Abstract
Background
The dysfunction of specific brain areas might account for the distortion of body image in patients with anorexia nervosa. The present study was designed to reveal brain regions that are abnormal in structure and function in patients with this disorder. We hypothesized, based on brain areas of altered activity in patients with anorexia nervosa and regions involved in pain processing, an interrelation of structural aberrations in the frontoparietal–cingulate network and aberrant functional activation during thermal pain processing in patients with the disorder.
Methods
We determined pain thresholds outside the MRI scanner in patients with anorexia nervosa and matched healthy controls. Thereafter, thermal pain stimuli were applied during fMRI imaging. Structural analyses with high-resolution structural T1-weighted volumes were performed using voxel-based morphometry and a surface-based approach.
Results
Twenty-six patients and 26 controls participated in our study, and owing to technical difficulties, 15 participants in each group were included in our fMRI analysis. Structural analyses revealed significantly decreased grey matter volume and cortical thickness in the frontoparietal–cingulate network in patients with anorexia nervosa. We detected an increased blood oxygen level–dependent signal in patients during the painful 45°C condition in the midcingulate and posterior cingulate cortex, which positively correlated with increased pain thresholds. Decreased grey matter and cortical thickness correlated negatively with pain thresholds, symptom severity and illness duration, but not with body mass index.
Limitations
The lack of a specific quantification of body image distortion is a limitation of our study.
Conclusion
This study provides further evidence for confined structural and functional brain abnormalities in patients with anorexia nervosa in brain regions that are involved in perception and integration of bodily stimuli. The association of structural and functional deviations with thermal thresholds as well as with clinical characteristics might indicate a common neuronal origin.
Introduction
Distortion of body image and disturbance in the accuracy of perception or cognitive interpretation of stimuli arising in the body are core symptoms of anorexia nervosa.1 It is intriguing whether stereotypical psychopathological phenomena in patients with anorexia nervosa might be related to the dysfunction of specific brain areas. However, any quest to identify an underlying brain pathology is hampered by the unsolved dilemma of whether regional aberrations in brain functioning and structure are the cause or result of prolonged starvation.
The parietal cortex is the brain region most consistently suggested to be involved in anorexia nervosa.2 Functional imaging indicates that parietal activity at rest is generally decreased before and increased after treatment.3,4 Symptom provocation further decreases parietal activity in currently ill patients.5 It is tempting to relate parietal dysfunction to symptoms of anorexia nervosa because the parietal cortex is the brain region where both proprioceptive and visual information of one’s own body are integrated and where appetitive and food-related behaviours are processed.6 Similarly, the cingulate cortex has been shown to be dysfunctional in patients with anorexia nervosa. Both the anterior (ACC) and posterior cingulate cortices (PCC) have been identified to function abnormally in these individuals.2,4,7–9 The third prominent brain region supposed to play a major role in anorexia nervosa is the insula.10–12 This part of the brain is involved in the processing of emotion, pain perception, interoception and cognitive functioning as well as in the regulation of bodily homeostasis.13,14 It has a wide set of connections with many cortical areas, such as the frontal, temporal and parietal cortices.15 Nunn and colleagues16 have suggested that a failure to integrate and regulate autonomic, sensory and affective stimuli caused by insular dysfunction might account for the disparate symptoms of anorexia nervosa. Aberrant insular functioning has recently been shown in patients with this disorder during the perception of pain.17 Reduced perception of pain is an additional feature of the disease18,19 and might be associated with distorted body image.20 Interestingly, all of the above-mentioned brain regions have been shown to constitute a brain network that is crucially involved in pain processing.21
In contrast to studies focusing on functional imaging, studies describing structural changes have yielded less consistent results.22 While some studies found loss of global grey matter,23,24 others found a loss of white matter.25 Brooks and colleagues26 reported no significant global differences in patients with anorexia nervosa. Moreover, circumscribed reductions in local grey matter were found in the ACC and PCC,26 the insula26 and the parietal cortex.23,25
Cortical surface parameters, such as cortical thickness, have not yet been studied in patients with anorexia. There is some evidence28 that cortical thickness might be more accurate than grey matter volume indices for the detection of structural abnormalities because it comprises a mixture of thickness, surface area and folding differences. As previously shown,29 the in vivo analysis of cortical thickness has the potential to provide more precise evidence for specific cortical alterations.
The present multimodal study was therefore designed to reveal brain regions that are abnormal in structure and function in patients with anorexia nervosa. We used thermal pain stimulation during fMRI acquisition to investigate the neural correlates of the body perception deficit. Based on previous studies, we hypothesized abnormal functional activation during thermal pain processing in association with aberrant grey matter volume and cortical thickness in the frontoparietal–cingulate network. We further expected to find a significant association between abnormal structure and abnormal pain processing on the one hand and clinical characteristics on the other.
