes | 58 (21.9%) | 81 (26.6%) | 0.19 |
| Hypertension | 197 (74.3%) | 195 (63.9%) | <0.01 |
| Congestive heart failure | 101 (38.1%) | 68 (22.3%) | <0.01 |
| Charlson comorbidity index, mean (SD) | 0.07 | ||
| Score = 0 | 84 (31.8%) | 124 (40.8%) | |
| Score = 1 | 70 (26.4%) | 76 (25.0%) | |
| Score = 2+ | 110 (41.7%) | 104 (34.2%) | |
| Missing | 1 | 1 | |
| self-report survey variables n (column %) |
|||
| Diagnoses associated with warfarin use | |||
| Atrial fibrillation | 89 (33.6%) | 136 (44.6%) | <0.01 |
| DVT | 31 (11.7%) | 42 (13.8%) | 0.45 |
| PE | 27 (10.2%) | 26 (8.5%) | 0.49 |
| Stroke | 27 (10.2%) | 20 (6.6%) | 0.12 |
| Heart valve replacement | 54 (20.4%) | 32 (10.5%) | 0.01 |
| Myocardial infarction | 13 (4.9%) | 17 (5.6%) | 0.71 |
| Joint replacement | 12 (4.5%) | 12 (3.9%) | 0.72 |
| CABG | 6 (2.3%) | 11 (3.6%) | 0.36 |
| Other | 17 (6.4%) | 23 (7.5%) | 0.61 |
| Race | 0.45 | ||
| American Indian or Alaska Native | 3 (1.2%) | 1 (0.3%) | |
| Asian | 3 (1.2%) | 6 (2.0%) | |
| Black/African American | 7 (2.7%) | 5 (1.6%) | |
| White/Caucasian | 243 (93.5%) | 287 (94.4%) | |
| Other | 4 (1.5%) | 2 (0.7%) | |
| Missing | 5 | 4 | |
| Care setting* | 0.12 | ||
| Anticoagulation clinic | 115 (46.4%) | 166 (55.1%) | |
| Primary care | 86 (34.7%) | 91 (30.2%) | |
| Cardiologist | 20 (8.1%) | 24 (8.0%) | |
| Other | 27 (10.9%) | 20 (6.6%) | |
| Missing | 17 | 4 | |
| Concomitant OTC Medications* | |||
| NSAID | 22 (10.2%) | 13 (4.3%) | 0.07 |
| Acetaminophen | 74 (32.6%) | 85 (28.1%) | 0.24 |
| Aspirin | 70 (32.6%) | 63 (21.1%) | <0.01 |
| Vitamin E | 49 (23.4%) | 47 (15.9%) | 0.02 |
| Annual household income* | 0.10 | ||
| <$25 000 | 40 (17.1%) | 46 (16.1%) | |
| $25 000–$49 999 | 102 (43.6%) | 96 (33.7%) | |
| $50 000–$74 999 | 45 (18.4%) | 79 (27.7%) | |
| $75 000–$99 999 | 23 (9.8%) | 33 (11.6%) | |
| ≥$100 000 | 24 (10.3%) | 31 (10.9%) | |
| Missing | 31 | 20 | |
| Alcohol drinking groups based on AUDIT-C scores* | 0.55 | ||
| Nondrinkers (AUDIT-C 0) | 89 (34.6%) | 119 (39.9%) | |
| Low level drinking (AUDIT-C 1–2 women, 1–3 men) | 103 (40.1%) | 108 (36.2%) | |
| Mild alcohol misuse (AUDIT-C 3–4 women, 4 men) | 47 (15.8%) | ||
| Moderate alcohol misuse (AUDIT-C 5–7 men and women) | 37 (14.4%) | 19 (6.4%) | |
| Severe alcohol misuse (AUDIT-C 8–12 men and women) | 21 (8.2%) 7 (2.7%) | 5 (1.7%) | |
| Missing | 8 | 7 | |
| Heavy episodic drinking based on AUDIT-C Question #3* | 0.02 | ||
| No heavy episodic drinking | 221 (83.4%) | 272 (89.2%) | |
| Heavy episodic drinking | 42 (16.6%) | 29 (10.8%) | |
| Missing | 2 | 4 |
Self-report items asked about in the one-year time period prior to the index date.
Bold p-values indicate statistically significant differences between cases and controls at α = 0.05.
