Abstract
Background
Bipolar disorder is associated with medical comorbidities that have been linked to systemic inflammatory mechanisms. There is, however, limited evidence supporting a role of neuroinflammation in bipolar disorder. Here we tested whether microglial activation and associated tissue remodelling processes are related to bipolar disorder by analyzing markers in cerebrospinal fluid (CSF) and serum from patients and healthy controls.
Methods
Serum was sampled from euthymic patients with bipolar disorder and healthy controls, and CSF was sampled from a large subset of these individuals. The levels of monocyte chemoattractant protein-1 (MCP-1), YKL-40, soluble cluster of differentiation 14 (sCD14), tissue inhibitor of metalloproteinases-1 (TIMP-1) and tissue inhibitor of metalloproteinases-2 (TIMP-2), were measured, and we adjusted comparisons between patients and controls for confounding factors.
Results
We obtained serum samples from 221 patients and 112 controls and CSF samples from 125 patients and 87 controls. We found increased CSF levels of MCP-1 and YKL-40 and increased serum levels of sCD14 and YKL-40 in patients compared with controls; these differences remained after controlling for confounding factors, such as age, sex, smoking, blood–CSF barrier function, acute-phase proteins and body mass index. The CSF levels of MCP-1 and YKL-40 correlated with the serum levels, whereas the differences between patients and controls in CSF levels of MCP-1 and YKL-40 were independent of serum levels.
Limitations
The cross-sectional study design precludes conclusions about causality.
Conclusion
Our results suggest that both neuroinflammatory and systemic inflammatory processes are involved in the pathophysiology of bipolar disorder. Importantly, markers of immunological processes in the brain were independent of peripheral immunological activity.
Introduction
Bipolar disorder is a severe psychiatric condition characterized by recurrent episodes of elevated (mania or hypomania) or depressed mood and interepisodic periods with no or few symptoms.1,2 The disorder has consistently been associated with inflammation-related medical comorbidities (e.g., cardiovascular diseases, obesity, insulin resistance, autoimmune disorders), and the peripheral pathophysiology of bipolar disorder appears to be related to systemic inflammatory mechanisms.3,4 Furthermore, inflammation has been proposed to be a causative factor for disease progression in patients with bipolar disorder.5
Evidence of immune system involvement in bipolar disorder comes mainly from blood analyses of circulating inflammatory markers, including cytokines, chemokines and cytokine receptors.6 Two recent meta-analyses listed increased serum levels of interleukin (IL)-4, IL-10, tumour necrosis factor (TNF)-α, sIL-2R, sIL-6R and sTNF-R1 as the most consistent findings in patients with bipolar disorder.7,8 However, previous studies on inflammatory markers have rarely accounted for potential confounders, such as body mass index (BMI), smoking and ongoing medication use. Moreover, the interpretation of the findings is hampered by the fact that elevated inflammatory markers in the blood cannot be presumed to reflect inflammatory processes in the central nervous system (CNS). There is actually limited evidence supporting a role of neuroinflammation in bipolar disorder. Findings of increased levels of microglia markers in the post-mortem frontal cortices of patients with bipolar disorder suggest, however, that microglia — the resident immune cells of the CNS — play a role in the pathophysiology of the disorder.9 In addition, increased binding of the PK11195 positron emission tomography (PET) ligand, which is indicative of activated microglia, has been reported in the hippocampus of patients with bipolar disorder.10 These studies suggest that the monocyte-T-cell theory of mood disorders initially proposed for major depression may also be applicable to bipolar disorder.11,12
In contrast to inflammatory marker levels in the blood, cerebrospinal fluid (CSF) marker levels accurately reflect immunological activity in the brain.13,14 In a small study involving 30 patients and 30 healthy controls, increased levels of IL-1 were reported in patients with bipolar disorder.15 However, cytokines are unstable proteins and sensitive to preanalytical factors (e.g., storage conditions, sample handling).16 In addition, current immunoassays lack sensitivity for reliable quantification of low abundant cytokines in the CSF.17 An increased blood–CSF barrier permeability in patients with bipolar disorder may also be an important confounder, as this leads to increased passage of plasma proteins to CSF resulting in increased CSF levels.18 Hence, studies of more stable and reliable CSF markers to assess neuroinflammation are warranted. Three such candidate markers are monocyte chemoattractant protein 1 (MCP-1; also called CCL-2), YKL-40 (also called chitinase-3-like protein 1) and soluble cluster of differentiation 14 (sCD14), which are all primarily secreted by cells of monocytic origin (e.g., microglia in the brain). Alterations in these markers have been associated with microglial activation in neurologic disorders, such as multiple sclerosis, Alzheimer disease and Parkinson disease.19 Activated microglia secrete proteolytic enzymes that can degrade the neighbouring extracellular matrix and neuronal cells.20 These enzymes include the matrix metalloproteinases (MMPs), whose activities are strictly regulated by the tissue inhibitors of metalloproteinases (TIMPs).21 Increased levels of MMPs and TIMPs in the CSF may reflect the degree of inflammation-related tissue remodelling in the CNS and have been demonstrated in several neuroinflammatory disorders.22–25
The aim of the present study was to test if microglial activation and associated tissue remodelling processes are related to bipolar disorder. We analyzed markers of these processes in both CSF and serum from patients and healthy controls. The large sample size allowed us to control for possible confounders, such as age, sex, smoking, blood–CSF barrier function, acute-phase proteins and BMI, and investigate associations with disease severity, subdiagnosis and current treatment.
