Abstract
Background
Magnetic resonance imaging (MRI) studies show reduced cortical thickness in patients with schizophrenia and bipolar disorder. These subtle brain abnormalities may provide insight into illness mechanisms. However, environmental and lifestyle-related factors, such as cigarette smoking, may contribute to brain structure changes. Cigarette smoking is highly prevalent in patients with severe mental illness. In nonpsychiatric samples, smoking has been associated with reduced thickness in the anterior (ACC) and posterior cingulate cortices, the insular cortex (INS), the dorsolateral prefrontal cortex and the orbitofrontal cortex.
Methods
We examined MRI scans from patients with schizophrenia, other psychotic disorders or bipolar disorder and healthy controls using FreeSurfer.
Results
We included 506 patients (49% smokers) and 237 controls (20% smokers) in our study. We found reduced cortical thickness in the left rostral ACC and the left INS in smoking patients compared with nonsmoking patients, but this difference was not found among healthy controls. No dose–response relationship was found between amount of smoking and cortical thickness in these regions. Among patients, maps of thickness along the whole cortical surface revealed reduced insular thickness but no effects in other regions. Among healthy controls, similar analyses revealed increased age-related cortical thinning in the left occipital lobe among smokers compared with nonsmokers.
Limitations
The causal direction could not be determined owing to the cross-sectional design and lack of detailed data on smoking addiction and smoking history.
Conclusion
The effect of cigarette smoking should be considered in MRI studies of patients with severe mental illness.
Introduction
Widespread reductions in cerebral cortical thickness have been documented in MRI studies of patients with first-episode1–3 and chronic schizophrenia.4,5 In patients with bipolar I disorder, a more limited regional cortical thinning has been reported.6–8 To the extent that these subtle brain abnormalities reflect etiological factors, they may hold clues to illness mechanisms. However, factors associated with having the illness, such as medication, alcohol and illicit substance use, cigarette smoking, sedentary lifestyle and nutrition, might also affect brain structure.9 Cigarette smoking is highly prevalent among patients with mental illness.10,11 For example, smokers with schizophrenia have higher nicotine consumption, report more craving and are less likely to quit than other smokers.12–14 High rates of smoking observed before illness onset suggest that, unlike medication effects, potential effects of smoking need not be confined to periods after illness onset.15
Voxel-based morphometry (VBM) studies in nonpsychiatric populations have shown differences in cortical grey matter between adult smokers and nonsmokers. Reduced grey matter volume in the anterior cingulate cortex (ACC) has been reported among smokers.16–18 Reduced grey matter volume in smokers has also been demonstrated in frontal cortex regions, including the dorsolateral prefrontal cortex (DLPFC) and the orbitofrontal cortex (OFC).16,17,19 In another study, grey matter volume reduction was reported in a number of brain areas, including the cingulate gyrus, frontal and occipital lobe areas.20 Regional cortical thinning in the medial OFC has also been demonstrated.21 Smoking has previously been shown to be associated with reduced insular thickness in a study of patients with alcohol addiction.22 In a recent study by Morales and colleagues,23 cumulative exposure to cigarettes correlated inversely with insular thickness among young, healthy smokers. In the same study, no difference emerged between smokers and nonsmokers, possibly suggesting that a certain degree of exposure is required to cause thinning in this region.
Regions of cortical thinning in healthy smokers do, to some extent, overlap with regions where thinner cortices are reported in patients with schizophrenia; the cingulate cortex, INS, DLPFC and OFC have rather consistently been found to be reduced and have therefore been proposed to play a key role in the illness.4,24,25 Reduced grey matter in these regions has also been demonstrated in studies of unmedicated patients with psychosis.26,27 In patients with bipolar disorder, reductions in the ACC and DLPFC6,28 and the OFC8 have been reported.
To our knowledge, 2 studies have previously examined associations between regional cortical morphology and smoking in patients with schizophrenia. In a VBM study, smokers showed increased grey matter volume of the lateral prefrontal and superior temporal gyri compared with nonsmokers.29 However, the sample was small, and potential confounders could not be ruled out. A recent study found smaller DLPFC volume among smoking patients,30 while exploratory analyses of the whole brain cortical surface showed thinning limited to a region in the right occipital lobe. Thus, findings have not been consistent.
The aim of the present study was to examine how smoking affects cortical thickness in patients with psychotic illness and/or bipolar disorders and healthy controls. Based on the literature on smoking and brain structure in healthy populations reviewed above,16–18,20,21 we selected 4 regions of interest (ROIs) in both hemispheres: the ACC/PCC, INS, DLPFC and OFC. Our main hypothesis was that smoking would be associated with thinner cortices in these regions and that this effect would be more pronounced in the patient group than the control group. We also hypothesized there would be a dose–response relationship between cigarette consumption and reduced cortical thickness as well as an age-dependent relationship where smokers in both groups would show thinner cortices with older age compared with nonsmokers. We aimed to rule out possible confounding variables affecting cortical thickness, including illness severity, alcohol or illicit substance use, medication and IQ. To examine the regional specificity of our findings, we also conducted analyses of cortical thickness across the whole brain surface.
Methods
Sample
The present study is part of the ongoing Thematically Organized Psychosis (TOP) Study, conducted at the Norwegian Centre for Mental Disorders Research (NORMENT). Patients with schizophrenia, schizoaffective disorder, other psychotic illness (including brief psychotic disorder, delusional disorder, psychotic disorder not otherwise specified and severe depression with psychotic features) and bipolar disorders were recruited from psychiatric clinics and hospitals in the Oslo region, Norway. Some patients (n = 21) were recruited from hospitals outside of the Oslo region. Healthy controls were randomly selected from the national population registry in the same catchment area. Differences in regional cortical thickness between patients with schizophrenia and bipolar disorder and healthy controls have previously been reported based on a partly overlapping sample.7 Inclusion criteria for the patient sample were age 18–65 years; no history of moderate to severe head injury; no history of neurologic disorder; IQ greater than 65; and ability to fully understand the information given about the study and provide informed consent, as judged by the study physician or clinical psychologist. Additional inclusion criteria for the healthy control sample were no current or previous psychiatric disorders, no family history of severe psychiatric disorders, no alcohol or substance dependence and no use of cannabis in the 3 months preceding assessment. All participants received information about the study orally and in writing before giving their consent. The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate.