Statistical analysis
We compared characteristics of cases and controls using chi-square tests, Fisher exact test for categorical variables, and the Student t-test for continuous variables. In addition, characteristics of patients with AUDIT-C ≥5 and <5 were compared, as well as patients with and without self-reported heavy episodic drinking (Online Supplement, eTables 3 and 4).
We used logistic regression to estimate the risk of major bleeding among warfarin users with moderate/severe alcohol misuse on the AUDIT-C compared to those with no/mild alcohol misuse, and heavy episodic drinking compared to no reported heavy episodic drinking, during the year prior to index date. Results are expressed as unadjusted and multivariate odds ratios (OR) with respective 95% confidence intervals (CI).
All demographic and clinical characteristics were considered for inclusion in the multivariate analyses. Pre-specified confounders were age, gender, race/ethnicity, duration of warfarin therapy, and household income, in accordance with prior reports of risk factors for alcohol misuse and major bleeding.49 We evaluated additional potential confounders hypothesized to be associated with alcohol misuse and major bleeding and variables associated with case status that might impact precision (Table 1). We only retained covariates that resulted in a >5% change in regression coefficients. Our final multivariate logistic regression model included the covariates provided in Table 2.50–52
Table 2.
Association between screening positive for moderate/severe alcohol misuse, or heavy episodic drinking and the risk of major bleeding, among warfarin therapy patients
| Alcohol use definition | Odds ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|
| All major bleeds (N = 265) |
Gastrointestinal major bleeds (N = 117) |
Other major bleeds (N = 148) |
||||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| Moderate/severe alcohol misuse by AUDIT-C score^ |
1.39 (0.83–2.32) | 2.10 (1.08–4.07) | 1.26 (0.67–2.35) | 1.80 (0.78–4.16) | 1.37 (0.75–2.50) | 1.94 (0.86–4.38) |
| Heavy episodic drinking# | 1.78 (1.08–2.95) | 2.36 (1.24–4.50) | 1.67 (0.92–3.06) | 3.24 (1.44–7.33) | 1.73 (0.96–3.10) | 1.73 (0.77–3.86) |
AUDIT-C score of ≥5 versus those with AUDIT-C score <5.
Report of 5+ drinks in one occasion on AUDIT-C item #3.
Multivariate model adjusted for: genetic status, race (white/other), duration of therapy (continuous), age (continuous), gender, warfarin indication (atrial fibrillation, deep vein thrombosis, pulmonary embolism, stroke, other clotting event, heart valve replacement, myocardial infarction, joint replacement, CABG), regular acetaminophen use, regular NSAID use, care setting (anticoagulation clinic/other setting), and annual household income (<$25 000, $25 000–$49 999, $50 000–$74 999, $75 000–$99 999, and $100 000+).
In stratified analyses, we assessed the association between both AUDIT-C score and heavy episodic drinking and major bleeding by: age, obesity, duration of warfarin treatment, and genetic status. Additionally, we assessed the association between AUDIT-C score and heavy episodic drinking stratified by major bleeding site (gastrointestinal/other).
Although INR is in the causal pathway between alcohol misuse and risk of major bleeding, and thus adjustment is not indicated, several prior warfarin major bleeding studies have adjusted for INR in statistical analyses.19,45 To allow comparison with these studies, we conducted INR-adjusted analyses among the subset of patients with INRs testing in the 30 days preceding the index date (Online Supplement, eTable 5).
We performed all analyses in STATA, version 12.0 (STATA Inc., Austin, TX).
RESULTS
There were significant differences between cases and controls in duration of warfarin therapy, diagnosis of hypertension and congestive heart failure, use of warfarin for atrial fibrillation and heart valve replacement, and self-reported regular aspirin and vitamin E use (Table 1). Cases and controls did not differ in AUDIT-C score results, but cases were more likely to report heavy episodic drinking (Table 1).
Association between alcohol misuse and major bleeding
Patients who screened positive for moderate/severe alcohol misuse or heavy episodic drinking had approximately two-fold increased odds of major bleeding in adjusted analyses (Table 2). Sub-group analyses demonstrated that the moderate/severe alcohol misuse bleeding association was similar for gastrointestinal and other bleeding, while the heavy episodic drinking association was stronger for gastrointestinal bleeding (Table 2).
The associations for moderate/severe alcohol misuse and heavy episodic drinking were similar in multivariate sub-group analysis adjusting for INR (AUDIT-C: OR = 3.13, 95% CI = 1.28–7.68; heavy episodic drinking: OR = 2.21, 95% CI = 0.96–5.10).