Methods
Study population
Patients were recruited from the St. Göran bipolar project, which includes patients from the bipolar unit at the Northern Stockholm Psychiatric Clinic, Stockholm, Sweden. The work-up and diagnostic assessments have been described in detail previously.26 The key clinical assessment instrument used was the Affective Disorder Evaluation (ADE), which was developed for the Systematic Treatment Enhancement Program of Bipolar Disorder (STEP-BD).27 The full diagnostic assessment was based on all available sources of information, including patient interviews, case records and, if possible, interviews with the next of kin. The diagnoses were established at a diagnostic case conference where all information available at the time of admission was presented. A consensus panel of experienced board-certified psychiatrists specialized in bipolar disorder made a best-estimate diagnostic decision. Using this procedure, the risk of bias in the inclusion process was reduced. To be included, patients were required to be 18 years or older and to meet DSM-IV criteria for bipolar-spectrum disorders (i.e., type I, type II or not otherwise specified). We collected information on age; sex; number of lifetime manic, hypomanic, depressive and total episodes; duration of illness, defined as years since first hypomanic or manic episode; age at onset of illness, defined as age at first hypomanic or manic episode; family history of bipolar disorder (first- or second-degree relatives with bipolar disorder); years of education; primary source of income; BMI; and previous psychotic episodes. The severity of bipolar disorder was rated using the Clinical Global Impression (CGI) rating scales and Global Assessment of Functioning (GAF). For ethical reasons, patients continued to take their prescribed medications at the time of CSF and blood sampling.
Population-based controls were randomly selected by Statistics Sweden (SCB) and contacted by mail. A research nurse contacted individuals who volunteered to participate, and a preliminary telephone screening was conducted to exclude individuals with severe mental health and neurologic problems and substance abuse. Eligible individuals were scheduled for a 1-day comprehensive assessment. Of the controls who received the invitation, 14% contacted the research team. This is on par with other studies of similar nature according to SCB. Controls underwent a psychiatric interview by experienced clinicians using the Mini-International Neuropsychiatric Interview to exclude psychiatric disorders.28 We screened for substance abuse in several ways: during the telephone interview with the nurse, during the psychiatric interview, using the Alcohol Use Disorders Identification Test (AUDIT) and Drug Use Disorders Identification Test (DUDIT) and by determining serum levels of carbohydrate-deficient transferrin (CDT).29 Overconsumption of alcohol, as revealed by CDT or responses indicating large consumption (> 8 standard drinks per time more than twice per week) and/or amnesia and/or loss of control more than once per month, resulted in the exclusion of these individuals from the study. Other exclusion criteria were neurologic conditions other than mild migraines, untreated endocrinological disorders, pregnancy, dementia, recurrent depressive disorder and suspected severe personality disorders (based on a psychiatric interview and assessment with the Structured Clinical Interview for DSM-IV Axis II Personality Disorders), and a family history of schizophrenia or bipolar disorder in first-degree relatives.
The Regional Ethics Committee in Stockholm approved our study, which we conducted in accordance with the latest Helsinki Protocol. All patients and controls consented orally and in writing to participate in the study.
CSF and blood sampling
Blood and CSF sampling (lumbar puncture) took place when the participants were euthymic. Sampling occurred between 9 and 10 am after an overnight fast. We collected a total volume of 12 mL of CSF and gently inverted it to avoid gradient effects. We obtained serum from blood samples after coagulation and centrifugation. Both serum and CSF samples were divided into aliquots that were stored at −80°C pending analysis at the Biobank at Karolinska Institutet, Stockholm. Sweden. Controls underwent an identical procedure. All samples in this study were thawed and refrozen once before analysis.
Analysis of CSF:serum albumin ratio
Serum and CSF levels of albumin were analyzed by immunonephelometry on an Immage immunochemistry system (Beckman Coulter Inc.) at the Clinical Neurochemistry Laboratory in Mölndal, Sweden, using a method accredited by the Swedish Board for Accreditation and Conformity Assessment (SWEDAC). Experienced and board-certified laboratory technicians who were blinded to clinical information performed all measurements. Intra- and interassay coefficients of variation were below 10%. To assess blood–CSF barrier function, we calculated the ratio between the albumin concentration in CSF (mg/L) and serum (g/L).30
Analysis of inflammatory markers
Blood levels of high-sensitivity C-reactive protein (hsCRP) were analyzed using an immunoturbimetric assay (Siemens Healthcare Diagnostics Inc. and Beckman Coulter Inc.) at Unilabs, Stockholm, Sweden. The limit of quantification was 0.2 mg/L. Further, MCP-1 and TIMP-1 were analyzed using commercial electrochemiluminescence enzyme-linked immunosorbant assays (ELISA; Human MCP-1 Ultra-Sensitive Kit and Human TIMP-1 kit, Meso Scale Discovery), and sCD14, YKL-40 and TIMP-2 were analyzed using commercial colorimetric ELISAs (Human sCD14 quantikine ELISA kit, Human chitinase-3 quantikine ELISA kit, Human TIMP-2 quantikine ELISA kit, R&D systems Inc.) at the Clinical Neurochemistry Laboratory in Mölndal, Sweden. All analyses were performed following the instructions from the manufacturer. Intra-assay coefficients of variation were below 10% for all assays. The staff performing the analyses were blinded to patient identity and diagnosis.
Statistics
We used SPSS Statistics software version 20 (IBM Corp.) in all statistical analyses. Data not showing normal distribution (as tested using a 1-sample Kolmogorov–Smirnov test) were log-transformed (log10) before parametric tests. We used multiple linear regression to analyze the effects of age, sex, BMI, smoking, clinical characteristics, diagnosis and current medications on CSF marker concentrations. Effect size was estimated using squared partial correlation (r2). We considered results to be significant at p < 0.05, 2-tailed. For post hoc analyses, we used false discovery rate (FDR) for correction of multiple testing.