Methods
Participants
We recruited patients who met the DSM-IV criteria for anorexia nervosa according to the Structured Clinical Interview for DSM-IV Axis I disorders from the specialized ward for eating disorders at our institution. Patients were investigated immediately after admission to avoid any interference with psychotherapeutic or pharmacological treatment. Patients who had a history of major depression or any further psychiatric disease were excluded from the study. Healthy controls matched for age and sex were recruited through a local newspaper advertisement. We screened them for any psychiatric and neurologic disease. In particular, the absence of a psychiatric or family history of an eating disorder was mandatory, and such individuals were excluded. Controls with past or current neurologic or psychiatric diseases and/or first-degree relatives with Axis I psychiatric disorders were also excluded from the study. All participants gave their written informed consent, and the ethics committee of the University Hospital in Jena, Germany, approved our study protocol.
Assessment of thermal pain threshold
The pain thresholds were assessed in the laboratory outside the MRI scanner. Thermal pain thresholds were determined by an ascending method of limits, as described previously (see the Appendix, available at jpn.ca, for details).30,31
Functional MRI experiment
We used a Peltier-type thermal stimulator (TSA-2001, Medoc) to deliver thermal pain stimuli to the volar surface of each participant’s right forearm. All stimuli were initiated from a baseline resting temperature of 32°C (OFF condition) and administered in 3 different ON conditions: 37°C, 42°C and 45°C thresholds. The ON phase was limited to 10 s to reduce head motion–related artifacts and to limit potential individual adaptation. The interstimulus interval was about 10.5 s. During the scanning procedure, participants were asked to relax, to keep their eyes closed and to concentrate on the sensation in their right arms.
Functional data were collected using a 1.5 T Siemens Magnetom Vision whole body system scanner. We obtained T2*-weighted images using a gradient-echo echo-planar imaging sequence (repetition time [TR] 2700 ms, echo time [TE] 60 ms, flip angle 90°) resulting in 24 contiguous transversal slices of 5 mm thickness and an in-plane resolution of 3.44 × 3.44 mm2. A series of 230 whole brain volumes were acquired in 1 run.
Functional data analysis
We used SPM8 software for image processing and statistical analyses. The procedure for data preprocessing was comparable to that used in our previous studies17,31 and is described in detail in the Appendix. We focused our statistical analysis on the comparison contrasting the painful thermal stimulation at 45°C with the nonpainful 37°C condition. To test the a priori hypothesis of cingulate, insular and parietal dysfunction in patients with anorexia nervosa during pain processing, we applied a predefined image mask including the whole cingulate cortex, the bilateral insula and the parietal cortex that we created with the WFU Pickatlas (www.fmri.wfubmc.edu/). The significance threshold for between-group differences in blood oxygen level–dependent (BOLD) activation was calculated using threshold-free cluster enhancement (TFCE). The TFCE approach takes a raw statistical image and produces an output image in which the voxel-wise values represent the amount of cluster-like local spatial support over the voxel threshold.32 Statistical significance and the correction for multiple comparisons is then determined with permutation testing so that clusters are determined without arbitrary height thresholds (i.e., “threshold free”). We performed 5000 permutations and set the significance threshold to the combined peak-cluster level of p = 0.05, family-wise error (FWE)–corrected.
Structural MRI
We obtained high-resolution structural T1-weighted volume scans in sagittal orientation (TR 15 ms, TE 5 ms, flip angle 30°, field of view 256 mm) with a voxel size of 1 × 1 × 1 mm3.
Voxel-based morphometry
The preprocessing and statistical analyses were performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm), as implemented in SPM8. Detailed information is provided in the Appendix.
We calculated the significance threshold for between-group differences in grey matter volume using the TFCE approach. We performed 5000 permutations and set the significance threshold to the combined peak-cluster level of p = 0.05, FWE-corrected. We used the Pearson product-moment correlation to test the association between total and local brain volume and body mass index (BMI), as well as illness duration in the patient group.
Cortical thickness
To calculate cortical thickness, we used the processing stream, as implemented in FreeSurfer version 5.3.0. Detailed information is provided in the Appendix. A general linear model (GLM) was used to estimate differences in cortical thickness between patients and controls at each vertex on the surface. Right and left hemispheres were tested separately. We performed a Monte Carlo simulation cluster analysis (5000 iterations) for multiple comparisons correction based on the AlphaSim algorithm.33 The significance threshold was set at p < 0.05. The p values of the resulting clusters of the original data, when expressed as a cluster-wise probability (CWP), represent the probability of identifying a maximum cluster of that size or larger during the simulation step.
