Abstract
Background
A construct calculated as the sum of items 13, 14, 15, 29, 30 of the Unified Parkinson’s Disease Rating Scale (UPDRS) has been used as an “Ambulatory Capacity Measure” (ACM in Parkinson disease (PD). Its construct validity has never been examined. A similar construct, consisting of the mean value of the same UPDRS items has been used under the acronym PIGD as a measure of postural instability and gait disorder in PD.
Objective
To examine the construct validity of the ACM and PIGD in PD.
Methods
We analyzed data in an existing database of 340 PD patients, Hoehn and Yahr stages (HYS) 1–5 who participated in a study of falls. Number of falls (NOF) was recorded over 4 weeks, and UPDRS (mental, ADL, and motor subscales), HYS, Activities Based Confidence Scale (ABC), Freezing of Gait Questionnaire (FOG), Five Times Sit-to-Stand (FTSS), Timed Up-and Go (TUG), Gait Velocity (GV), and Berg Balance Scale (BBS) evaluations were performed. Internal consistency was assessed by Cronbach’s alpha. Construct validity was assessed through correlations of the ACM and PIGD to these measures and to their summed-ranks. A coefficient of determination was calculated through linear regression.
Results
Mean age was 71.4, mean age at diagnosis 61.4 years; 46% were women; mean UPDRS subscale scores were: mental 3.7; ADL 15.7; motor: 27.1; mean ACM was 6.51, and mean PIGD 1.30. Cronbach’s alpha was 0.78 for both ACM and PIGD. Spearman correlation coefficients between the ACM/PIGD and ABC, FOG, TUG, GV and BBS were 0.69, 0.72, 0.67, 0.58, and 0.70 respectively. Correlation between the ACM/PIGD and summed-ranks of HYS, NOF, ABC, FOG, FTSS, TUG, GV and BBS was high (Spearman r=0.823, p <0.0001); 68% of the variability in the summed-ranks was explained by ACM/PIGD.
Conclusion
The ACM and the PIGD are valid global measures and accurately reflect the combined effects of the various components of ambulatory capacity in PD patients with HY stages 1–4.
Keywords: Idiopathic Parkinson Disease, Ambulation, Scales, Balance, Falls, PIGD, Ambulatory Capacity
INTRODUCTION
Ambulatory capacity is impaired in Parkinson disease (PD) as a result of the progression of the motor symptoms, apprehension, and fear of falling. Severe restriction and eventual loss of ambulatory capacity constitutes a major source of disability for PD patients, affecting activities of daily living, and may lead to falls and injuries, medical complications, increased medical costs, and ultimately institutionalization and death [1–4]. Developing an accurate measure of ambulatory capacity in PD has been challenging, because of the heterogeneity of causes and symptoms that may lead to its decline: bradykinesia may impair the individual’s ability to rise from the seated position, while freezing may interfere with effectiveness of ambulation and leads to falls. The loss of postural reflexes impairs the patient’s ability to prevent a fall once their static or dynamic balance has been perturbed, while an increasing number of falls may undermine the patient’s confidence in their own abilities. The combined effect of these processes is ultimately wheelchair-bound status [5].
The NIH Exploratory Trials in Parkinson Disease Long-Term Study 1 (LS-1) [6] was designed as a 5-year double blind, placebo controlled trial to examine the disease-modifying potential of creatine in PD. As a primary outcome measure it utilizes a composite measure that takes into account ambulatory capacity, activities of daily living, cognitive status, and global disability. Ambulatory capacity was measured in LS-1 using a construct (Ambulatory Capacity Measure – ACM) derived as the sum of the scores of the Unified Parkinson’s Disease Rating Scale (UPDRS) items 13 (falling), 14 (freezing), 15 (walking), 29 (gait), and 30 (postural stability). A higher score reflects a greater impairment of ambulatory capacity. A similar construct has been extensively used in the literature under the acronym PIGD (for Postural Instability – Gait Disturbance) [7], which is the average value of the same items from the UPDRS. Although inter-rater and test-retest reliability can be established on the basis of such assessments of the entire UPDRS done in the past [8–10], the ACM/PIGD’s construct validity and internal consistency has never been demonstrated.