Results
Participants
This study comprised serum measurements from 221 patients with bipolar disorder (84 men and 137 women) and 112 healthy controls (50 men and 62 women). We obtained CSF measurements from a subset of this sample population — 125 patients (50 men and 75 women) and 87 controls (39 men and 48 women) — who consented to lumbar puncture in addition to blood sampling. The demographic and clinical characteristics of participants are shown in Table 1. Patients had a higher median BMI and CSF:serum albumin ratio than controls, and the proportion of smokers was greater among patients than controls. The hsCRP levels did not differ between patients and controls. Patients’ current medication was classified as use or nonuse of different classes of psychoactive drugs. Many patients were taking a combination of medications.
Table 1.
Demographic and clinical characteristics of the study population
| CSF, no. (%) or median [IQR]* | Serum, no. (%) or median [IQR]* | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | Control (n = 87) | Bipolar disorder (n = 125) | p value | Control (n = 112) | Bipolar disorder (n = 221) | p value |
| Sex, no. male:female | 39:48 | 50:75 | 0.48‡ | 50:62 | 84:137 | 0.29‡ |
| Primary source of income | ||||||
| Employment/education | 85 (97.8) | 62 (54.9) | — | 109 (97.3) | 115 (59.3) | — |
| Sickness benefit | 1 (1.1) | 44 (38.9) | — | 1 (0.9) | 67 (34.5) | — |
| Supplementary benefit | — | 3 (2.7) | — | 1 (0.9) | 4 (2.1) | — |
| Relative’s or own capital | 1 (1.1) | 4 (3.5) | — | 1 (0.9) | 8 (4.1) | — |
| Smokers | 11 (12.6) | 37 (32.5) | 0.001‡ | 17 (15.2) | 66 (33.3) | < 0.001‡ |
| Family history of BD | — | 38 (38.0) | — | — | 52 (29.1) | — |
| Diagnosis | ||||||
| BD type I | — | 62 (49.6) | — | — | 111 (50.2) | — |
| BD type II | — | 43 (34.4) | — | — | 82 (37.1) | — |
| BD NOS | — | 20 (16.0) | — | — | 28 (12.7) | — |
| Previous psychotic episodes | — | 62 (50.8) | — | — | 103 (48.6) | — |
| Medication | ||||||
| Lithium | — | 73 (58.4) | — | — | 128 (60.7) | — |
| Lamotrigine | — | 26 (20.8) | — | — | 48 (22.7) | — |
| Valproate | — | 16 (12.8) | — | — | 24 (11.4) | — |
| Antidepressants | — | 57 (45.6) | — | — | 93 (44.1) | — |
| Benzodiazepines | — | 24 (19.2) | — | — | 38 (18.0) | — |
| Antipsychotics | — | 32 (25.6) | — | — | 49 (23.2) | — |
| Age, yr | 34 [27–46] | 36 [28–50] | 0.51§ | 34 [27–44] | 36 [28–47] | 0.36§ |
| Education, yr† | 14 [12–15] | 14 [12–15] | 0.73§ | 14 [12–15] | 14a [12–15] | 0.28§ |
| BMI | 23.4 [21.6–25.7] | 24.7 [22.2–27.7] | 0.027§ | 23.3 [21.7–25.4] | 24.7 [22.2–27.7] | 0.007§ |
| CSF:serum albumin ratio | 4.8 [3.7–6.0] | 5.1 [4.2–6.5] | 0.049§ | — | — | — |
| hsCRP† | 0.74b [0.35–1.60] | 0.90a [0.27–2.80] | 0.39§ | 0.73c [0.36–1.70] | 0.80d [0.29–2.60] | 0.79§ |
| Duration of illness, yr† | — | 12e [4–19] | — | — | 10f [4–19] | — |
| Age at onset, yr† | — | 23e [18–32] | — | — | 23f [18–32] | — |
| No. of episodes | ||||||
| Manic† | — | 1g [0–2] | — | — | 1b [0–2] | — |
| Depressive† | — | 4h [3–10] | — | — | 5i [3–10] | — |
| Total† | — | 10g [5–20] | — | — | 10a [5–20] | — |
| YMRS score† | — | 0 [0–2j] | — | — | 0 [0–2k] | — |
| MADRS score† | — | 4 [0–11l] | — | — | 4 [0–11m] | — |
| GAF score† | — | 68b [60–72] | — | — | 66b [60–75] | — |
| CGI score† | — | 4h [4–5] | — | — | 4n [4–5] | — |
BD = bipolar disorder; BMI = body mass index; CGI = Clinical Global Impressions scale; CSF = cerebrospinal fluid; GAF = Global Assessment of Functioning; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range; NOS = not otherwise specified.
Unless otherwise indicated.
Missing data: an = 12, bn = 9, cn = 11, dn = 19, en = 7, fn = 18, gn = 3, hn = 4, in = 13, jn = 20, kn = 30, ln = 25, mn = 28, nn = 11.
Mann–Whitney U test.