Patients and healthy controls who participated in the TOP study between 2004 and 2012 and had completed both the clinical assessment and MRI scan were selected for the present study. The median time period between the clinical assessment and MRI scan was 118 days. The clinical assessment was performed by trained physicians or clinical psychologists. We verified diagnoses using the Structured Clinical Interview for the DSM-IV axis-I disorders (SCID).31 For the patient sample, we collected data on current use of tobacco products, number of cigarettes smoked per day and years of daily smoking using structured interviews designed for the study. Patients who smoked daily at the time of inclusion were considered current smokers. Healthy controls were asked through retrospective phone interviews if they smoked at the time of inclusion. We excluded users of smokeless tobacco products from the sample.
MRI image acquisition
All participants underwent MRI scanning on the same 1.5 T Siemens Sonata scanner (Siemens Medical Solutions) equipped with a standard head coil. After a conventional 3-plane localizer, 2 sagittal T1-weighted magnetization-prepared rapid gradient echo volumes were acquired with the Siemens tfl3d1_ns pulse sequence (echo time 3.93 ms, repetition time 2730 ms, inversion time 1000 ms, flip angle 7°, field of view 24 cm, voxel size 1.33 × 0.94 × 1 mm, 160 partitions). Acquisition parameters were optimized for increased grey/white matter contrast. No acceleration by means of parallel imaging was applied. Patients and controls were scanned interchangeably. There was no scanner hardware upgrade during the study period, but routine software updates were performed. All scans were evaluated by a neuroradiologist, and participants with scans showing minor brain pathology were excluded from the study.
MRI image processing
We used FreeSurfer 5.3.0 software (http://surfer.nmr.mgh.harvard.edu/) to create a 3-dimensional (3D) model of the cortical surface for cortical thickness measurements. Briefly, this processing includes motion correction and averaging, removal of non–brain tissue, automated Talairach transformation, segmentation of subcortical structures, intensity normalization, tessellation of the grey/white matter boundary, automated topology correction and surface deformation following intensity gradients to optimally place the grey/white matter and grey matter/cerebrospinal fluid (CSF) boundaries.32–35 The method uses both intensity and continuity information from the entire 3D MRI volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the grey/white matter boundary to the grey matter/CSF boundary at each vertex of the tessellated surface.33 Quality control and editing was performed by trained research assistants under the supervision of an experienced FreeSurfer user. If image quality problems due to motion or image artifacts were detected, each of the 2 acquired T1 images were processed separately and the volume of highest quality was selected by visual inspection. All volumes were visually inspected for segmentation errors. If found, segmentation errors were corrected using manual editing and/or control points. Two individuals were excluded owing to failed quality control.
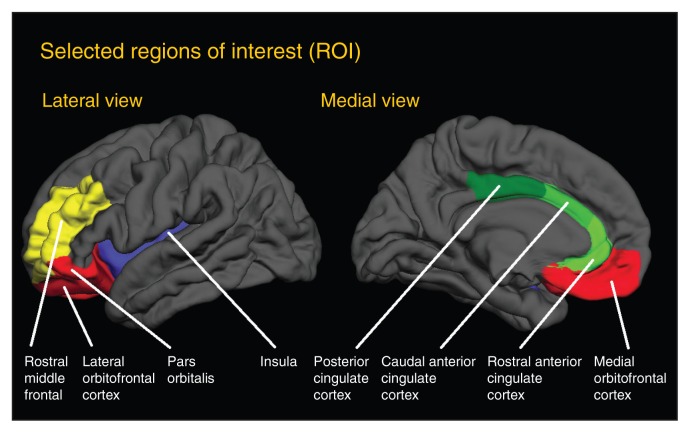
For the ROI analyses, we selected 3 parcellated regions in the cingulate cortex (caudal ACC, rostral ACC, PCC), 1 in the INS, 1 in the DLPFC (rostral middle frontal cortex) and 3 in the OFC (lateral OFC, medial OFC and pars orbitalis) in the left and right hemisphere (Fig. 1). All parcellations were defined from the Desikan–Killiany atlas, which is included in the FreeSurfer package.36
Fig. 1.
Cortical map showing the selected parcellated regions based on the Desikan–Killiany atlas. Regions in the cingulum (anterior cingulate cortex, posterior cingulate cortex) are shown in green. Regions in the orbitofrontal cortex are shown in red. The insula is shown in blue and the dorsolateral prefrontal cortex is shown in yellow.
Statistical analyses
We assessed differences in demographic and clinical data between smokers and nonsmokers in both groups using Student t or χ2 tests. We analyzed the selected ROIs using a general linear model (analysis of covariance [ANCOVA]) with cortical thickness as a dependent variable, smoking status as a categorical variable of interest and age and sex as covariates. Before examining the main effects of smoking, we performed initial analyses to test if main effects of smoking would differ between patients and controls. We included smoking × group (patient, control) interaction terms in the models and included the whole sample in these analyses. Since we found significant smoking × group interaction effects in the left rostral ACC (F = 3.89, p = 0.049) and in the left INS (F = 4.21, p = 0.041) as well as at a trend level in the left PCC (F = 3.60, p = 0.06), we decided to stratify further analyses of main effects (i.e., analyze patients and healthy controls separately). For more information about the initial analyses, see the Appendix, available at jpn.ca.