In defining the concept of ambulatory capacity, and as a first step towards assessing the ACM’s construct validity, we accepted that ambulatory capacity depends on the degree of gait impairment, the amount of freezing of gait experienced by the PD patient, the frequency of falling, the balance impairment, and the patient’s self-confidence and fear of falling. Since the PIGD has been used extensively in the literature, we elected to examine the validity of both constructs simultaneously. Because of the similarity of the two constructs, when reporting and discussing analyses that were parallel or results that were identical for the two constructs we will use the combined acronym ACM/PIGD. However, it should be understood that two sets of analyses were performed in parallel, one set for each construct.
MATERIALS AND METHODS
Participants
We utilized data from the Gait and Balance Initiative (GABI) cohort made available by the Struthers Parkinson’s Center (a convenience sample). The database contains data from 340 participants in a research study of falling in PD [11]. Participants were recruited from the outpatient PD population at the Struthers Parkinson’s Center in a sequential fashion, regardless of history of falling. The experimental protocol was approved by The Institutional Review Board of the Park Nicollet Institute. All participants fulfilled the United Kingdom PD Society brain bank clinical diagnostic criteria for PD [12]. Patients with HYS between 1 and 5 were included. After providing informed consent, participants completed falls diaries over a period of 4 weeks, at the end of which they underwent a battery of assessments, including objective and self-reported measures of both motor and non-motor impairment. Relevant to the present analysis, the following data on measures of PD severity, motor function and mobility were extracted from the GABI database, and utilized for the assessment of construct validity of the ACM/PIGD: items 13, 14, 15, 29, and 30 of the UPDRS [13] to calculate the ACM/PIGD; Hoehn and Yahr state while ON (HYS) as a measure of disease severity [14] ; number of falls in the preceding 4 weeks (NOF); Activities Based Confidence Scale (ABC) [15] as a measure of the subjects’ confidence in their own ability to ambulate safely; Freezing of Gait Questionnaire (FOG) [16] as a measure of freezing; Five Times Sit-to-Stand (FTSS) [17, 18], Timed Up-and Go (TUG) [19, 20], Gait Velocity (GV) [21], as global measures of bradykinesia and overall mobility; and, Berg Balance Scale (BBS) [22,23] as a measure of balance. Briefly, all objective assessments took place in the ON state, according to standardized protocols. Subjects were asked to perform the requested tasks at a comfortable speed. For each test a single trial was allowed. Subjects were allowed to use a gait assistive device, if they did so routinely during the performance of their daily activities. A 10-meter walk was used for GV testing. All objective testing was performed by trained staff. With regard to the definition of fall, this was defined as an instance of loss of balance that ended with the subject at a lower level than the one intended (e.g. from standing to sitting or to the floor, or from sitting to the floor).
Approach and hypotheses
There is currently no “gold standard” for measuring ambulatory capacity in PD. One might argue that the concept of ambulatory capacity is vague and ill-defined. As mentioned earlier, we proposed that ambulatory capacity would depend on the degree of gait impairment, the amount of freezing of gait experienced by the PD patient, the frequency of falling, the balance impairment, and the patient’s self-confidence and fear of falling. We hypothesized that there would be strong correlations between the ACM/PIGD and the individual objective and self-reported measures of freezing, falling, gait, and postural stability contained in the GABI assessment battery. Since the ACM/PIGD construct consists of items that address these related, yet not completely overlapping components of mobility in PD, we expected that correlation of ACM/PIGD with the individual measures would be moderately high (Spearman correlation coefficient r > 0.50), whereas, the correlation between ACM/PIGD and a composite of the mobility measures was expected to be higher (r > 0.75).