CSF marker in patients compared with controls
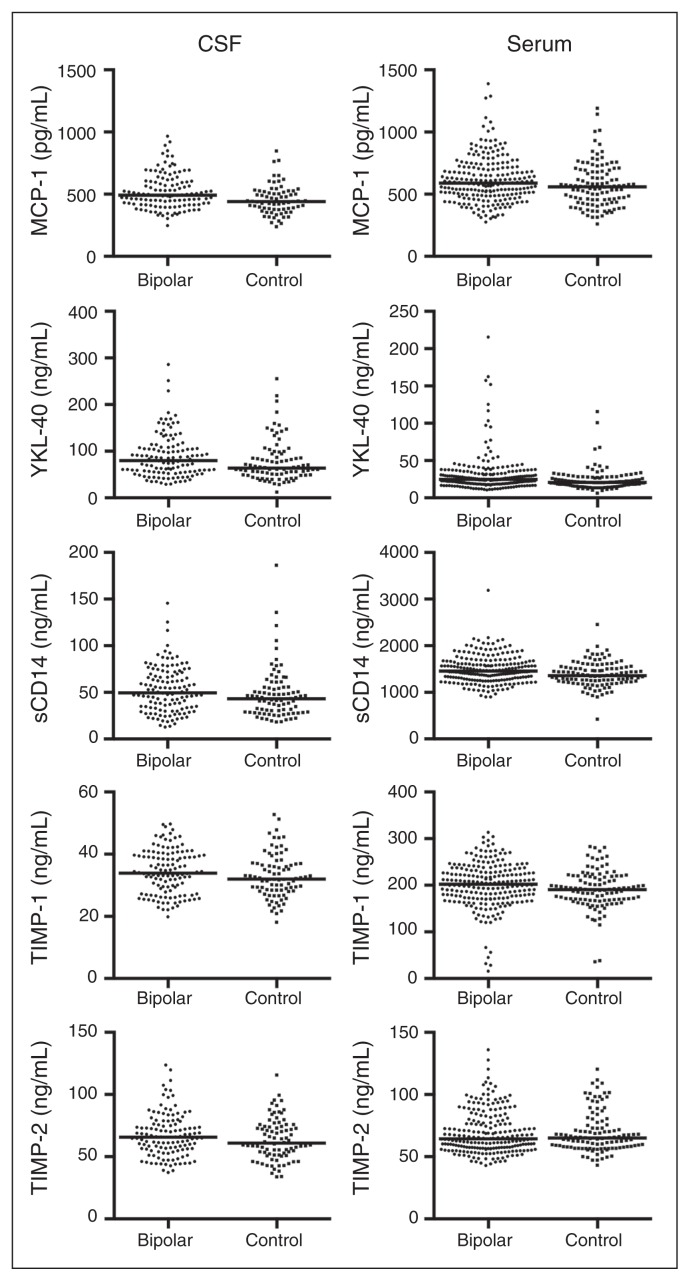
The CSF levels of MCP-1, YKL-40, sCD14, TIMP-1 and TIMP-2 in patients and controls are displayed in Figure 1. The MCP-1, YKL-40 and TIMP-2 levels were significantly higher in patients with bipolar disorder than controls when adjusting for age and sex (Table 2 and Appendix, Table S1, available at jpn.ca). Increased CSF levels in patients may be secondary to differences between patients and controls in confounding factors, such as BMI, increased blood–CSF barrier permeability (assessed using the CSF:serum albumin ratio), smoking or unspecific infections (assessed using hsCRP; Table 1). Thus, we adjusted for these covariates (Table 2 and Appendix, Table S2). The CSF:serum albumin ratio correlated with all CSF markers, and sCD14 correlated with BMI and smoking. Smoking also correlated with TIMP-1 and TIMP-2. High-sensitivity CRP was not significantly associated with any of the CSF markers. The CSF levels of MCP-1 and YKL-40 were the only ones that remained significantly higher in patients than in controls when adjusting for all confounders.
Fig. 1.
Grouped scatter plot showing the serum and cerebrospinal fluid (CSF) concentrations of MCP-1, YKL-40, sCD14, TIMP-1, and TIMP-2 in patients and controls. The median is shown as a straight line. Samples with extreme high values have been excluded from the graphs (i.e., 1 patient from the CSF MCP-1, 1 patient from theserum TIMP-1 and 1 patient from the serum TIMP-2 samples). MCP-1 = monocyte chemoattractant protein-1; sCD14 = soluble cluster of differentiation; TIMP = tissue inhibitor of metalloproteinases; YKL-40 = Chitinase-3-like protein-1.
Table 2.
Comparisons of cerebrospinal fluid marker levels between patients with bipolar disorder and healthy controls.
| Group, no. (median) [IQR] | Analysis 1* | Analysis 2† | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Marker | Control | Bipolar disorder | β | p value | r2 | β | p value | r2 |
| MCP-1, pg/mL | 71 (440) [377–521] | 125 (493) [417–602] | 0.213 | 0.001 | 0.057 | 0.201 | 0.004 | 0.052 |
| YKL-40, ng/mL | 87 (63.5) [49.3–94.5] | 125 (80.0) [55.9–107.7] | 0.121 | 0.011 | 0.030 | 0.132 | 0.014 | 0.035 |
| sCD14, ng/mL | 87 (43.3) [29.0–55.4] | 125 (49.5) [33.5–69.6] | 0.095 | 0.08 | 0.015 | 0.069 | 0.19 | 0.010 |
| TIMP-1, ng/mL | 87 (32.0) [28.4–36.8] | 125 (33.9) [27.8–39.7] | 0.096 | 0.08 | 0.014 | 0.062 | 0.19 | 0.010 |
| TIMP-2, ng/mL | 87 (60.9) [51.5–74.3] | 125 (65.6) [56.1–76.0] | 0.107 | 0.047 | 0.019 | 0.075 | 0.13 | 0.013 |
BMI = body mass index; CSF = cerebrospinal fluid; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range; MCP-1 = monocyte chemoattractant protein-1; sCD14 = soluble cluster of differentiation; TIMP = tissue inhibitor of metalloproteinases; YKL-40 = Chitinase-3-like protein-1.
Comparison of 125 patients and 87 controls (except for MCP-1: n = 71 controls) using linear regression with age and sex as covariates.
Comparison of 104 patients and 77 controls (except for MCP-1: n = 63 controls) using linear regression with age, sex, smoking, hsCRP, CSF:serum albumin ratio and BMI as covariates.