Next, we examined interaction effects between smoking and age to assess whether the effects of smoking depended on age in a similar way. First, we performed initial analyses to determine whether age × smoking status interaction effects would differ between patients and controls. This was done by adding 3-way interaction terms (age × smoking × group) to models tested in the whole sample. Significant 3-way interaction effects were found in several areas, including the left (F = 3.1, p = 0.041) and right caudal ACC (F = 3.7, p = 0.024), left (F = 4.4, p = 0.012) and right medial OFC (F = 4.8, p = 0.008), right lateral OFC (F = 4.2, p = 0.016) and, at a trend level, the right rostral ACC (F = 2.9, p = 0.06). Consequently, we stratified these analyses by group (patient v. control). For more information about these initial interaction analyses, see the Appendix. Since previous studies have shown regional cortical thickness differences between patients with schizophrenia and bipolar disorder,37 we adjusted for diagnosis (coded as a categorical variable with 3 levels: schizophrenia, bipolar disorder, other psychosis) in our within-patients analyses.
We then examined associations between degree of smoking (no. of cigarettes/d) and cortical thickness among patients when data were available. Since the number of cigarettes/d was not symmetrically distributed, and such a distribution could not be obtained by log transformation, we decided to divide the degree of smoking into 3 categories (light: 1–9, moderate: 10–19 heavy: ≥ 20 cigarettes/d).38 We then performed an ANCOVA to test whether cortical thickness differed between these groups.
Based on previously published data, we selected the following 5 variables a priori and examined them as potential confounds: duration of illness, defined by time of initial illness episode to date of MRI;39 symptom severity based on Positive and Negative Syndrome Scale (PANSS) score;40 antipsychotic exposure (see the Appendix for details); alcohol and/or illicit substance use;19,41 and general cognitive ability, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI).42–44 Alcohol use was defined as the mean number of alcohol units consumed per week. Illicit substance use was analyzed collectively as use of any substance (dichotomized) and separately as use of cannabis, amphetamines and cocaine. Because illicit substance use was among the exclusion criteria for healthy controls, analysis of substance use was done in for the patient sample only. We examined the influence of each potential confounder by including each variable in the model, with age, sex and (for patients) diagnosis as covariates, and assessing their influence on the model.
For all ROI analyses, we used an α level of p < 0.05, 2-tailed. We applied the Benjamini–Hochberg method to correct for multiple comparisons.45 Based on observed p values from 32 tests, the procedure resulted in a corrected threshold of p = 0.003. We performed our analyses using SPSS software, version 20 (IBM).
For vertex-based cortical thickness analyses, we created statistical p maps by fitting general linear models with cortical thickness as a dependent variable to each vertex across the cortical mantle. Statistical maps were first spatially smoothed using a 20 mm full-width at half-maximum (FWHM) Gaussian kernel. We also examined post hoc how different smoothing thresholds (FWHM of 10, 15, 25 and 30 mm) affected significant results. Similar to the aforementioned analyses, smoking status was entered as a variable of interest, while age, sex and (for patients) diagnosis were entered as covariates in the models. We also included age × smoking status interactions in the models. Results are reported using a false-discovery rate (FDR) set at p < 0.05, 2-tailed. We also report findings from whole brain analysis using a threshold of p < 0.01, 2-tailed, uncorrected. These analyses were performed in FreeSurfer using the general linear model function.
Results
Participants
We selected 506 patients and 237 healthy controls to participate in our study; 250 patients (49%) and 48 controls (20%)were smokers. Characteristics of the sample are shown in Table 1. Users of smokeless tobacco products (n = 22) were excluded from the sample. One patient who smoked both pipe tobacco and cigarettes was included as a cigarette smoker. There were significantly more men than women among the smoking controls, but there were no sex differences among patients. In both groups, smokers used more alcohol than nonsmokers. Among patients, smokers had more often used cannabis and other illicit substances than nonsmokers. In both groups, smokers had lower WASI scores than nonsmokers. For detailed information about medication use, see the Appendix.
Table 1.