Statistical analysis
Internal consistency of the ACM/PIGD was assessed by Cronbach’s coefficient alpha. The construct validity of the ACM/PIGD was assessed by estimating (r) between the ACM/PIGD and other measures of mobility (HYS, NOF, ABC, FOG, FTSS, TUG, GV and BBS). The null hypothesis H0: r ≤ 0.50 was tested at alpha of 0.05 (one-sided) using Fisher’s Z transformation. The signs of ABC, TUG, and GV were reversed, so that for all measures, as well as for the ACM/PIGD, higher scores would reflect a greater impairment. In order to assess the correlation of ACM/PIGD with all the mobility measures jointly, HYS, NOF, ABC, FOG, FTSS, TUG, GV and BBS were combined into a summed rank score following the approach for O’Brien’s nonparametric Global Statistic Test (GST) [24]. Specifically, after coding each outcome in the same direction, each participant was ranked on each outcome (HYS, NOF, ABC, FOG, FTSS, TUG, GV and BBS). Next, the ranks were summed for each participant, and the correlation between the summed-ranks and ACM/PIGD was estimated with Spearman correlation coefficient. A linear regression of the summed-ranks as the dependent variable with the ACM/PIGD as the regressor variable was utilized in order to obtain a coefficient of determination (R2).
RESULTS
Descriptive Statistics
Demographics, disease characteristics, and measures are summarized in Table 1. A total of 46% of participants were women. Only 7 participants had HYS 5 during ON. ACM/PIGD results were received from 329 of the 340 patients, and the proportions of missing data from ACM/PIGD (3.2%) were lower than the missing data for HYS, ABC, FOG, FTSS, TUG, GV and BBS (5.3–10.3%) and were higher than NOF (0.9%). Figure 1 shows the distribution of ACM scores: less than 10% people had ACM over 12 and 59.3% of people had ACM greater than 3 and less than 10. Distribution is not shown for PIGD, but similarly, less than 10% people had PIGD over 2.4 and 59.3% of people had PIGD greater than 0.6 and less than 2. Table 2 shows ACM/PIGD by year since diagnosis; the average and median ACM/PIGD score increases as number of years since diagnosis increases. Likewise, the average ACM/PIGD scores increase with age category (Table 3). Although ACM/PIGD scores approached a normal distribution, few participants demonstrated ACM/PIGD scores at the high end of the range (Figure 1). There was no evidence of either a floor or a ceiling effect.
Table 1.
Demographics and disease characteristics
| Variable | N | Mean | Std Dev | 25th Pctl | Median | 75th Pctl |
|---|---|---|---|---|---|---|
| Age at diagnosis | 340 | 61.40 | 11.50 | 54 | 61.5 | 70 |
| Age at assessment | 340 | 71.40 | 9.06 | 65.47 | 72.29 | 78.14 |
| UPDRS mental | 337 | 3.67 | 2.49 | 2 | 3 | 5 |
| UPDRS ADL | 338 | 15.73 | 6.65 | 11 | 15 | 20 |
| UPDRS motor | 328 | 27.07 | 11.12 | 19 | 27 | 35 |
| HYS | 320 | 2.63 | 0.98 | 2 | 3 | 3 |
| NOF | 337 | 2.13 | 4.90 | 0 | 1 | 2 |
| ABC (%) | 305 | 59.43 | 22.85 | 78.13 | 60.00 | 42.19 |
| FOG | 322 | 9.28 | 5.46 | 5 | 9 | 14 |
| FTSS (sec) | 313 | 19.47 | 12.27 | 13.1 | 16 | 22.4 |
| TUG (sec) | 313 | 16.53 | 11.19 | 10.31 | 12.5 | 18.83 |
| GV (m/s) | 314 | 0.99 | 0.31 | 1.24 | 1.00 | 0.76 |
| BBS | 316 | 43.38 | 11.78 | 53 | 48 | 37 |
| ACM | 329 | 6.51 | 3.53 | 4 | 6 | 9 |
| PIGD | 329 | 1.30 | 0.76 | 0.8 | 1.2 | 1.8 |
| HYS | Frequency | Percent |
|---|---|---|
| 1 | 65 | 20.31 |
| 2 | 34 | 10.63 |
| 3 | 183 | 57.19 |
| 4 | 31 | 9.69 |
| 5 | 7 | 2.19 |
Figure 1.