Serum markers in patients compared with controls
The serum levels of MCP-1, YKL-40, sCD14, TIMP-1 and TIMP-2 in patients and controls are displayed in Figure 1. All serum marker levels except TIMP-2 were significantly higher in patients with bipolar disorder than controls when adjusting for age and sex (Table 3 and Appendix, Table S3). We next analyzed differences between patients and controls adjusting for BMI, hsCRP and smoking status (Table 3 and Appendix, Table S4). We found that hsCRP correlated with YKL-40, sCD14, TIMP-1 and TIMP-2, whereas smoking correlated with MCP-1. Serum levels of YKL-40 and sCD14 were still significantly higher in patients than in controls when adjusting for all confounders.
Table 3.
Comparisons of serum marker levels between patients with bipolar disorder and healthy controls
| Group, no. (median) [IQR] | Analysis 1* | Analysis 2† | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Marker | Control | Bipolar disorder | β | p value | r2 | β | p value | r2 |
| MCP-1, pg/mL | 112 (559) [446–684] | 221 (589) [492–715] | 0.213 | 0.040 | 0.013 | 0.094 | 0.11 | 0.009 |
| YKL-40, ng/mL | 112 (20.8) [15.8–27.7] | 221 (24.7) [19.0–32.8] | 0.184 | < 0.001 | 0.039 | 0.176 | 0.002 | 0.035 |
| sCD14, ng/mL | 112 (1360) [1225–1534] | 221 (1455) [1288–1632] | 0.156 | 0.004 | 0.026 | 0.148 | 0.009 | 0.024 |
| TIMP-1, ng/mL | 112 (191.0) [168.6–218.7] | 221 (202.8) [172.2–231.9] | 0.096 | 0.047 | 0.012 | 0.062 | 0.19 | 0.010 |
| TIMP-2, ng/mL | 112 (65.0) [57.7–75.4] | 221 (64.5) [56.9–78.7] | −0.006 | 0.92 | 0.000 | 0.075 | 0.13 | 0.013 |
BMI = body mass index; hsCRP = high-sensitivity C-reactive protein; IQR = interquartile range; MCP-1 = monocyte chemoattractant protein-1; sCD14 = soluble cluster of differentiation; TIMP = tissue inhibitor of metalloproteinases; YKL-40 = Chitinase-3-like protein-1.
Comparison of 221 patients and 112 controls using linear regression with age and sex as covariates.
Comparison of 183 patients and 101 controls using linear regression with age, sex, smoking, hsCRP and BMI as covariates.
Associations between serum and CSF markers
As both CSF and serum markers were higher in patients than controls, it is conceivable that increased levels of CSF markers reflect a systemic increase in inflammatory markers rather than specific inflammatory processes in the brain. Thus, we investigated if serum levels had any impact on the CSF levels. Serum levels of both MCP-1 and YKL-40 correlated with the CSF levels (MCP-1: β = 0.353, t = 5.955, p < 0.001, r2 = 0.158, adjusted for age, sex, CSF:serum albumin ratio and diagnosis; YKL-40: β = 0.182, t = 3.597, p < 0.001, r2 = 0.063, adjusted for age, BMI, smoking, CSF:serum albumin ratio and diagnosis). We found no association between serum and CSF levels of sCD14, TIMP-1 and TIMP-2 (data not shown). The difference between patients and controls in CSF levels of MCP-1 and YKL-40 remained significant after adjusting for serum levels (MCP-1: β = 0.164, t = 2.855, p = 0.005, r2 = 0.041; YKL-40: β = 0.098, t = 2.062, p = 0.041, r2 = 0.015).
Effects of current medications and clinical status on marker levels
We next tested if alterations in serum and CSF markers in the patient group were associated with current medications, bipolar disorder subtype and/or variables reflecting disease intensity/severity (i.e., history of psychosis, duration of illness, age at onset, family history of bipolar disorder, education, primary source of income, number of episodes [total, manic and depressive], CGI score, GAF score; Appendix, Tables S5 and S6). Lithium, benzodiazepines and antipsychotics were positively associated with CSF levels of MCP-1, whereas antidepressant treatment was negatively associated with CSF levels of MCP-1 when controlling for age, sex and CSF:serum albumin ratio. Previous psychotic episodes were positively associated with CSF levels of sCD14. We found no other associations between CSF markers and medications or clinical status variables. None of the significant associations remained after correction for multiple testing. In serum, sCD14 was positively associated with the number of manic episodes and negatively associated with employment or education as the primary source of income. These associations remained significant after correction for multiple testing.
Discussion
Both systemic inflammation and neuroinflammation may be important components in the pathophysiology of bipolar disorder. While there is evidence of peripheral inflammation in bipolar disorder, the potential role of neuroinflammation and microglial activation is inconclusive. In this study, we analyzed markers of monocyte and microglial activation and associated tissue remodelling processes in CSF and serum from a large and carefully phenotyped sample population of patients with bipolar disorder and healthy controls. The primary aim was to investigate the role of CNS immune activation in bipolar disorder, and the secondary aim was to investigate the role of systemic inflammatory processes. Our results suggest that both neuroinflammatory and systemic inflammatory processes play a role in the pathophysiology of bipolar disorder.