Modification of the alcohol-related risk of major bleeding
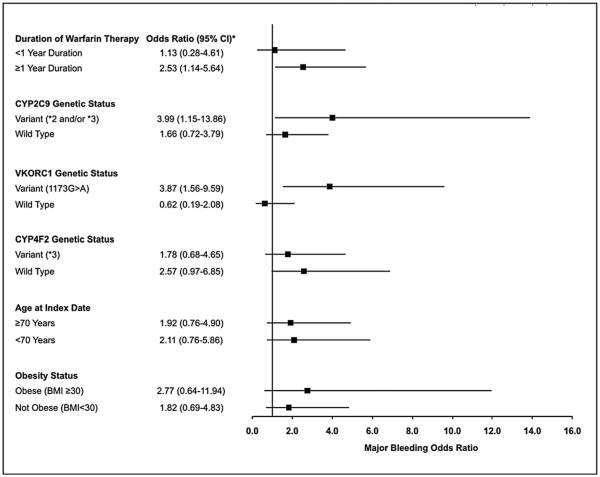
In stratified analyses, the association between moderate/severe alcohol misuse and major bleeding risk was significant in patients on warfarin for ≥1 year, and those with CYP2C9*2/*3 and VKORC1(173G>A) genetic variants, but not those in other sub-groups evaluated (Figure 1). A test of multiplicative interaction was statistically significant for VKORC1 genetic status (p = 0.04), but interactions were not significant for duration of warfarin use (p = 0.52) or CYP2C9 genetic status (p = 0.39).
Figure 1.
Odds of major bleeding associated with moderate/severe alcohol misuse among warfarin therapy patients
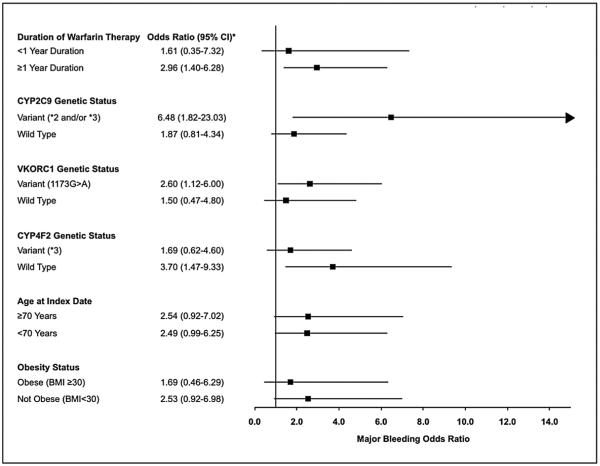
For heavy episodic drinking and major bleeding risk, associations were significant for patients on warfarin for ≥1 year, those with CYP2C9*2/*3 and VKORC1(1173G>A) variants, and CYP4F2 wild type patients, but not those in other sub-groups (Figure 2). However, all tests of multiplicative interaction with heavy episodic drinking were non-significant (duration of warfarin use, p = 0.35; CYP2C9 status, p = 0.70; VKORC1 status, p = 0.64; and CYP4F2 status, p = 0.43).
Figure 2.
Odds of major bleeding associated with heavy episodic drinking among warfarin therapy patients
DISCUSSION
We investigated the association between results on commonly used alcohol screening questionnaires, the AUDIT-C and a single item alcohol screen about heavy episodic drinking, and the risk of major bleeding in a community sample of warfarin patients. We found that patients who screened positive for moderate/severe alcohol misuse or reported heavy episodic drinking were at increased risk for major bleeding. Moreover, the risk with alcohol use was particularly high among patients that received warfarin for more than 1 year and those with genetic variants involved in warfarin metabolism and the anticoagulation cascade.18,45,53,54
This study has important implications for clinical care. Our findings that patients with alcohol misuse may be at increased risk of major bleeding should be taken into consideration when discussing the benefit–risk tradeoffs of warfarin therapy and particularly in patients with known alcohol misuse that are unwilling to reduce alcohol intake. Repeated discussions are likely necessary to remind warfarin patients about alcohol use as a risk factor for major bleeding and support patients that are attempting to decrease consumption. Those who continue to drink should be advised to do so at a low level consistent with AHA recommendations.55
The study also has important implications for improving medication safety. Warfarin had a black box warning about concomitant use of alcohol, but that warning was removed, potentially because of lack of evidence to support the risk of alcohol–warfarin interactions. This study suggests that patients should be warned about alcohol-related warfarin risks. Also, despite widespread alcohol use, remarkably little is known about risks because of drug–alcohol interactions in practice. This study suggests that widespread alcohol screening based on the U.S. Preventive Services Task Force recommendations22,35 will provide an opportunity for improved understanding of alcohol-related medication risks. There is tremendous opportunity to integrate alcohol misuse screening information into electronic systems and to leverage that information to assess and mitigate the risk of drug–alcohol interactions at the point of care.