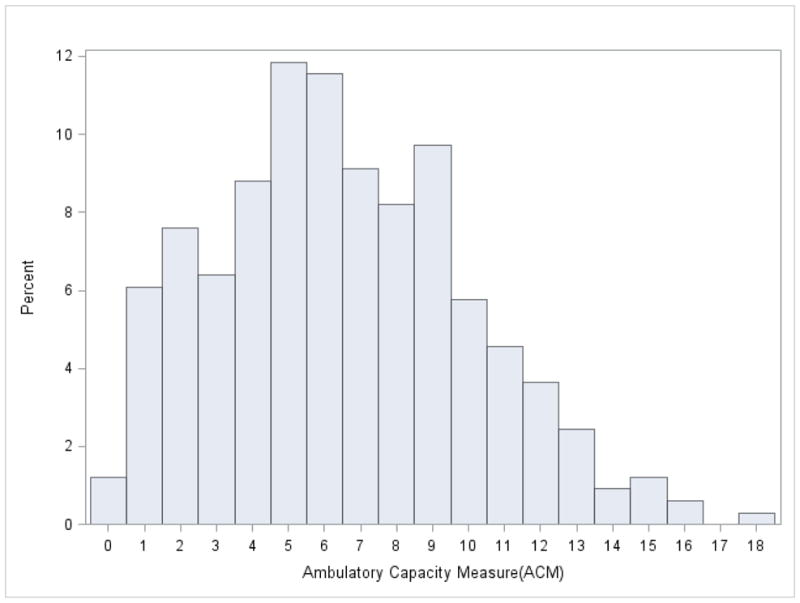
Distribution of ACM scores in a sample of PD patients (N=329)
Table 2.
ACM and PIGD by year since diagnosis
| Years since diagnosis | N | Mean | Std Dev | 25th Pctl | Median | 75th Pctl |
|---|---|---|---|---|---|---|
| ACM | ||||||
|
| ||||||
| <5 | 86 | 5.24 | 3.36 | 2 | 5 | 7 |
| 5–9.9 | 112 | 6.39 | 3.32 | 4 | 6 | 9 |
| 10–14.9 | 63 | 6.92 | 3.63 | 4 | 7 | 9 |
| ≥15 | 68 | 7.91 | 3.48 | 6 | 8 | 10 |
|
| ||||||
| PIGD | ||||||
|
| ||||||
| <5 | 86 | 1.05 | 0.13 | 0.4 | 1 | 1.4 |
| 5–9.9 | 112 | 1.28 | 0.13 | 0.8 | 1.2 | 1.8 |
| 10–14.9 | 63 | 1.38 | 0.15 | 0.8 | 1.4 | 1.8 |
| ≥15 | 68 | 1.58 | 0.14 | 1.2 | 1.6 | 2 |
Table 3.
ACM and PIGD by age at assessment
| Age | N | Mean | Std Dev | 25th Pctl | Median | 75th Pctl |
|---|---|---|---|---|---|---|
| ACM | ||||||
|
| ||||||
| <50 | 8 | 5.50 | 2.62 | 4 | 6 | 6.5 |
| 50–59 | 27 | 4.85 | 3.41 | 2 | 4 | 6 |
| 60–69 | 97 | 5.98 | 3.91 | 2 | 5 | 9 |
| 70–79 | 140 | 6.99 | 3.49 | 4 | 7 | 9 |
| >=80 | 57 | 7.16 | 2.75 | 5 | 7 | 9 |
|
| ||||||
| PIGD | ||||||
|
| ||||||
| <50 | 8 | 1.10 | 0.10 | 0.8 | 1.2 | 1.3 |
| 50–59 | 27 | 0.97 | 0.14 | 0.4 | 0.8 | 1.2 |
| 60–69 | 97 | 1.20 | 0.16 | 0.4 | 1 | 1.8 |
| 70–79 | 140 | 1.40 | 0.14 | 0.8 | 1.4 | 1.8 |
| >=80 | 57 | 1.43 | 0.11 | 1 | 1.4 | 1.8 |
Internal Consistency
Cronbach’s coefficient alphas are presented in Table 4. The full scales for both ACM and PIGD demonstrated good internal consistency with an overall Cronbach’s coefficient alpha of 0.78. To determine how much each item affects the reliability of the constructs, Cronbach’s coefficient alpha was calculated after deleting each individual item (UPDRS question 13, 14, 15, 29, and 30). The coefficient alphas calculated without the deleted variable decreased slightly as compared to the overall coefficient alpha, which suggests each item is moderately positively correlated with the other items. The corrected-item total correlation coefficients of each item with the total of the remaining items ranged from 0.52–0.61.