Demographic and clinical characteristics of the study sample
| Patients, mean ± SD or no. (%)* | Healthy controls, mean ± SD or no. (%)* | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Characteristic† | Smokers, n = 250, 49% | Nonsmokers, n = 256, 51% | Statistic | p value | Smokers, n = 48, 20% | Nonsmokers, n = 189, 80% | Statistic | p value |
| Age, yr | 32.8 ± 10.2 | 33.0 ± 10.8 | t = −0.2 | 0.84 | 35.2 ± 9.9 | 35.9 ± 9.7 | t = −0.5 | 0.64 |
| Sex, m:f | 131:119 | 130:126 | χ2 = 0.13 | 0.72 | 34:14 | 99:90 | χ2 = 5.3 | 0.021 |
| No. cigarettes/d | 14.2 ± 8.0 | — | — | — | n/a | — | — | — |
| Smoking, yr | 13.2 ± 9.0 | — | — | — | n/a | — | — | — |
| Alcohol consumption‡ | 9.0 ± 12.6 | 4.3 ± 7.6 | t = 5.2 | < 0.001 | 5.9 ± 5.5 | 4.6 ± 4.8 | t = 1.7 | 0.09 |
| Substance use | ||||||||
| Any§ | 140 (56) | 38 (15) | χ2 = 94.0 | < 0.001 | 0 (0) | 0 (0) | — | — |
| Cannabis¶ | 125 (50) | 35 (14) | χ2 = 73.5 | < 0.001 | 2 (1) | 3 (2) | — | — |
| Amphetamines | 50 (20) | 7 (3) | χ2 = 35.5 | < 0.001 | 0 (0) | 0 (0) | — | — |
| Cocaine | 42 (17) | 8 (3) | χ2 = 24.5 | < 0.001 | 0 (0) | 0 (0) | — | — |
| Other illicit drugs | 37 (15) | 6 (2) | χ2 = 23.5 | < 0.001 | 0 (0) | 0 (0) | — | — |
| BMI | 25.9 ± 4.5 | 26.2 ± 5.0 | t = 0.7 | 0.49 | 24.8 ± 3.7 | 24.9 ± 3.5 | t = 0.2 | 0.85 |
| WASI verbal score | 101.8 ± 14.2 | 104.5 ± 14.7 | t = 1.9 | 0.05 | 107.0 ± 11.9 | 111.7 ± 9.2 | t = 2.5 | 0.013 |
| WASI performance score | 105.1 ± 14.2 | 107.7 ± 14.2 | t = 1.9 | 0.05 | 113.3 ± 10.7 | 116.3 ± 9.6 | t = 1.9 | 0.06 |
| WASI total score | 103.8 ±14.0 | 106.8 ± 14.3 | t = 2.2 | 0.027 | 111.1 ± 10.3 | 115.7 ± 8.5 | t = 3.2 | 0.002 |
| Diagnosis | χ2 = 1.7 | 0.42 | — | — | — | — | ||
| Schizophrenia | 102 (41) | 115 (45) | — | — | — | — | — | — |
| Bipolar disorder | 96 (38) | 84 (33) | — | — | — | — | — | — |
| Other psychotic illness | 52 (21) | 57 (20) | — | — | — | — | — | — |
| Age at onset, yr | 21.8 ± 8.7 | 21.9 ± 8.2 | t = 0.1 | 0.92 | — | — | — | — |
| Duration of illness, yr | 11.1 ± 8.6 | 11.0 ± 9.4 | t = −0.1 | 0.89 | — | — | — | — |
| No. of admissions (total) | 2.0 ± 2.6 | 1.9 ± 3.0 | t = −0.6 | 0.57 | — | — | — | — |
| No. of psychotic episodes | 1.7 ± 2.5 | 1.5 ± 1.5 | t = −1.3 | 0.20 | — | — | — | — |
| PANSS positive subscale | 12.9 ± 5.0 | 12.5 ± 5.1 | t = 1.0 | 0.34 | — | — | — | — |
| PANSS negative subscale | 12.8 ± 5.8 | 13.1 ± 5.8 | t = 0.7 | 0.52 | — | — | — | — |
| PANSS total score | 55.1 ± 16.3 | 53.9 ± 15.6 | t = 0.9 | 0.39 | — | — | — | — |
| Current medication | ||||||||
| Antipsychotic | 149 (63) | 164 (66) | χ2 = 0.4 | 0.53 | — | — | — | — |
| CPZ equivalent dose (mg) | 328 | 282 | t = −1.5 | 0.12 | — | — | — | — |
| Lithium | 20 (8) | 16 (6) | χ2 = 0.7 | 0.39 | — | — | — | — |
| Other mood stabilizer | 60 (25) | 53 (21) | χ2 = 1.2 | 0.28 | — | — | — | — |
| Antidepressant | 72 (30) | 70 (28) | χ2 = 0.3 | 0.36 | — | — | — | — |
BMI = body mass index; CPZ = chlorpromazine; n/a = not available; PANSS = Positive and Negative Symptom Scale; SD = standard deviation; WASI = Wechsler Abbreviated Scale of Intelligence.
Unless otherwise indicated.
Data missing for BMI (n = 5), WASI (n = 66), PANSS (n = 5), medication (n = 19), admissions (n = 8), no. of episodes (n = 42), alcohol use (n = 32) and substance use (n = 13).
In patients this refers to the average no. of alcohol units consumed per week during the last 2 years; in healthy controls it refers to the no. of units consumed per week during the last 2 weeks.
Use of any illicit substance (cannabis, cocaine, amphetamines, heroin, ecstasy, other) during the last 2 years.
Cannabis use in the last 3 months before assessment was an exclusion criterion for healthy controls. However, 5 healthy controls who reported minimal use (1–2 times) of cannabis during the month before MRI scan were kept in the sample.
Main effects of smoking on thickness of the ACC/PCC, INS, DLPFC and OFC
Smoking patients showed reduced cortical thickness in the left rostral ACC and the left INS compared with nonsmoking patients (Table 2). In addition, we observed reductions in the right rostral ACC, the PCC bilaterally, the left rostral middle frontal cortex and the left lateral OFC among patients who smoked, but these findings did not remain significant after correction for multiple testing. There were no significant differences between smokers and nonsmokers among healthy controls (Table 2). Subgroup analyses showed similar trends across diagnostic subgroups (schizophrenia, bipolar disorder, other psychosis) in the left rostral ACC and left INS, with cortical thickness reductions among smokers ranging from 1.6%–3.3% and 0.9%–2.2%, respectively. However, sample size was smaller in these analyses, and no finding was significant after correction for multiple testing (Appendix).
Table 2.