Association between abnormal function, structure, mean pain threshold and clinical parameters
To illustrate the overlap between the abnormal BOLD signal and decreased grey matter volume in patients with anorexia nervosa, we inclusively masked the significant group difference in the fMRI analysis with the mask image created from the significant grey matter volume group difference in the voxel-based morphometry (VBM) analysis.
We correlated grey matter volume, the clinical parameters and the mean pain threshold using multiple regression analysis in SPM8. We focused solely on brain regions that showed significant grey matter volume difference between patients and controls. The statistical threshold was set at the uncorrected voxel level of p < 0.001. Subsequently, the average grey matter values were extracted from the significant cluster and used to create scatter plots of the significant correlations.
The associations between cortical thickness, the mean pain threshold and the clinical parameters of illness duration, the Eating Disorder Inventory-2 (EDI-2) and BMI were computed by means of a multiple regression analysis using Freesurfer. We masked the analyses with the previous results of the group analysis to focus only on those regions with significantly decreased cortical thickness in patients. We performed a Monte Carlo simulation cluster analysis (5000 iterations) for multiple comparisons correction. The significance threshold was set at p < 0.05. Patients for whom the EDI-2 scores and duration of illness were missing were excluded from our correlation analyses between these variables and grey matter volume and cortical thickness.
Results
Participants
Twenty-six patients (3 men, 23 women, restrictive subtype) who met the DSM-IV criteria for anorexia nervosa according to the Structured Clinical Interview for DSM-IV Axis I disorders and 26 healthy controls participated in our study. The demographic and clinical characteristics of the sample are provided in Table 1. Owing to technical problems with the thermal stimulation during fMRI scanning, only 15 patients and 15 matched healthy controls were ultimately included in the fMRI analysis (Table 1).
Table 1.
Demographic and clinical characteristics of the studied total sample and the fMRI subsample
| Group, mean ± SD | |||
|---|---|---|---|
|
|
|||
| Characteristic | Anorexia nervosa | Control | p value |
| Whole sample, n = 26 per group | |||
| Male:female | 3:23 | 3:23 | > 0.99 |
| Age, yr | 22.96 ± 4.97 | 24.00 ± 1.92 | 0.33 |
| BMI | 16.97 ± 1.46 | 21.72 ± 1.50 | < 0.001 |
| EDI-2 | 32.7 ± 11.4 | — | — |
| EAT-26 | 59.5 ± 26.7 | — | — |
| Duration of current episode, mo | 22.4 ± 14.8 | — | — |
| No. hospital admissions for anorexia nervosa | 3 ± 2.2 | — | — |
| BDI | 20.00 ± 12.05 | < 5 | — |
| Thermal pain threshold, °C | |||
| Right hand | 46.91 ± 1.84 | 44.83 ± 2.71 | 0.002 |
| Left hand | 46.72 ± 1.72 | 44.98 ± 2.78 | 0.009 |
| Whole brain tissue volumes (VBM), cm3 | — | — | — |
| Grey matter | 646.66 ± 57.59 | 701.07 ± 61.63 | 0.002 |
| White matter | 469.36 ± 54.59 | 489.2 ± 51.22 | 0.18 |
| Total | 1331.33 ± 108.16 | 1384.71 ± 117.25 | 0.09 |
| fMRI subsample, n = 15 per group | |||
| Male:female | 3:12 | 3:12 | > 0.99 |
| Age, yr | 22.8 ± 5.8 | 24.3 ± 2.06 | 0.35 |
| BMI | 17.2 ± 1.2 | 22.0 ± 1.35 | < 0.001 |
| EDI-2 | 32.1 ± 11.11 | — | — |
| EAT-26 | 59.2 ± 30.68 | — | — |
| Duration of current episode, mo | 22.5 ± 14.1 | — | — |
| No. hospital admissions for anorexia nervosa | 2 ± 3.4 | — | — |
| BDI | 21.4 ± 12.9 | < 5 | — |
| Thermal pain threshold, °C | |||
| Right hand | 47.0 ± 1.67 | 45.0 ± 2.45 | 0.015 |
| Left hand | 46.9 ± 1.73 | 44.6 ± 2.71 | 0.010 |
BDI = Beck Depression Inventory; BMI = body mass index; EAT = Eating Attitudes Test; EDI = Eating Disorder Inventory; SD = standard deviation; VBM = voxel-based morphometry.
Thermal pain thresholds
As shown in Table 1, the 26 patients with anorexia nervosa showed a significantly higher mean thermal pain threshold on the left (46.7°C v. 45°C, p = 0.009) and on the right hand (46.9°C v. 44.8°C, p = 0.002) than the 26 controls.
Grey matter volume
Patients with anorexia nervosa had significantly decreased total grey matter volume (p = 0.002) compared with healthy controls (Table 1). However, we found no significant differences in the total white matter or in the whole brain volume.