Table 4.
Cronbach’s coefficient alphas for the ACM/PIGD
| Overall Cronbach’s Coefficient Alpha = 0.78
| |
|---|---|
| Deleted Variable | Cronbach’s Coefficient Alpha Without the Deleted Variable |
| UPDRS 13 | 0.75 |
| UPDRS 14 | 0.75 |
| UPDRS 15 | 0.74 |
| UPDRS 29 | 0.72 |
| UPDRS 30 | 0.75 |
Construct Validity
Spearman correlations between the scores of the ACM/PIGD and HY, NOF, ABC, FOG, FTSS, TUG, GV and BBS are presented in Table 5. The correlation coefficient for ACM/PIGD and the comparison measures ranged from 0.45–0.72. The null hypothesis of H0: r ≤0.50 was rejected for the Spearman correlation between ACM/PIGD and ABC, FOG, TUG, GV and BBS. A positive correlation (Spearman r =0.823, p-value<0.0001) was found between the ACM/PIGD and summed-ranks of HY, NOF, ABC, FOG, FTSS, TUG, GV and BBS. In a simple linear regression, 68% of the variability in the summed-ranks of HY, NOF, ABC, FOG, FTSS, TUG, GV and BBS is explained by ACM/PIGD (R2 = 0.68).
Table 5.
Spearman correlations between the ACM/PIGD and other measures of ambulatory capacity
| Variable | N | Correlation Estimate (r) | 95% Confidence Limits on r | |
|---|---|---|---|---|
| FOG | 314 | 0.72** | 0.60 | 0.73 |
| BBS | 306 | 0.70** | 0.67 | 0.77 |
| ABC | 298 | 0.69** | 0.63 | 0.75 |
| TUG | 305 | 0.67** | 0.64 | 0.76 |
| GV | 306 | 0.58* | 0.50 | 0.65 |
| HYS | 312 | 0.50 | 0.35 | 0.53 |
| NOF | 327 | 0.48 | 0.39 | 0.56 |
| FTSS | 304 | 0.45 | 0.42 | 0.58 |
The null hypothesis H0: r ≤ 0.50 was rejected at p = .025.
The null hypothesis H0: r −≤ 0.50 was rejected at p < .0001.
The signs of ABC, TUG, and GV were reversed, so that for all measures, as well as for the ACM/PIGD, higher scores would reflect a greater impairment.
DISCUSSSION
In this analysis, we demonstrate the construct validity and internal consistency of the ACM and PIGD constructs as easures of ambulatory capacity in PD patients in Hoehn and Yahr stages 1–4. Currently there is no gold standard for ambulatory capacity in PD, therefore we adopted a hypothesis-driven approach to the validation process: we hypothesized that the ACM/PIGD would show good correlations with objective and self-reported measures of overlapping, but not identical determinants of ambulatory capacity, and an even stronger association to a combination of these measures. Our analysis confirmed our hypothesis: as expected, the ACM and PIGD were highly correlated with HY, NOF, ABC, FOG, FTSS, TUG, GV and BBS. The majority of the scales were statistically significantly correlated with ACM and PIGD, by a correlation of more than 0.50, the pre-specified hypothesis. More to the point, the ACM and PIGD were highly correlated with the summed-rank of these comparison measures.