Main effects of smoking status on cortical thickness
| Patients | Healthy controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Brain region | Smokers n = 250 Estimated Mean (mm) |
Nonsmokers n = 256 Estimated Mean (mm) |
% Difference between smokers and nonsmokers | F | p value* | Smokers n = 48 Estimated Mean (mm) |
Nonsmokers n = 189 Estimated Mean (mm) |
% Difference between smokers and nonsmokers | F | p value |
| ACC/PCC | ||||||||||
| Left caudal anterior | 2.59 | 2.63 | −1.52 | 3.31 | 0.07 | 2.62 | 2.63 | −0.38 | 0.03 | 0.87 |
| Left rostral anterior | 2.79 | 2.85 | −2.11 | 12.61 | < 0.001† | 2.86 | 2.87 | −0.35 | 0.12 | 0.73 |
| Left posterior | 2.43 | 2.45 | −0.82 | 4.52 | 0.034 | 2.43 | 2.46 | −1.23 | 1.19 | 0.28 |
| Right caudal anterior | 2.42 | 2.45 | −1.22 | 1.65 | 0.20 | 2.44 | 2.38 | 2.46 | 2.83 | 0.09 |
| Right rostral anterior | 2.71 | 2.75 | −1.82 | 5.61 | 0.018 | 2.74 | 2.72 | 0.73 | 0.96 | 0.33 |
| Right posterior | 2.35 | 2.37 | −0.84 | 4.01 | 0.046 | 2.39 | 2.38 | 0.42 | 0.52 | 0.47 |
| INS | ||||||||||
| Left insula | 2.93 | 2.97 | −1.35 | 9.95 | 0.002† | 2.98 | 3.01 | −1.01 | 0.23 | 0.63 |
| Right insula | 2.94 | 2.97 | −1.01 | 3.76 | 0.05 | 2.99 | 3.00 | −0.33 | 0.00 | 0.99 |
| DLPFC | ||||||||||
| Left rostral middle frontal | 2.22 | 2.25 | −1.35 | 5.59 | 0.018 | 2.28 | 2.31 | 1.22 | 1.52 | 0.22 |
| Right rostral middle frontal | 2.18 | 2.20 | −0.96 | 3.43 | 0.07 | 2.24 | 2.26 | 0.71 | 0.53 | 0.47 |
| OFC | ||||||||||
| Left lateral | 2.52 | 2.55 | −0.84 | 5.35 | 0.021 | 2.59 | 2.57 | 0.77 | 1.33 | 0.25 |
| Left medial | 2.38 | 2.39 | −1.18 | 0.83 | 0.36 | 2.40 | 2.38 | 0.83 | 2.27 | 0.13 |
| Left pars orbitalis | 2.54 | 2.57 | −0.42 | 3.66 | 0.06 | 2.62 | 2.60 | 0.76 | 0.88 | 0.35 |
| Right lateral | 2.42 | 2.43 | −1.17 | 1.97 | 0.16 | 2.48 | 2.48 | 0.00 | 0.03 | 0.86 |
| Right medial | 2.31 | 2.33 | −0.41 | 0.82 | 0.37 | 2.36 | 2.32 | 1.69 | 3.47 | 0.06 |
| Right pars orbitalis | 2.52 | 2.54 | −0.86 | 2.45 | 0.12 | 2.62 | 2.59 | 1.15 | 1.29 | 0.26 |
ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; INS = insular cortex; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex.
Unadjusted for multiple comparisons.
Remained significant after Benjamini–Hochberg correction for multiple testing.
Association between smoking severity and cortical thickness in patients
There were no significant differences among light, moderate and heavy smokers (Appendix), with the exception of a thinner cortex in the left medial OFC among heavy smokers compared with light or moderate smokers. This finding did not remain significant after correction for multiple testing.
Interaction effects of age and smoking status
There were no interaction effects between smoking status and age that remained significant after correction for multiple testing. We observed nominally significant findings in some regions. Among patients, interaction effects indicating thinner cortices with increasing age in smokers compared with nonsmokers were observed in several regions: the left and the right caudal ACC (F = 4.71, p = 0.031 and F = 5.68, p = 0.018), the left PCC (F = 5.24, p = 0.022), the left INS (F = 6.18, p = 0.013), and the right lateral and the right medial OFC (F = 5.95, p = 0.015 and F = 4.28, p = 0.039). Scatter plots showing raw values with age slopes for the left INS are shown in the Appendix, Figure S1A. With age × smoking interaction terms in the models, there were no significant main effects; however, there were effects at trend levels in the right caudal ACC (F = 3.53, p = 0.06) and the right lateral OFC (F = 3.60, p = 0.06).
In the control group, nominally significant interaction effects indicated reduced cortical thickness with increasing age in nonsmokers, but not in smokers, in the right rostral ACC (F = 6.65, p = 0.011) and left and right medial OFC (F = 6.95, p = 0.009 and F = 5.44, p = 0.021, respectively). In addition, there were significant main effects of smoking, indicating that when this age-dependent effect was controlled for, cortical thickness was reduced among smokers in the same areas. Scatter plots of raw values with age slopes for the right rostral ACC are shown in the Appendix, Figure S1B.
Control for possible confounding variables
The main effect of smoking observed in the left rostral ACC among patients remained significant with correction for multiple testing after any of the potential confounders were added to the model. Similarly, the main effect observed in the left INS remained significant, with 2 exceptions: when alcohol and illicit substance use were included in the models, and with correction for multiple testing, reductions in the left INS were significant at trend levels only (both unadjusted p = 0.005). Neither alcohol nor illicit substance use was significantly associated with cortical thickness, and the change observed after entering them in the models was small. None of the other potential confounders affected the finding in the left INS. Analyses of the effects of potential confounding variables on nominally significant results are provided in the Appendix.