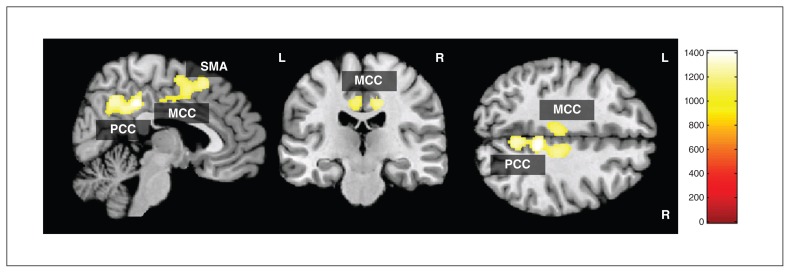
A test for differences in local grey matter volume in patients (Fig. 1 and Table 2) revealed significantly decreased grey matter in 1 significant cluster consisting of the right ventral PCC and precuneus and the left and right posterior midcingulate cortex (pMCC)/dorsal PCC, extending to the supplementary motor area (SMA). The grey matter volume differences remained significant, even after controlling for age and BMI. No significantly increased local grey matter volume was observed in patients with anorexia nervosa compared with healthy controls.
Fig. 1.
Voxel-based morphometry grey matter analysis showing regions of significant grey matter reduction in patients with anorexia nervosa compared with healthy controls (threshold-free cluster enhancement, p < 0.05, family-wise error–corrected). MCC = midcingulate cortex; PCC = posterior cingulate cortex; SMA = supplementary motor area.
Table 2.
Montreal Neurological Institute coordinates of the group differences in the grey matter volume
| MNI coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Brain area | Right/Left | Broadmann area | Cluster size, mm2 | x | y | z | TFCE | p value* |
| Posterior cingulate cortex | R | 31 | 3708 | 8 | −43 | 42 | 1409 | 0.011 |
| Precuneus | R | 7 | — | 5 | −61 | 43 | 1272 | 0.018 |
| Midcingulate cortex | L | 23/24 | — | −5 | −26 | 44 | 1151 | 0.026 |
| Midcingulate cortex | R | 23/24 | — | 12 | −26 | 41 | 1204 | 0.023 |
| Supplementary motor area | R | 6 | — | 2 | 0 | 56 | 1238 | 0.020 |
FWE = family-wise error; MNI = Montreal Neurological Institute; TFCE = threshold-free cluster enhancement.
Regions of significant grey matter reduction in patients with anorexia nervosa compared with controls (TFCE, p < 0.05, FWE-corrected).
Cortical thickness
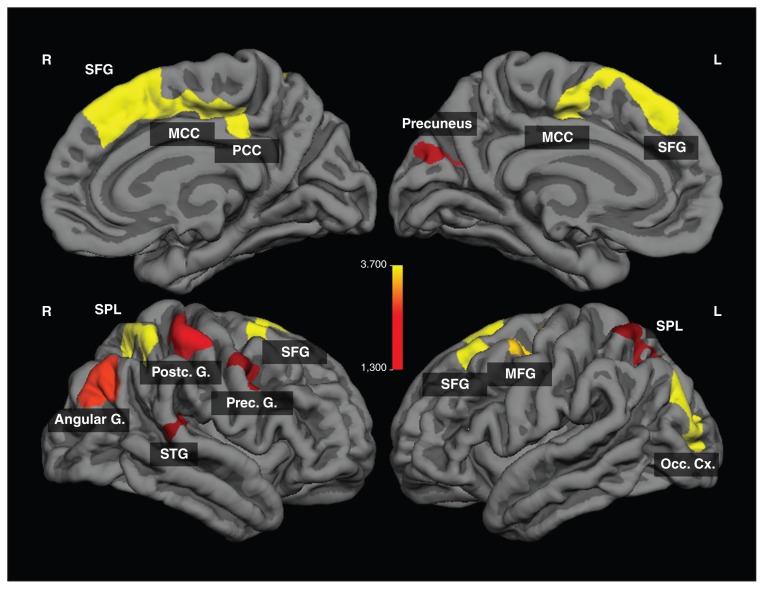
We observed significantly lower cortical thickness in patients with anorexia nervosa mainly in the frontocingulate and parietal regions (Fig. 2 and Table 3). The differences in cortical thickness remained significant, even after controlling for age and BMI. No significantly increased cortical thickness was observed in patients with anorexia nervosa compared with healthy controls.
Fig. 2.
Regions of significant difference in cortical thickness between patients with anorexia nervosa and healthy controls (p < 0.05, cluster-level corrected). The colour bar indicates logarithmized p values (−log(p)). G = gyrus; MCC = midcingulate cortex; MFG = middle frontal gyrus; Occ Cx = occipital cortex; PCC = posterior cingulate cortex; SFG = superior frontal gyrus; SPL = superior parietal lobule; STG = superior temporal gyrus.