The overall Cronbach’s alpha of ACM/PIGD (0.78) was within the range that is recommended in order to demonstrate good internal consistency, 0.70–0.90. [25,26]. A low Cronbach’s alpha indicates lack of correlation between items of the scale suggesting that they should not be combined, whereas a very high Cronbach’s alpha indicates redundancy [26]. The range of the overall Cronbach’s alpha and the similarity of Cronbach’s alpha calculated for each deleted item with the overall Cronbach’s alpha, suggested that the ACM/PIGD is measuring a uni-dimensional construct [27].
Very few patients scored in the upper range of the ACM/PIGD, and no patient had an ACM score of 20 (or 4 for the PIGD construct), the maximum possible score. This may be the result of inconsistencies in the scaling of the individual items of the UPDRS as has been discussed previously [28], or due to floor effects of the individual UPDRS items comprising the ACM/PIGD. It would be intriguing to assess the validity of similar constructs derived from the Movement Disorders Society optimized version of the UPDRS [29].
Our analysis has the strength of analyzing data from a well characterized cohort of PD patients who were evaluated with many bedside measures of various aspects of the disease. These patients, spanning from HYS 1 to 4 represent early, moderate, and advanced stages of the disease. The lack of a sufficient number of participants in HYS 5 constitutes a limitation of the applicability of our conclusions. HYS 5 represents mainly non-ambulatory or minimally ambulatory patients, and, conceivably, their inclusion would not be likely to alter the findings. The distribution of HYS in this cohort, may not be typical for a cross-sectional sample of PD. Based on previous studies there appears to be an over-representation of HYS 3, and an underrepresentation of HYS 2 in the GABI cohort [30]. It is unclear, however, how this skew in the data could have had an impact on our conclusions. A further limitation is that because we used a convenience sample, we were not able to evaluate the ACM/PIGD against a different, and, possibly, more comprehensive battery of instruments measuring aspects of ambulatory capacity. The GABI cohort has focused on bedside assessments of gait and balance. It will be interesting in the future to evaluate ACM/PIGD against instrumented measures of gait, balance, and ambulatory activity [31, 32]. For the same reasons, we had to include the available self-reported measures as comparators for validation, which may arguably compromise the objectivity of the assessments. Inter-rater and test-retest reliability could not be determined in this analysis as we utilized a convenience cohort. Although such reliability can presumed on the basis of previous validations of the UPDRS as a whole, the inter-rater and test-retest reliability of the specific construct will need to be further assessed.
We conclude that the ACM, a score comprised of UPDRS items 13, 14, 15, 29, and 30, and the PIGD, consisting of the average score of the same UPDRS items, are valid measures of gait and ability to ambulate for PD patients with HY stages 1–4.
Acknowledgments
SOURCES OF SUPPORT
This analysis was funded by the NINDS (U01NS043127); the data used for this analysis was collected through a grant from the National Parkinson Foundation, and was kindly provided by the investigators of the GABI study.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflict of interest to report.
References
- 1.Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA. Falls and injuries resulting from falls among patients with Parkinson’s disease and other parkinsonian syndromes. Mov Disord. 2005;20:410–415. doi: 10.1002/mds.20347. [DOI] [PubMed] [Google Scholar]
- 2.Matinolli M, Korpelainen JT, Sotaniemi KA, Myllylä VV, Korpelainen R. Recurrent falls and mortality in Parkinson’s disease: a prospective two-year follow-up study. Acta Neurol Scand. 2011;123:193–200. doi: 10.1111/j.1600-0404.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaltenboeck A, Johnson SJ, Davis MR, Birnbaum HG, Carroll CA, Tarrants ML, Siderowf AD. Direct costs and survival of Medicare beneficiaries with early and advanced Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:321–326. doi: 10.1016/j.parkreldis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Allyson Jones C, Wayne Martin WR, Wieler M, King-Jesso P, Voaklander DC. Incidence and mortality of Parkinson’s disease in older Canadians. Parkinsonism Relat Disord. 2012;18:327–331. doi: 10.1016/j.parkreldis.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Brozova H, Stochl J, Roth J, Ruzicka E. Fear of falling has greater influence than other aspects of gait disorders on quality of life in patients with Parkinson’s disease. Neuro Endocrinol Lett. 2009;30:453–457. [PubMed] [Google Scholar]