We found 2 lines of evidence suggesting that microglia and neuroinflammation are involved in the pathophysiology of bipolar disorder. First, we found higher CSF levels of MCP-1 in patients with bipolar disorder than controls, and this difference remained after controlling for a range of possible confounding factors, such as age, sex, peripheral inflammation, smoking status, blood–CSF barrier dysfunction and serum levels of MCP-1. Second, we found higher CSF levels of YKL-40 in patients than controls, and this difference also persisted after controlling for confounding factors. In response to proinflammatory cytokines, the expression of MCP-1 increases in various cell types, including microglia, astrocytes and neurons, and the increased expression is primarily involved in the recruiting of microglia to sites of injury or inflammation and not increased expression/secretion of proinflammatory cytokines.31 In addition, the function of MCP-1 may be more complex than previously thought, as MCP-1 also possesses neuromodulatory functions.32 Thus, the increased MCP-1 levels in CSF may be indicative of both neuroprotective and neurotoxic processes. Another example demonstrating the complexity in interpreting MCP-1 results is that the neuroinflammatory condition multiple sclerosis has been associated with relatively low CSF levels of MCP-1, but high levels of another chemokine CXCL10.33 In contrast to MCP-1, YKL-40 appears to be more expressed in microglia than in neurons or astrocytes.34 Increased YKL-40 expression is characteristic of many chronic inflammatory conditions, such as rheumatoid arthritis and irritable bowel syndrome, that are associated with destruction of the extracellular matrix and tissue remodelling.35 It is therefore conceivable that YKL-40 causes remodelling of the extracellular matrix in the brain that could lead to neuronal dysfunction.
In the present study, post hoc analyses identified several weak associations with ongoing treatments and CSF levels of MCP-1. Use of lithium, benzodiazepines and antipsychotics was positively associated with MCP-1, whereas use of antidepressants was negatively associated with MCP-1 levels. These associations appear to be brain-specific, as no associations were observed between medications and serum levels of MCP-1. In contrast, no medication was associated with YKL-40 levels. The effects of medications on microglia activity have been assessed in other models and partially contrast our observations. For instance, lithium has been shown to inhibit microglial activation and cytokine/chemokine secretion in rats.36,37 In addition, mood stabilizers and antidepressants have an anti-inflammatory effect on isolated monocytes.38,39 In this cross-sectional study, however, we are not able to make causal inferences with respect to pharmacodynamic effects.
The CSF levels of sCD14, TIMP-1 and TIMP-2 were higher in patients than controls at a trend level. The levels of these markers were strongly associated with the CSF:serum albumin ratio, and adding this factor as a covariate reduced the differences between patients and controls. We have previously reported a higher CSF:serum albumin ratio in patients with bipolar disorder and an association with antipsychotic treatment.18 An increase in the CSF:serum albumin ratio indicates impaired blood–CSF barrier function due to increased cerebral capillary permeability or altered CSF production/flow.30 Independent of underlying mechanism, an increased CSF:albumin ratio is accompanied with increased concentrations of plasma proteins in CSF. On the other hand, increased CSF:albumin ratio may be driven by inflammatory mechanisms.40 It is thus possible that the MMP/TIMP system has a role in inflammation-mediated tissue remodelling (i.e., reorganization of the extracellular matrix) in patients with bipolar disorder but that this process is obscured by the association between CSF levels of TIMPs and the CSF:serum albumin ratio.
We also found indications of systemic inflammation, as serum levels of sCD14 and YKL-40 were higher in patients than controls. Previous studies have suggested that sCD14 is secreted both by monocytes/macrophages and by hepatocytes as an acute phase protein.41,42 We found that the serum levels of sCD14 were positively correlated with the acute phase protein hsCRP, but that the difference between patients and controls was independent of this correlation. High-sensitivity CRP is predominantly secreted by the liver (monocytes also express CRP but likely to a minor extent compared with sCD14).43 Thus, our results indicate that the increase in sCD14 is related to an increased secretion by monocytes rather than increased secretion by the liver. Furthermore, sCD14 is involved in the innate immune response to infections and mediates phagocytosis by binding to bacterial ligands, such as lipopolysaccharide (LPS).44 Intestinal mucosal dysfunction has been suggested to play a role in the pathophysiology of depression.45 This dysfunction is related to increased exposure of enterobacteria to the systemic circulation, a process known as bacterial translocation. It has been suggested that sCD14 is a marker of bacterial translocation, and it has recently also been assessed in serum from patients with schizophrenia and bipolar disorder.46 In line with our findings, these authors found higher sCD14 concentrations in patients with bipolar disorder and those with schizophrenia than in healthy controls. We found that, like sCD14, serum levels of YKL-40 were positively correlated with the acute phase protein hsCRP, but the difference between patients and controls was independent of this correlation. Serum YKL-40 is increased in many diseases, which may be explained by its role as an acute phase protein. Post hoc analyses indicated that serum levels of YKL-40 and sCD14 do not reflect identical processes (i.e., YKL-40 was not associated with number of manic episodes). One possible explanation is that increased CSF levels of YKL-40 contribute to the higher serum levels. This explanation is supported by the fact that the YKL-40 levels are about 3 times higher in CSF than in serum.
No differences between patients and controls in serum levels of MCP-1, TIMP-1 and TIMP-2 were found when adjusting for covariates. The serum levels of MCP-1 have previously been assessed in 2 different cohorts of euthymic patients.47,48 The results in these studies diverged, with the latter study reporting increased serum levels in patients48 and the former reporting no difference between patients and controls.47 We found a positive association between increased serum MCP-1 levels and smoking status that might explain these discrepancies. In a multianalyte profiling study of plasma from patients with schizophrenia and major depression, MMPs and TIMPs emerged as significant markers discriminating patients from controls.49 Our results indicate that serum TIMPs do not differentiate patients with mood-stabilized bipolar disorder from healthy controls.