Table 3.
Montreal Neurological Institute coordinates of group differences in cortical thickness
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Brain area | Right/Left | Brodmann area | Cluster size, mm2 | x | y | z | Cluster-wise p value* |
| Superior frontal gyrus | R | 6 | 2418.96 | 8 | 15 | 61 | < 0.001 |
| Superior frontal gyrus | L | 8 | 1484.47 | −23 | 23 | 51 | < 0.001 |
| Middle occipital gyrus | L | 19 | 1083.07 | −31 | −87 | 13 | < 0.001 |
| Inferior parietal lobule | R | 40 | 915.12 | 36 | −49 | 57 | < 0.001 |
| Middle frontal gyrus | L | 6 | 872.26 | −37 | 1 | 48 | < 0.001 |
| Angular gyrus | R | 39 | 723.65 | 40 | −76 | 28 | 0.003 |
| Postcentral gyrus | R | 3 | 594.44 | 47 | −27 | 56 | 0.011 |
| Posterior cingulate/precuneus | L | 7/31 | 481.64 | −20 | −60 | 19 | 0.027 |
| Precentral gyrus | R | 6/9 | 465.99 | 39 | 3 | 40 | 0.036 |
| Superior parietal lobule | L | 7 | 447.84 | −31 | −55 | 62 | 0.037 |
| Superior temporal gyrus | R | 42 | 448.16 | 57 | −35 | 15 | 0.043 |
MNI = Montreal Neurological Institute.
Significant clusters for the left and right hemisphere, cluster size in mm2, p values from the Monte Carlo simulation (5000 iterations) and clustering as cluster-wise probability resulting from the vertex-wise comparison of cortical thickness between the patients with anorexia nervosa and healthy controls.
Functional MRI analysis: group comparison of the 45°C versus 37°C contrast
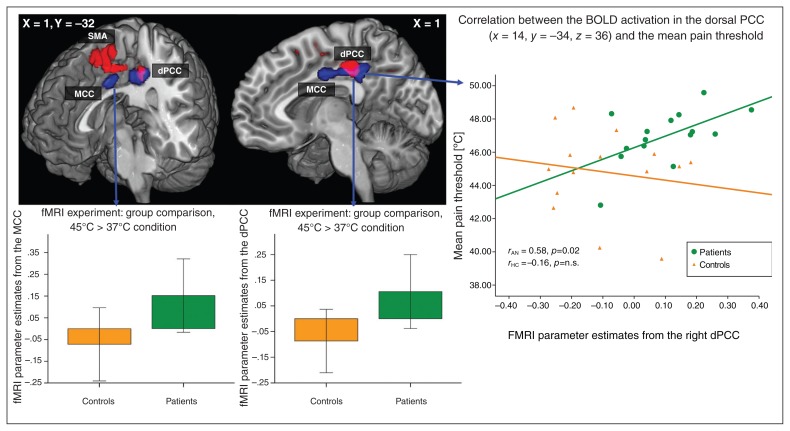
As illustrated in Figure 3, we observed a significant BOLD signal increase in patients with anorexia nervosa relative to controls in a cluster (cluster extent k = 1075 voxels) comprising the right (Brodmann area [BA] 23/31; Montreal Neurological Institute (MNI) coordinates: x, y, z = 14, −32, 36; TFCE = 688, p = 0.022, FWE-corrected) and the left dorsal PCC (dPCC; BA23/31; MNI coordinates: x, y, z = −10, −36, 38; TFCE = 621, p = 0.030, FWE-corrected) and the MCC (BA24, MNI coordinates: x, y, z = 8, −8, 34; TFCE = 618, p = 0.030, FWE-corrected). No significant BOLD signal differences were observed in the insular or parietal cortices. Masking this significant fMRI group analysis with the image of the significantly decreased grey matter volume from the VBM group analysis, the right dPCC (BA23/31, MNI coordinates: x, y, z = 14, −32, 36; cluster size = 93) and the left dPCC (BA23/31, MNI coordinates: x, y, z = −10, −34, 40; cluster size = 70) showed significantly decreased grey matter volumes and abnormal BOLD activation in patients with anorexia nervosa (Fig. 3). The fMRI parameter estimates, as extracted from the right dPCC (MNI coordinates: x, y, z = 14, −32, 36), showed a highly significant correlation with pain thresholds in patients (r = 0.58, p = 0.024), but not in controls (r = −0.16, p = 0.57).
Fig. 3.