- 6.Elm JJ NINDS NET-PD Investigators. Design innovations and baseline findings in a long-term Parkinson’s trial: the National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson’s Disease Long-Term Study-1. Mov Disord. 2012;27:1513–1521. doi: 10.1002/mds.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott MP, Jankovic J, Carter J, Fahn S, Gauthier S, Goetz CG, Golbe LI, Koller W, Lang AE, Olanow CW, Shoulson I, Stern MB, Tanner CM, Weiner WJ. Factors predictive of the need for levodopa therapy in early, untreated Parkinson’s disease. Arch Neurol. 1995;52:565–570. doi: 10.1001/archneur.1995.00540300037010. [DOI] [PubMed] [Google Scholar]
- 8.Richards M, Marder K, Cote L, Mayeux R. Interrater reliability of the Unified Parkinson’s Disease Rating Scale motor examination. Mov Disord. 1994;9:89–91. doi: 10.1002/mds.870090114. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 10.Siderowf A, McDermott M, Kieburtz K, Blindauer K, Plumb S, Shoulson I Parkinson Study Group. Test-retest reliability of the unified Parkinson’s disease rating scale in patients with early Parkinson’s disease: results from a multicenter clinical trial. Mov Disord. 2002;17:758–763. doi: 10.1002/mds.10011. [DOI] [PubMed] [Google Scholar]
- 11.Parashos SA, Erickson-Davis C, Krohn SA, Wielinski CL. Gait and balance initiative (GABI): Phenomenology of falls in Parkinson’s disease. Mov Disord. 2008;23:S332–S333. [Google Scholar]
- 12.Gibb WR, Lees AJ. A comparison of clinical and pathological features of young- and old onset Parkinson’s disease. Neurology. 1988;38:1402–1406. doi: 10.1212/wnl.38.9.1402. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Elton RL . UPDRS program members. Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinsons disease. Vol. 2. Macmillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 14.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 15.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol Med Sci. 1995;50:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 16.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 17.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 18.Duncan RP, Leddy AL, Earhart GM. Five times sit-to-stand test performance in Parkinson’s disease. Arch Phys Med Rehabil. 2011;92:1431–1436. doi: 10.1016/j.apmr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. JAGS. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 20.Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors. J Neurol. 2005;252:1310–1315. doi: 10.1007/s00415-005-0855-3. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka H, Tanaka N, Eng M, Saeki K, Kiriyama T, Eura N, Ikeda M, Izumi T, Kitauti T, Furiya Y, Sugie K, Ikada Y, Ueno S. Risk of falling in Parkinson’s disease at the Hoehn-Yahr stage III. Eur Neurol. 2011;66:298–304. doi: 10.1159/000331635. [DOI] [PubMed] [Google Scholar]
- 22.Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehab. 1992;73:1073–1080. [PubMed] [Google Scholar]
- 23.Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson’s disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. 2005;86:789–792. doi: 10.1016/j.apmr.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- 25.Nunnally JC, Bernstein IH. Psychometric theory. 3. McGraw-Hill; New York: 1994. [Google Scholar]
- 26.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Yu CH. An introduction to computing and interpreting Cronbach Coefficient Alpha in SAS. Proceedings of the 26th SAS User Group International Conference; 2001. pp. 1–7. [Google Scholar]
- 28.Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 29.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N Movement Disorder Society UPDRS Revision Task Force . Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YJ, Wee HL, Chan YH, Seah SH, Au WL, Lau PN, Pica EC, Li SC, Luo N, Tan LC. Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Mov Disord. 2010;25:710–716. doi: 10.1002/mds.22875. [DOI] [PubMed] [Google Scholar]
- 31.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman T, Weiss A, Brozgol M, Giladi N, Hausdorff JM. Identifying axial and cognitive correlates in patients with Parkinson’s disease motor subtype using the instrumented Timed Up and Go. Exp Brain Res. 2014;232:713–721. doi: 10.1007/s00221-013-3778-8. [DOI] [PubMed] [Google Scholar]