This is the first study to our knowledge to evaluate the association between common alcohol screening instruments and major bleeding risk in a community sample of warfarin patients. A single previous study of veteran patients on warfarin assessed whether screening positive for any (mild) alcohol misuse on AUDIT-C (≥3 points women or 4 points men) was associated with anticoagulation control or bleeding and found no association.16 Our findings for the association between screening positive for moderate/severe alcohol misuse and major bleeding are consistent with a variety of established behavioral and biologic effects. First, alcohol misuse is associated with decreased medication adherence,56,57 potentially leading to warfarin dose escalation, and resulting in over-anticoagulation. Additionally, alcohol can alter plasma protein binding of drugs and thus could alter the available free fraction of warfarin, increasing anticoagulant effects.58 Through these mechanisms, warfarin patients with alcohol misuse could be put at increased risk of major bleeding.16
Our sub-group analysis findings of significant major bleeding risk among patients receiving warfarin for 1 or more years, and among patients with low-dose genotypes, are also important. Patients taking warfarin for longer durations could be at greater risk for alcohol-associated major bleeding for several reasons. First, patients who recently began warfarin might be more likely to recall advice about limiting alcohol use, making them more likely to underreport alcohol consumption than those on warfarin for longer. Alternatively, patients who have decreased alcohol use when initiating warfarin therapy might slowly increase the amount they drink over time while under-reporting alcohol consumption on the AUDIT-C. Additionally, CYP2C9 and VKORC1 variants and CYP4F2 wild-type patients misusing alcohol could be at increased risk of major bleeding because of complex interactions between biological and psychosocial factors. For example, patients with reduced warfarin clearance and lower hepatic VKOR content may be more sensitive to the biochemical changes imposed by alcohol than in patients without this genetic variant who exhibit faster hepatic metabolism of warfarin.
This study has a number of limitations. First, all case–control studies can be limited by systematic differences between cases and controls. However, we minimized potential bias by randomly sampling controls from the same population who were using warfarin on the same dates as cases’ major bleeding events, and adjusting for an extensive list of potential confounders. Similarly, the generalizability of observational studies can be limited if the characteristics of study participants systematically differ from those of the general patient population under study. This concern is mitigated by our findings that participants and non-participants were very similar in the key attributes of age, gender, duration of health plan enrollment, and Charlson comorbidity index score. Additionally, response bias would likely move the odds ratios toward the null, as heavier drinkers are less likely to respond to surveys about alcohol use.59 It should also be noted that this study was conducted in a single regional health care system and evaluated alcohol screening results collected for research and not placed in patients’ medical records, potentially limiting generalizability. Finally, our stratified analysis findings should be interpreted with caution given that they were exploratory analyses and had limited statistical power. Further research is needed to verify these results in other populations and settings where the results of alcohol screening are available in patients’ medical records.
CONCLUSIONS
This is the first study to assess whether validated screens for moderate/severe alcohol misuse and heavy episodic drinking are associated with major bleeding on warfarin verified by chart review. We identified novel associations between alcohol misuse and the risk of major bleeding in warfarin patients monitored in a community setting. Risks may be highest in patients on long-term warfarin and those with CYP2C9 and/or VKORC1 genetic variants, although these findings deserve confirmation in future studies. Clinicians should consider routine use of brief alcohol misuse screening questionnaires to inform selection of patients for warfarin and warfarin monitoring, and further research on alcohol–pharmacogenomic interactions in major bleeding outcomes among warfarin users is warranted.
Supplementary Material
KEY POINTS.
Little is known about the impact of alcohol consumption on warfarin safety.
We conducted the first study to assess the association between moderate/severe alcohol misuse and major bleeding risk on warfarin, and interactions with key demographic, clinical, and genetic factors.
We demonstrate a strong association between alcohol misuse and major bleeding risk.