Functional MRI analysis including 15 patients and 15 controls in the 45°C versus the 37°C condition. Blue regions depict significant blood oxygen–level dependent (BOLD) signal differences between patients with anorexia nervosa and healthy controls (threshold-free cluster enhancement [TFCE], p < 0.05, family-wise error [FWE]–corrected) during thermal pain stimulation. Red regions depict significant grey matter reduction in patients with anorexia nervosa compared with healthy controls (TFCE, p < 0.05, FWE-corrected) to illustrate the overlap between abnormal BOLD signal and reduced grey matter volume (pink). On the right, a scatter plot depicts BOLD activation in the dorsal posterior cingulate cortex (dPCC; abnormal BOLD activation and decreased grey matter volume) and mean pain thresholds in patients and controls. pMCC = posterior midcingulate cortex.
Correlation between grey matter volume, BMI, mean pain threshold and clinical parameters
No significant correlations were detected between BMI or Beck Depression Inventory score and total grey matter, white matter and whole brain volume or decreased local grey matter volume in patients with anorexia nervosa.
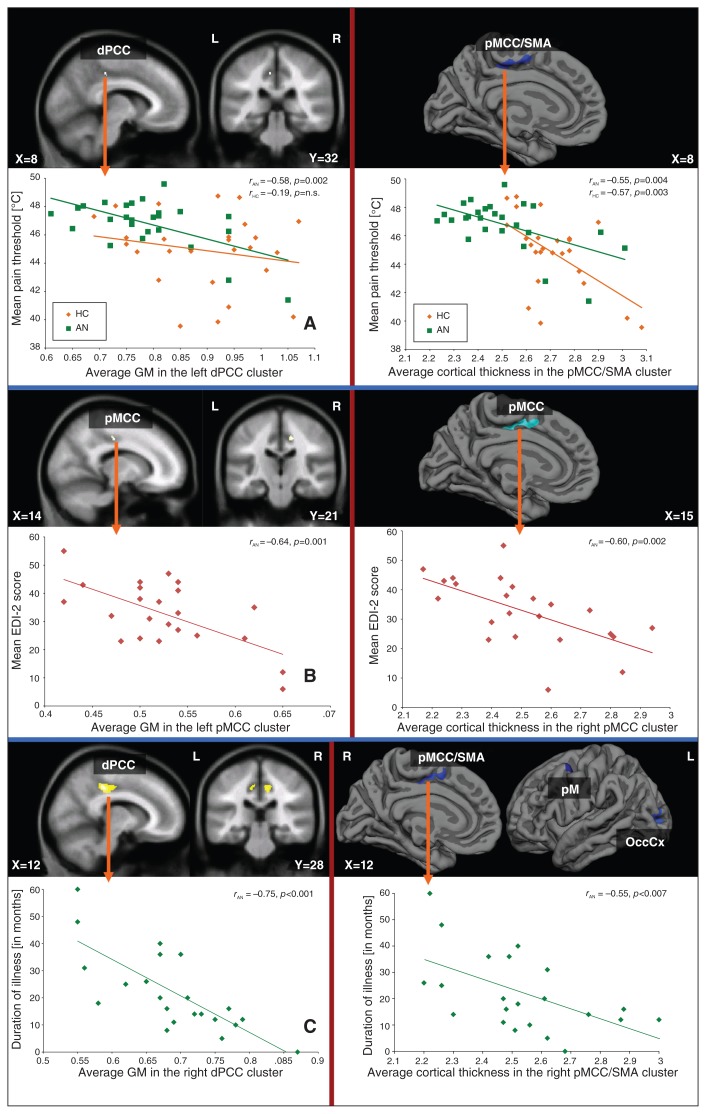
As illustrated in Figure 4A, we observed a significant negative correlation between grey matter volume and the mean pain threshold in the left dPCC (BA23, MNI coordinates: x, y, z = −8, −31, 43; t = 3.49, p < 0.001, uncorrected, cluster size = 4) in patients, but not in controls (r = −0.19).
Fig. 4.
Correlation analyses between pain threshold, clinical variables and grey matter (GM) volume as well as cortical thickness. Whole brain regression analysis between the (left) grey matter volume and (right) cortical thickness and (A) mean pain threshold, (B) symptom severity and (C) duration of illness (current episode) in patients with anorexia masked with the significant results of the group analysis. AN = anorexia nervosa; dPCC = dorsal posterior cingulate cortex; EDI-2 = Eating Disorder Inventory-2; HC = healthy controls; OccCx = occipital cortex; pM = premotor cortex; pMCC = posterior midcingulate cortex; SMA = supplementary motor area.