Whole brain analyses of cortical thickness
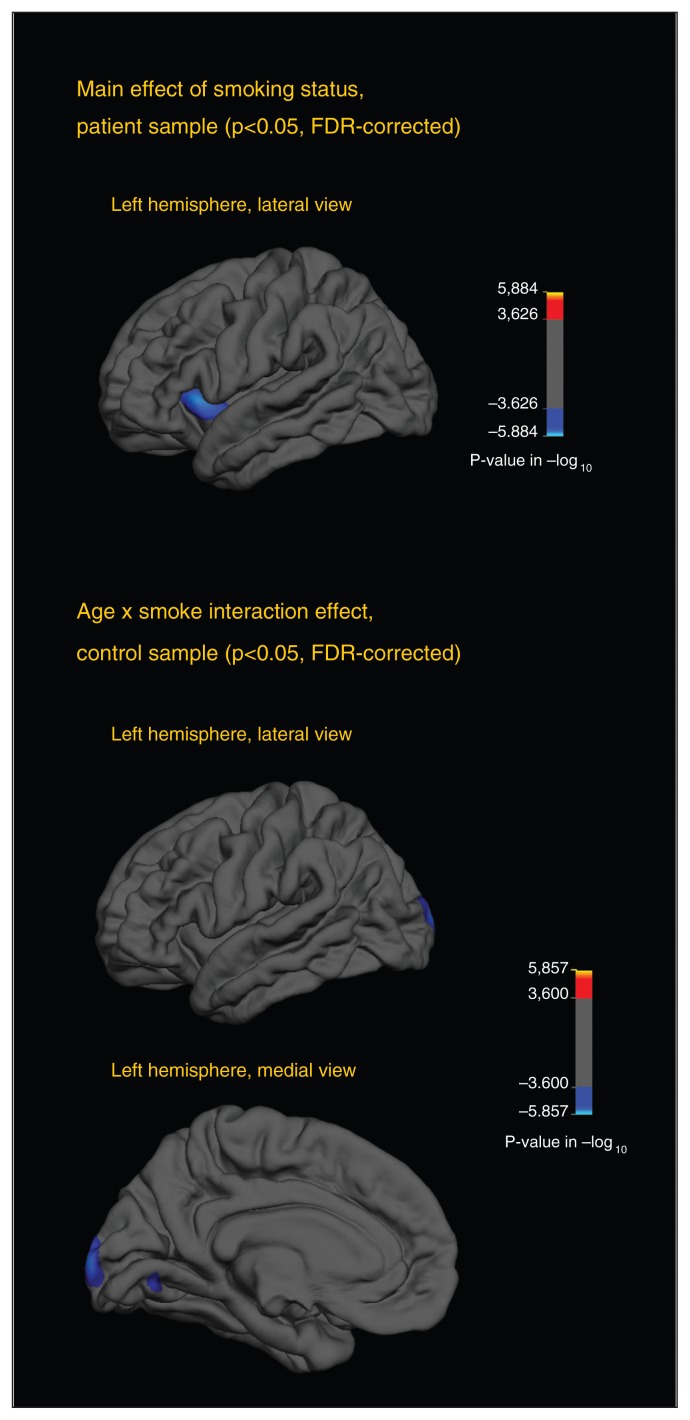
Statistical maps showing main effects of smoking and interaction effects between smoking and age are shown in Fig. 2. Among patients, cortical thinning was observed in a region in the left INS. This effect was observed across varying smoothing thresholds (FWHM of 10–30 mm). We observed no further regional cortical thickness differences between smokers and nonsmokers.
Fig. 2.
The top statistical map illustrates differences in cortical thickness between smoking and nonsmoking patients. The blue area indicates a significantly thinner cortex (p < 0.05, false-discovery rate [FDR]–corrected) in smokers compared with nonsmokers. Since no areas were significantly different in the right hemisphere and no differences emerged in the medial view, only the lateral view of the left hemisphere is provided. The 2 bottom statistical maps illustrate different age slopes between smoking and nonsmoking healthy controls. Blue areas indicate a steeper slope, with increased thinning with older age among smokers (p < 0.05, FDR-corrected). All analyses were adjusted for age, sex and diagnosis (patients only) and were performed in FreeSurfer using the general linear model function.
Among healthy controls, no main effect of smoking was observed. A significant age × smoking status interaction effect in a region in the left occipital cortex indicated increased cortical thinning with greater age among smokers. This effect was not changed by applying smoothing kernels of 15 mm and 25 mm, but when p maps were smoothed using the lowest (10 mm FWHM) or highest (30 mm FWHM) thresholds, the results did not remain significant after FDR correction.
In the ROI analyses, we found reduced thickness in the left ACC. In the same region, reduced thickness was among the findings observed in uncorrected p maps (p < 0.01), but did not survive FDR correction. For completeness, the uncorrected p maps are provided and the results are described in the Appendix.
Discussion
The main finding of the present study was reduced cortical thickness in regions of the cingulate and insular cortices among smoking patients compared with nonsmoking patients. These findings were largely unaffected by adjusting for potential confounders. Some of the reduction observed in the left INS was found to be attributable to alcohol and illicit substance use, although their effects were small; no confounding effect was found in the left rostral ACC. No reduced cortical thickness in these regions was demonstrated in healthy controls.
The cingulate and insular cortices have previously been implicated in smoking addiction among healthy smokers in resting state fMRI studies,46 and these regions contain high densities of nicotinic acetylcholine receptors (nAChR).47 These receptors have been shown to be upregulated among smokers, both in healthy samples and in patients with schizophrenia.48–50 In animal models, prolonged exposure to nicotine in early development, adolescence and, to some extent, in adulthood has been shown to lead to desensitization and lasting deleterious effects on cholinergic synaptic transmission.51,52 Although it is possible this could result in cortical thinning, other neurotoxic effects, such as oxidative stress or apoptosis, cannot be excluded.53 Regions in the cingulate and insular cortices are considered central nodes in the salience network,54 which based on resting-state fMRI studies is implicated in psychosis.26,55 Two recent studies suggest that an overlapping circuitry may be involved in schizophrenia and smoking addiction.56,57 Interestingly, nAChR genes, which have been previously associated with smoking addiction, may confer risk for schizophrenia.58 Cognitive processes that are often reported to be impaired in patients with schizophrenia, such as attention and working memory, have been implicated in research on cortical thinning in patients with severe mental illness59–61 as well as in research on the effects of smoking on brain microstructure.43,44 Furthermore, these cognitive processes are modulated by the cholinergic system,62 and there may be lower nAChR availability in several brain regions in individuals with schizophrenia.63 Future studies should examine the interplay between smoking-related cortical thinning, cognitive deficits in patients with severe mental illness and a potential modulation by nAChR gene variants.