We observed correlations between serum and CSF levels of YKL-40 and MCP-1, but found no correlations between serum and CSF levels of sCD14, TIMP-1 and TIMP-2. Furthermore, none of the CSF markers correlated with hsCRP, suggesting that these CSF markers do not directly reflect peripheral inflammatory processes. However, it is well established that bacterial and viral infections cause sickness behaviour, including depressive symptoms and anxiety, and it has been suggested that these effects are mediated by pro-inflammatory cytokines.50 Inflammatory mediators affect neurotransmitter metabolism and neural plasticity, but also communicate with the brain indirectly by activating other pathways, such as the hypothalamic–pituitary–adrenal axis. Thus, lack of correlation between CSF and serum inflammatory markers may not completely rule out the possibility that peripheral inflammation affects the brain.
Previous studies on peripheral inflammatory markers have suggested that an increase of inflammatory markers is more prominent during active mood states.7,8 Here, we observed increased levels of inflammatory markers in euthymic patients, which suggests that low-grade chronic peripheral and central inflammation is a characteristic of euthymic patients with bipolar disorder. However, it is also possible that elevated levels of inflammatory markers reflect a recent or approaching mood episode without any apparent mood symptoms. Furthermore, the clinical relevance of elevated inflammatory markers in euthymic patients is unclear. Future studies analyzing associations between biomarker levels and clinical outcomes, imaging, genetics and cognition might clarify this relevance.
Limitations
Our study has limitations that should be considered. First, the cross-sectional study design precludes conclusions about causality. Second, low statistical power and multiple testing issues in post hoc analyses (e.g., associations with medications, subdiagnosis) may result in false-negative and false-positive findings, respectively. Third, even though we adjusted for single medication class use in the statistical analyses, there is a risk that either a combination of medications or previous drug intake may have influenced the concentrations of biomarkers. Fourth, the markers examined are proxies for, not direct measures of, microglial activation. Analysis of more inflammatory markers is needed to obtain a more complete picture of the inflammatory profile. There are, however, several important strengths of this study. First, we analyzed CSF markers that, in contrast to serum/plasma markers, relate to processes in the brain. Second, we selected markers whose levels are well within the dynamic range of the quantitative assays used. Third, samples from both patients and controls were drawn between 9 and 10 am, minimizing circadian variations. Finally, the relatively large sample size allowed us to control for a range of possible confounders and to perform post hoc analyses.
Conclusion
Both neuroinflammatory and systemic inflammatory processes may play a role in the pathophysiology of bipolar disorder. This work also stresses the importance of complementing blood analyses with CSF analyses to elucidate brain-specific inflammatory mechanisms and the importance of controlling for potential confounding factors when comparing patients with controls.
Acknowledgements
This research was supported by grants from the Swedish foundation for Strategic Research, the Swedish Medical Research Council (K2014-62X-14647-12-51 and K2010-61P-21568-01-4), the Swedish Brain foundation, the Swedish Federal Government under the LUA/ALF agreement (ALF 20130032, ALFGBG-142041), Märta Lundqvists stiftelse, Demensfonden, Tore Nilssons stiftelse, and Gun och Bertil Stohnes stiftelse. We thank Åsa Källén, Monica Christiansson, Lobna Almasalmeh, Sara Hullberg, and Dzemila Secic for excellent technical assistance, the St. Göran bipolar affective disorder unit, including coordinator Martina Wennberg, study nurses Agneta Carlswärd-Kjellin, Lena Lundberg, and Benita Gezelius, and data managers Haydeh Olofsson and Mathias Kardell. Yngve Hallström is acknowledged for performing lumbar punctures. Kristoffer Bäckman and Erik Joas are acknowledged for statistical support. We are also thankful to the patients and controls participating in this study.
Footnotes
Competing interests: C.-J. Ekman declares receiving lecture honoraria from Medivir AB outside the scope of the submitted work. K. Blennow has received personal fees and research support from Roche Diagnostics outside the scope of the submitted work. M. Landén declares personal fees received from Biophausia, Servier Sweden, AstraZeneca and Lundbeck outside the scope of the submitted work. No other competing interests declared.
Contributors: J. Jakobsson, M. Bjerke and M. Landén designed the study. J. Jakobsson, M. Bjerke, C.-J. Ekman, C. Sellgren, B. Olsson, H. Zetterberg, K. Blennow and M. Landén acquired the data, which J. Jakobsson, M. Bjerke, S. Sahebi, A. Isgren, B. Olsson, E. Pålsson, H. Zetterberg, K. Blennow and M. Landén analyzed. J. Jakobsson wrote the article, which all authors reviewed and approved for publication.