Our findings have important implications for selecting patients for warfarin therapy, as well as refining dosing and monitoring practices.
ACKNOWLEDGEMENTS
This study was supported by grants from the NIGMS (1UO1GM092676-01), NIEHS (P30ES007033), and AHRQ (1K12HS022982-01). The authors thank Annika Hanson for her technical assistance on this manuscript.
Footnotes
No portion of this manuscript has been submitted for publication nor has it been published in whole or in part elsewhere. This study was presented at the International Conference on Pharmacoepidemiology on October 26, 2014.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The Group Health Human Subjects institutional review board approved this study.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web site.
REFERENCES
- 1.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5(5):615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Walraven C, Oake N, Wells PS, Forster AJ. Burden of potentially avoidable anticoagulant-associated hemorrhagic and thromboembolic events in the elderly. Chest. 2007;131(5):1508–1515. doi: 10.1378/chest.06-2628. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Diener HC, Frison L, et al. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293(6):690–698. doi: 10.1001/jama.293.6.690. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Investigators S. Stroke prevention in atrial fibrillation: pooled analysis of SPORTIF III and V trials. Am J Manag Care. 2004;10(14 Suppl):S462–469. discussion S469-473. [PubMed] [Google Scholar]
- 5.Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med. 2006;166(8):853–859. doi: 10.1001/archinte.166.8.853. [DOI] [PubMed] [Google Scholar]
- 6.Olsson SB. Executive Steering Committee of the SIIII. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362(9397):1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 9.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 10.Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, alcohol misuse screening and major bleeding in warfarin therapy apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453–460. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 11.Cove CL, Hylek EM. An updated review of target-specific oral anticoagulants used in stroke prevention in atrial fibrillation, venous thromboembolic disease, and acute coronary syndromes. JAHA. 2013;2(5):e000136. doi: 10.1161/JAHA.113.000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Caterina R, Husted S, Wallentin L, et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease position paper. J Am Coll Cardiol. 2012;59(16):1413–1425. doi: 10.1016/j.jacc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest. 2006;130(5):1390–1396. doi: 10.1378/chest.130.5.1390. [DOI] [PubMed] [Google Scholar]
- 14.Berwaerts J, Webster J. Analysis of risk factors involved in oral-anticoagulant-related intracranial haemorrhages. QJM: monthly journal of the Association of Physicians. 2000;93(8):513–521. doi: 10.1093/qjmed/93.8.513. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Banerjee A, Lagrenade I, Lane DA, Taillandier S, Fauchier L. Assessing the risk of bleeding in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation project. Circ Arrhythm Electrophysiol. 2012;5(5):941–948. doi: 10.1161/CIRCEP.112.972869. [DOI] [PubMed] [Google Scholar]
- 16.Efird LM, Miller DR, Ash AS, et al. Identifying the risks of anticoagulation in patients with substance abuse. J Gen Intern Med. 2013;28(10):1333–1339. doi: 10.1007/s11606-013-2453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107(12):1692–1711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 18.Higashi MK. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287(13):1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 19.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African– American and European–American patients on warfarin. Clin Pharmacol Ther. 2008;83(2):312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol. 2009;75(6):1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth JA, Boudreau D, Fujii MM, et al. Genetic risk factors for major bleeding in warfarin patients in a community setting. Clin Pharmacol Ther. 2014 Jun;95(6):636–643. doi: 10.1038/clpt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157(9):645–654. doi: 10.7326/0003-4819-157-9-201211060-00544. [DOI] [PubMed] [Google Scholar]
- 23.Saunders KWDR, Stergachis A. Pharmacoepidemiology. John Wiley and Sons; West Sussex, England: 2005. [Google Scholar]
- 24.Garg RK, Glazer NL, Wiggins KL, et al. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiol Drug Saf. 2011;20(3):313–316. doi: 10.1002/pds.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38(7–8):1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 26.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 27.White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep-venous thrombosis. Am J Med. 1999;107(5):414–424. doi: 10.1016/s0002-9343(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 28.Langholz B, Goldstein L. Risk set sampling in epidemiologic cohort studies. Stat Sci. 1996;11(1):35–53. [Google Scholar]
- 29.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 30.Ekdahl AW, Andersson L, Wirehn AB, Friedrichsen M. Are elderly people with co-morbidities involved adequately in medical decision making when hospitalised? A cross-sectional survey. BMC Geriatr. 2011;11:46. doi: 10.1186/1471-2318-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diokno AC, Brown MB, Herzog A. Sexual function in the elderly. Arch Intern Med. 1990;150(1):197–200. [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 34.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 35.Moyer VA. Preventive Services Task F. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159(3):210–218. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- 36.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 37.Rose HL, Miller PM, Nemeth LS, et al. Alcohol screening and brief counseling in a primary care hypertensive population: a quality improvement intervention. Addiction. 2008;103(8):1271–1280. doi: 10.1111/j.1360-0443.2008.02199.x. [DOI] [PubMed] [Google Scholar]
- 38.Group Health Cooperative Unhealthy Drinking Screening & Intervention Guideline: Adults. Part 1: Screening and Brief Interventions. 2013 https://http://www.ghc.org/all-sites/guidelines/alcohol-adult.pdf. Accessed Aug 13, 2013.