We observed no main effect of smoking in healthy controls in the selected regions. Several explanations are possible. First, healthy controls are likely to have smoked less than patients.64 Previous studies have reported that patients with schizophrenia consume more cigarettes per day and extract more puffs and nicotine from each cigarette than healthy controls.14,65 Unfortunately, we lacked data on smoking intensity among controls and could not investigate this further. Second, we could not examine whether patients and controls started smoking at the same age. In the medial OFC, we found an opposite age association in controls and patients; a main effect of smoking indicated reduced thickness in the left medial OFC, but significant interaction effects suggested that less reduction was present with older age, although none of these findings survived correction for multiple testing. It could be speculated that a greater proportion of older controls had previously smoked. Taking into account the smaller sample size of smoking controls (n = 48), it is difficult to draw firm conclusions; whether patients are more vulnerable to smoking-related cortical reductions than the population in general remains to be answered.
The rate of smoking in our patient sample was close to 50%, which is in line with previous studies reporting rates of about 60% in patients with schizophrenia.64,66 Daily smoking rates in the Norwegian population have declined from 22% in 2007 to 16% in 2012 (www.ssb.no/en/royk). In line with this, 20% of our healthy controls were smokers.
Limitations
Although our study had a large sample size, allowing us to control for a range of possible confounders, including substances that may affect brain structure independent of smoking, there are limitations to consider. First, the study was not primarily designed to examine the effects of smoking, and we had limited data available on smoking behaviour, particularly for the healthy controls. A more detailed assessment of smoking severity, history and dependence could shed more light on the differences observed between patients and controls. We did not find a dose–response relationship between smoking intensity and cortical thinning. However, without a more detailed smoking history, it is difficult to conclude that no such relationship exists. Second, smoking data were collected through personal interviews with patients and retrospective telephone interviews with controls. However, this limitation does not affect the interpretation of within-group findings among patients. Third, the time period between assessment of smoking behaviour and MRI scan (about 4 months) is a limitation. However, smoking status is known to be quite stable, with low cessation rates among patients.12 Fourth, FDR correction methods were applied in both the ROI and the whole brain cortical thickness analyses. Although we consider that this balances risks of false-positive versus false-negative results, family-wise error correction methods, such as Bonferroni correction, would have been more conservative.
Conclusion
Cigarette smoking is associated with reduced cingulate and insulate thickness among patients with severe mental illness. The degree to which smoking may confound findings in MRI studies of psychiatric disorders depends on 2 factors: the causal relationship and the degree of association. First, cigarette smoking can act as a confounder only if it causes reduced cortical thickness, but it may be premature to draw conclusions about causality. Second, our findings do not indicate large effects, with smokers showing reductions of up to about 0.05 mm in limited regions (left rostral ACC) within the patient group. The difference between patients and healthy controls was estimated to be about 0.075 mm in the same region after adjusting for smoking and the smoking × group interaction. Further, in a previous study of schizophrenia involving an overlapping sample, we reported cortical thinning in more widespread frontotemporal regions.37 Thus, although cigarette smoking may play a role, it explains only a limited portion of the cortical thickness reductions that have been demonstrated in previous studies of these disorders. Until the causal relationship has been clarified, future studies should take cigarette smoking into account together with other potential confounders.
Acknowledgements
The authors thank the study participants and clinicians involved in the recruitment and assessment in the Norwegian Research Center for Mental Disorders (NORMENT) and Stener Nerland for valuable help and advice regarding analyses in FreeSurfer.
Footnotes
Funding: The study was supported by grants from the Research Council of Norway (grant numbers 190311/V50, 167153/V50, 223273), the South-Eastern Norway Regional Health Authority (grant numbers 2012100, 2011092, 2011096, 2009037) and the K.G. Jebsen Foundation. The funding sources had no further role in the design of the study; in the collection, analysis, and interpretation of the data; in writing the manuscript; or in the decision to submit the paper for publication.
Competing interests: O. Andreassen has received speaker fees from Osaka, GlaxoSmithKline and Lundbeck. No other competing interests declared.
Contributors: K. Jørgensen, I. Skjærvø and I. Agartz designed the study. K. Jørgensen, U. Haukvik, E. Lange, I. Melle, O. Andreassen and I. Agartz acquired the data, which K. Jørgensen, I. Skjærvø, L. Mørch-Johnsen and I. Agartz analyzed. K. Jørgensen and I. Skjærvø wrote the article, which all authors reviewed and approved for publication.