References
- 1.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–86. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141:1–10. doi: 10.1016/j.jad.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magalhães PV, Kapczinski F, Nierenberg AA, et al. Illness burden and medical comorbidity in the Systematic Treatment Enhancement Program for Bipolar Disorder. Acta Psychiatr Scand. 2012;125:303–8. doi: 10.1111/j.1600-0447.2011.01794.x. [DOI] [PubMed] [Google Scholar]
- 5.Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein BI, Kemp DE, Soczynska JK, et al. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70:1078–90. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 7.Modabbernia A, Taslimi S, Brietzke E, et al. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Munkholm K, Brauner JV, Kessing LV, et al. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–33. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Rao JS, Harry GJ, Rapoport SI, et al. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–92. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haarman BC, Riemersma-Van der Lek RF, de Groot JC, et al. Neuroinflammation in bipolar disorder — a [C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun. 2014;40:219–25. doi: 10.1016/j.bbi.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–6. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- 12.Beumer W, Gibney SM, Drexhage RC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92:959–75. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- 13.Bromander S, Anckarsater R, Kristiansson M, et al. Changes in serum and cerebrospinal fluid cytokines in response to non-neurological surgery: an observational study. J Neuroinflammation. 2012;9:242. doi: 10.1186/1742-2094-9-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier B, Laurer HL, Rose S, et al. Physiological levels of pro- and anti-inflammatory mediators in cerebrospinal fluid and plasma: a normative study. J Neurotrauma. 2005;22:822–35. doi: 10.1089/neu.2005.22.822. [DOI] [PubMed] [Google Scholar]
- 15.Söderlund J, Olsson SK, Samuelsson M, et al. Elevation of cerebrospinal fluid interleukin-1ss in bipolar disorder. J Psychiatry Neurosci. 2011;36:114–8. doi: 10.1503/jpn.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dugué B, Leppanen E, Grasbeck R. Preanalytical factors and the measurement of cytokines in human subjects. Int J Clin Lab Res. 1996;26:99–105. doi: 10.1007/BF02592351. [DOI] [PubMed] [Google Scholar]
- 17.Malekzadeh A, de Groot V, Beckerman H, et al. Challenges in multi-plex and mono-plex platforms for the discovery of inflammatory profiles in neurodegenerative diseases. Methods. 2012;56:508–13. doi: 10.1016/j.ymeth.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Zetterberg H, Jakobsson J, Redsäter M, et al. Blood-cerebrospinal barrier dysfunction in patients with bipolar disorder in relation to antipsychotic treatment. Psychiatry Res. 2014;217:143–6. doi: 10.1016/j.psychres.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Lautner R, Mattsson N, Scholl M, et al. Biomarkers for microglial activation in Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:939426. doi: 10.4061/2011/939426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lively S, Schlichter LC. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J Neuroinflammation. 2013;10:75. doi: 10.1186/1742-2094-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai HC, Shi MH, Lee SS, et al. Expression of matrix metalloproteinases and their tissue inhibitors in the serum and cerebrospinal fluid of patients with meningitis. Clin Microbiol Infect. 2011;17:780–4. doi: 10.1111/j.1469-0691.2010.03393.x. [DOI] [PubMed] [Google Scholar]
- 23.Shukla V, Shakya AK, Dhole TN, et al. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of children with Japanese encephalitis virus infection. Arch Virol. 2013;158:2561–75. doi: 10.1007/s00705-013-1783-7. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzl S, Albers DS, LeWitt PA, et al. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003;207:71–6. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- 25.Bjerke M, Zetterberg H, Edman A, et al. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimers Dis. 2011;27:665–76. doi: 10.3233/JAD-2011-110566. [DOI] [PubMed] [Google Scholar]
- 26.Rydén E, Johansson C, Blennow K, et al. Lower CSF HVA and 5-HIAA in bipolar disorder type 1 with a history of childhood ADHD. J Neural Transm. 2009;116:1667–74. doi: 10.1007/s00702-009-0300-3. [DOI] [PubMed] [Google Scholar]
- 27.Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–42. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 29.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 30.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–90. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 31.Hinojosa AE, Garcia-Bueno B, Leza JC, et al. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mélik-Parsadaniantz S, Rostene W. Chemokines and neuromodulation. J Neuroimmunol. 2008;198:62–8. doi: 10.1016/j.jneuroim.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Moreira MA, Tilbery CP, Monteiro LP, et al. Effect of the treatment with methylprednisolone on the cerebrospinal fluid and serum levels of CCL2 and CXCL10 chemokines in patients with active multiple sclerosis. Acta Neurol Scand. 2006;114:109–13. doi: 10.1111/j.1600-0404.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 34.Bonneh-Barkay D, Bissel SJ, Wang G, et al. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am J Pathol. 2008;173:130–43. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. 2006;55:221–227. doi: 10.1007/s00011-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Li Q, Du X, et al. Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells. J Cereb Blood Flow Metab. 2011;31:2106–2115. doi: 10.1038/jcbfm.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knijff EM, Breunis MN, Kupka RW, et al. An imbalance in the production of IL-1beta and IL-6 by monocytes of bipolar patients: restoration by lithium treatment. Bipolar Disord. 2007;9:743–53. doi: 10.1111/j.1399-5618.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 39.Ichiyama T, Okada K, Lipton JM, et al. Sodium valproate inhibits production of TNF-alpha and IL-6 and activation of NF-kappaB. Brain Res. 2000;857:246–51. doi: 10.1016/s0006-8993(99)02439-7. [DOI] [PubMed] [Google Scholar]
- 40.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–97. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bas S, Gauthier BR, Spenato U, et al. CD14 is an acute-phase protein. J Immunol. 2004;172:4470–9. doi: 10.4049/jimmunol.172.7.4470. [DOI] [PubMed] [Google Scholar]
- 42.Marcos V, Latzin P, Hector A, et al. Expression, regulation and clinical significance of soluble and membrane CD14 receptors in pediatric inflammatory lung diseases. Respir Res. 2010;11:32. doi: 10.1186/1465-9921-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haider DG, Leuchten N, Schaller G, et al. C-reactive protein is expressed and secreted by peripheral blood mononuclear cells. Clin Exp Immunol. 2006;146:533–9. doi: 10.1111/j.1365-2249.2006.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 45.Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol Lett. 2008;29:117–24. [PubMed] [Google Scholar]
- 46.Severance EG, Gressitt KL, Stallings CR, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–7. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brietzke E, Kauer-Sant’Anna M, Teixeira AL, et al. Abnormalities in serum chemokine levels in euthymic patients with bipolar disorder. Brain Behav Immun. 2009;23:1079–82. doi: 10.1016/j.bbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Drexhage RC, Padmos RC, de Wit H, et al. Patients with schizophrenia show raised serum levels of the pro-inflammatory chemokine CCL2: Association with the metabolic syndrome in patients? Schizophr Res. 2008;102:352–5. doi: 10.1016/j.schres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Domenici E, Wille DR, Tozzi F, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE. 2010;5:e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]