We further detected a significant negative association between the grey matter volume in the right pMCC (BA24, MNI coordinates: x, y, z = 17, −21, 42; t = 3.89, p < 0.001, uncorrected, cluster size = 22) and the severity of anorexia nervosa, as assessed using EDI-2 (Fig. 4B). We observed a significant negative correlation between grey matter volume and illness duration (Fig. 4C) in the right (BA23, MNI coordinates: x, y, z = 12, −34, 43; t = 5.04, p = 0.010, FWE-corrected, cluster size = 387), the left dPCC (BA23, MNI coordinates: x, y, z = −6, −28, 46; t = 3.79, p < 0.001, uncorrected, cluster size = 41) and the precuneus (BA7, MNI coordinates: x, y, z = 2, −67, 36; t = 4.22, p < 0.001, uncorrected, cluster size = 17).
Correlation between cortical thickness, BMI, mean pain threshold and clinical parameter
No significant association was observed between BMI and decreased cortical thickness in patients with anorexia nervosa.
We observed a significant negative correlation between cortical thickness in the region extending between the pMCC and the SMA and the mean pain threshold (BA6/24, MNI coordinates: x, y, z = 8, −5, 57; CWP = 0.0056, cluster size = 295.55 mm2) in patients. The average cortical thickness of controls extracted from this cluster also correlated significantly with the mean pain threshold (r = −0.57, p = 0.003; Fig. 4A).
A significant negative correlation was detected between cortical thickness in the right pMCC (BA24, MNI coordinates: x, y, z = 15, −17, 42; CWP = 0.0002, cluster size = 402.23 mm2) and the severity of anorexia nervosa (using EDI-2; Fig. 4B).
We further observed a significant negative correlation between illness duration and reduced cortical thickness in the right pMCC (BA24, MNI coordinates: x, y, z = 12, −8, 48; CWP = 0.006, cluster size = 311.73 mm2), the premotor cortex (BA6, MNI coordinates: x, y, z = −38, 2, 50; CWP = 0.04, cluster size = 209.6mm2) and the left middle occipital gyrus (BA29, MNI coordinates: x, y, z = −24, −90, 3; CWP = 0.003, cluster size = 315.62 mm2; Fig. 4C).
More importantly, there were no significant correlations between illness duration and EDI-2 score, nor between the pain threshold and the 2 clinical measurements. This indicates that the observed significant correlations with reduced grey matter volume and with cortical thickness are not based on the shared variance between clinical variables.
Discussion
In accordance with previous studies,26 we did not observe a global reduction of brain volume in patients with anorexia nervosa. We found a circumscribed loss of grey matter volume in a brain network consisting of the pMCC, the ventral PCC and dPCC, the precuneus and the SMA using an improved methodological approach for image registration and separation of grey and white matter, as implemented in SPM8.34 Furthermore, we could demonstrate for the first time a distinct pattern of lower cortical thickness in patients with anorexia nervosa in frontocingulate and parietal areas as well as in the pre- and postcentral gyrus, confirming and extending the results of our VBM analysis. Measures of cortical thickness fit surfaces to the grey/white and pial boundaries, resulting in a low between-subjects variance.35 This may have a higher accuracy to detect focal cortical alterations. Contrary to our initial hypothesis, we did not observe any structural changes in the insula of patients with anorexia nervosa. In agreement with our hypothesis, circumscribed decreased grey matter volumes and reduced cortical thickness were most notably located in the junction between the dPCC and the pMCC. We also observed a relative hyperactivation in this region during thermal pain processing in patients, and this hyperactivation positively correlated with pain thresholds. This activation difference might be caused by the lack of suppression, as observed in healthy controls.
Moreover, structural alterations in this junction were significantly and negatively correlated with pain thresholds, symptom severity and duration of illness. Our findings therefore point toward an involvement of this region in the pathophysiology of anorexia nervosa. There is evidence to support this assumption.
First, taking the functional relevance of these brain regions into account, it is remarkable that the precuneus and the PCC belong to the neural network involved in self-referential processing and reflection on one’s own physical traits.36,37 In particular, functional alterations in the precuneus, the PCC and the inferior parietal lobule have been shown to account for the perceptive component of body image distortion in patients with anorexia nervosa.11,38,39 Our study therefore supports the argument made by Gaudio and Quattrocchi11 that a specific neural basis exists that potentially generates the altered estimation and identification of body image in patients with the disease. In agreement with the present results, Gaudio and colleagues40 recently reported a significant grey matter reduction in the precuneus as well as in the parietal cortex and midcingulate cortex in adolescents with anorexia nervosa. In addition, the precuneus is associated with impaired self-knowledge41 and a cognitive bias for food images42 in individuals with this disease.
Moreover, it is noteworthy that the dorsal part of the PCC and the whole pMCC have been considered to be crucially involved in visuospatial processing and body orientation owing to high inputs from the inferior parietal cortex and strong interactions with premotor, supplementary motor and somatosensory areas, which we found to be additionally altered in structure and function in patients with anorexia nervosa. Furthermore, recent functional connectivity studies43,44 demonstrated that this part of the cingulate cortex shows strong connectivity to a range of functionally distinct brain networks: the cognitive frontoparietal executive network, the dorsal attentional network, the sensorimotor network (SMN) and the salience network. Altogether, these findings point toward the importance of this transitional brain area for orchestrating coordinated interactions between various networks. One may therefore expect that structural changes in the pMCC/dPCC would lead to disturbances across specific cognitive domains.