- 39.Taj N, Devera-Sales A, Vinson DC. Screening for problem drinking: does a single question work? J Fam Pract. 1998;46(4):328–335. [PubMed] [Google Scholar]
- 40.Williams R, Vinson DC. Validation of a single screening question for problem drinking. J Fam Pract. 2001;50(4):307–312. [PubMed] [Google Scholar]
- 41.Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2013;37(Suppl 1):E253–E259. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24(7):783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111(8):4106–4112. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limdi NA, Beasley TM, Crowley MR, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African–Americans and European–Americans. Pharmacogenomics. 2008;9(10):1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Limdi NA, Wiener H, Goldstein JA, Acton RT, Beasley TM. Influence of CYP2C9 and VKORC1 on warfarin response during initiation of therapy. Blood Cells Mol Dis. 2009;43(1):119–128. doi: 10.1016/j.bcmd.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limdi NA, Veenstra DL. Expectations, validity, and reality in pharmacogenetics. J Clin Epidemiol. 2010;63(9):960–969. doi: 10.1016/j.jclinepi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lund K, Gaffney D, Spooner R, Etherington AM, Tansey P, Tait RC. Polymorphisms in VKORC1 have more impact than CYP2C9 polymorphisms on early warfarin International Normalized Ratio control and bleeding rates. Br J Haematol. 2012;158(2):256–261. doi: 10.1111/j.1365-2141.2012.09150.x. [DOI] [PubMed] [Google Scholar]
- 48.Bejarano-Achache I, Levy L, Mlynarsky L, Bialer M, Muszkat M, Caraco Y. Effects of CYP4F2 polymorphism on response to warfarin during induction phase: a prospective, open-label, observational cohort study. Clin Ther. 2012;34(4):811–823. doi: 10.1016/j.clinthera.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Dawson DA, Archer LD. Relative frequency of heavy drinking and the risk of alcohol dependence. Addiction. 1993;88(11):1509–1518. doi: 10.1111/j.1360-0443.1993.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 50.Palareti G, Cosmi B. Bleeding with anticoagulation therapy—who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102(2):268–278. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 51.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GYH. Performance of the HEM-ORR2HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60(9):861–867. doi: 10.1016/j.jacc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Margaglione M, Colaizzo D, D’Andrea G, et al. Genetic modulation of oral anticoagulation with warfarin. Thromb Haemost. 2000;84(5):775–778. [PubMed] [Google Scholar]
- 54.Aithal GP, Day CP, Kesteven PJL, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353(9154):717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 55.Fiumara K, Goldhaber SZ. Cardiology patient pages. A patient’s guide to taking coumadin/warfarin. Circulation. 2009;119(8):e220–e222. doi: 10.1161/CIRCULATIONAHA.108.803957. [DOI] [PubMed] [Google Scholar]
- 56.Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795–804. doi: 10.7326/0003-4819-149-11-200812020-00004. [DOI] [PubMed] [Google Scholar]
- 57.Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose–response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 58.Havrda DE, Mai T, Chonlahan J. Enhanced antithrombotic effect of warfarin associated with low-dose alcohol consumption. Pharmacotherapy. 2005;25(2):303–307. doi: 10.1592/phco.25.2.303.56955. [DOI] [PubMed] [Google Scholar]
- 59.Bradley KA, Bush KR, McDonell MB, Malone T, Fihn SD. Screening for problem drinking: comparison of CAGE and AUDIT. J Gen Intern Med. 1998;13(6):379–388. doi: 10.1046/j.1525-1497.1998.00118.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.