References
- 1.Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–19. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 2.Crespo-Facorro B, Roiz-Santiáñez R, Pérez-Iglesias R, et al. Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med. 2011;41:1449–60. doi: 10.1017/S003329171000200X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz CC, Koch K, Wagner G, et al. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116:204–9. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–88. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 5.Nesvåg R, Lawyer G, Varnäs K, et al. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Rimol LM, Hartberg CB, Nesvåg R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Foland-Ross LC, Thompson PM, Sugar CA, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar type I disorder using cortical pattern matching. Am J Psychiatry. 2011;168:530–9. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39:1363–72. doi: 10.1093/schbul/sbs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 11.Birkenaes AB, Sogaard AJ, Engh JA, et al. Sociodemographic characteristics and cardiovascular risk factors in patients with severe mental disorders compared with the general population. J Clin Psychiatry. 2006;67:425–33. doi: 10.4088/jcp.v67n0314. [DOI] [PubMed] [Google Scholar]
- 12.Diaz FJ, Rendon D, Velásquez D, et al. Datapoints: smoking and smoking cessation among persons with severe mental illnesses. Psychiatr Serv. 2006;57:462. doi: 10.1176/ps.2006.57.4.462. [DOI] [PubMed] [Google Scholar]
- 13.Lo S, Heishman SJ, Raley H, et al. Tobacco craving in smokers with and without schizophrenia. Schizophr Res. 2011;127:241–5. doi: 10.1016/j.schres.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JM, Gandhi KK, Lu S-E, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res. 2010;12:855–9. doi: 10.1093/ntr/ntq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiser M, Reichenberg A, Grotto I, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical prospective cohort study. Am J Psychiatry. 2004;161:1219–23. doi: 10.1176/appi.ajp.161.7.1219. [DOI] [PubMed] [Google Scholar]
- 16.Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 17.Liao Y, Tang J, Liu T, et al. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2012;17:977–80. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu R, Zhao L, Lu L. Regional grey and white matter changes in heavy male smokers. PLoS ONE. 2011;6:e27440. doi: 10.1371/journal.pone.0027440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales AM, Lee B, Hellemann G, et al. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–8. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–50. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- 21.Kühn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry. 2010;68:1061–5. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Durazzo TC, Mon A, Gazdzinski S, et al. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict Biol. 2013;18:379–91. doi: 10.1111/j.1369-1600.2011.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales AM, Ghahremani D, Kohno M, et al. Cigarette exposure, dependence and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.48. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fornito A, Yücel M, Patti J, et al. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–13. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung M, Cheung C, Yu K, et al. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornito A, Yücel M, Wood SJ, et al. Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. Br J Psychiatry. 2009;194:426–33. doi: 10.1192/bjp.bp.107.049205. [DOI] [PubMed] [Google Scholar]
- 29.Tregellas JR, Shatti S, Tanabe JL, et al. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophr Res. 2007;97:242–9. doi: 10.1016/j.schres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Schneider CE, White T, Hass J, et al. Smoking status as a potential confounder in the study of brain structure in schizophrenia. J Psychiatr Res. 2014;50:84–91. doi: 10.1016/j.jpsychires.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 32.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–96. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ségonne F, Dale A, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Rimol LM, Hartberg CB, Nesvag R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 38.Okuyemi KS, Ahluwalia JS, Richter KP, et al. Differences among African American light, moderate, and heavy smokers. Nicotine Tob Res. 2001;3:45–50. doi: 10.1080/14622200020032097. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, Wood SJ, Soulsby B, et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr Res. 2009;108:49–56. doi: 10.1016/j.schres.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Padmanabhan JL, Tandon N, Haller CS, et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu075. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nesvåg R, Frigessi A, Jonsson EG, et al. Effects of alcohol consumption and antipsychotic medication on brain morphology in schizophrenia. Schizophr Res. 2007;90:52–61. doi: 10.1016/j.schres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Wechsler D. Wechsler abbreviated scale of intelligence (WASI) Norwegian manual supplement. Stockholm, Sweden: Harcourt Assessment; 2007. [Google Scholar]
- 43.Cullen KR, Wallace S, Magnotta VA, et al. Cigarette smoking and white matter microstructure in schizophrenia. Psychiatry Res. 2012;201:152–8. doi: 10.1016/j.pscychresns.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gons RA, van Norden AG, de Laat KF, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain. 2011;134:2116–24. doi: 10.1093/brain/awr145. [DOI] [PubMed] [Google Scholar]
- 45.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statistic Soc B. 1995;57:289–300. [Google Scholar]
- 46.Hong LE, Hodgkinson CA, Yang Y, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–14. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picard F, Sadaghiani S, Leroy C, et al. High density of nicotinic receptors in the cingulo-insular network. Neuroimage. 2013;79:42–51. doi: 10.1016/j.neuroimage.2013.04.074. [DOI] [PubMed] [Google Scholar]
- 48.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–65. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esterlis I, Ranganathan M, Bois F, et al. In vivo evidence for b2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry. 2014;76:495–502. doi: 10.1016/j.biopsych.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staley JK, Krishnan-Sarin S, Cosgrove KP, et al. Human tobacco smokers in early abstinence have higher levels of b2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–14. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Slotkin TA, Ryde IT, Seidler FJ. Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res Bull. 2007;74:91–103. doi: 10.1016/j.brainresbull.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Ferrea S, Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42:255–65. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- 54.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palaniyappan L, Simmonite M, White Thomas P, et al. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–28. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moran LV, Sampath H, Kochunov P, et al. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013;39:1373–81. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moran LV, Sampath H, Stein EA, et al. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–9. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong LE, Yang X, Wonodi I, et al. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav. 2011;10:530–5. doi: 10.1111/j.1601-183X.2011.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cobia DJ, Csernansky JG, Wang L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr Res. 2011;133:68–76. doi: 10.1016/j.schres.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartberg CB, Sundet K, Rimol LM, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc. 2011;17:1080–93. doi: 10.1017/S1355617711001081. [DOI] [PubMed] [Google Scholar]
- 61.Ehrlich S, Brauns S, Yendiki A, et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr Bull. 2012;38:1050–62. doi: 10.1093/schbul/sbr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jasinska AJ, Zorick T, Brody AL, et al. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84:111–22. doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62:1564–73. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Tidey JW, Rohsenow DJ, Kaplan GB, et al. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend. 2005;80:259–65. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Chapman S, Ragg M, McGeechan K. Citation bias in reported smoking prevalence in people with schizophrenia. Aus N Z J Psychiatry. 2009;43:277–82. doi: 10.1080/00048670802653372. [DOI] [PubMed] [Google Scholar]