Consistent with this notion, previous studies have observed neuropsychological deficits in patients with anorexia nervosa in attentional, visuospatial, motor and problem solving tasks, but not in memory tasks.45–47 Grunwald and colleagues48,49 observed a deficit in the reproduction of haptic stimuli in patients with anorexia nervosa. They interpreted these findings in terms of a deficit in somatosensory integration processing of the parietal cortex. Our finding of decreased cortical thickness in the parietal cortex, in the postcentral gyrus and in the dPCC/MCC together with its correlation with the pain threshold fits well with this notion of a somatosensory deficit in anorexia nervosa.50 The abnormal BOLD activation in the dPCC during thermal pain processing and its significant association with increased pain threshold provide additional evidence for this hypothesis.
Thus, these neuropsychological findings are in agreement with the view that the dPCC coordinates specific neural networks during the processing of external information.
On the other hand, the dPCC is strongly interconnected with the adjacent ventral part of the PCC, which has been considered to be an integral part of the default mode network (DMN) and is associated with internally focused processes.
Thus, the structural changes in the dPCC may further lead to a breakdown of the dynamic interplay between internally and externally focused processes in patients. Our results are supported by the findings of McFadden and colleagues,8 who reported reduced resting state activity in the ACC, in the precuneus and in several regions of the SMN in patients with anorexia nervosa. Cowdrey and colleagues51 observed an abnormal functional connectivity in the precuneus and lateral prefrontal regions in recovered patients with anorexia nervosa. Interestingly, previous single photon emission computed tomography studies have also revealed decreased perfusion, predominantly in the PCC, the pre- and postcentral gyrus, the precuneus and the lateral prefrontal cortex.4,52 In the present fMRI experiment, a lack of BOLD activation suppression was observed in patients with anorexia nervosa in the PCC during the processing of painful thermal stimuli, indicating disturbed DMN activity. Thus, previous reports8,51 and our results may point toward the possibility that the observed circumscribed structural deficits might represent the neural basis of the reported functional disturbances.
In addition, the cingulate cortex is one of the most frequently activated regions during acute pain perception.21 Increased cerebral blood flow in the midcingulate cortex was shown during pain stimulation in patients with lower back pain.53 It was suggested that chronic pain with a somatic etiology critically involves the MCC and may selectively disrupt early orientation responses.54 A recent study suggested that increased sympathetic modulation might play an important role in pain inhibition in individuals with the disease.17 Because the PCC is known to be part of the central autonomic network and is mainly involved in parasympathetic modulation,55 a decreased inhibition of sympathetic modulation could be assumed, leading to increased pain thresholds. Altered pain thresholds might also lead to differences in activation in the PCC area during pain perception. This assumption is supported by the observed positive correlation between individual pain thresholds and activation during pain perception in this area (Fig. 4).
Thus, in accordance with our hypothesis, we found structural evidence of aberrant pain processing in patients with anorexia nervosa, which might be associated with the emergence of core symptoms of the disease.
Furthermore, the strong correlation between the volume and thickness of the pMCC/dPCC and symptom severity, as assessed using the EDI, indicates that distorted body image and deficient interoceptive awareness may have the same neural basis as abnormal pain processing.
It is an interesting question as to how changes in the nutritional state might influence decreases in grey matter volume and cortical thickness in our patients. The lack of a significant correlation between BMI and reduced grey matter volume and cortical thickness might argue against a simple association between nutritional state and abnormal cortical structure, although some studies have demonstrated a normalization of the total grey matter volume associated with long-term recovery (> 1 yr) from the anorexia.56 Our findings point toward specific cerebral morphological impairments, which can be considered not as a direct consequence of malnutrition, but rather as a premorbid symptom of anorexia nervosa that accompanies neuropsychological impairments. However, we cannot determine from the present study whether the observed grey matter and cortical thickness reductions precede the disease or develop during its course. The correlation between grey matter and cortical thickness reduction in the pMCC/dPCC and the duration of the disease suggests at the very least an ongoing process.
Limitations
A potential limitation of our study is the lack of a specific quantification of body image distortion. Patients were investigated in the acute state of the disease, and future studies need to determine state and trait characteristics of our findings. In addition, the number of patients who participated in heat pain testing was rather small, and the results of the functional analysis need to be repeated with a larger group of patients. Furthermore, although we recorded the duration of the current episode in months and the number of previous hospital admissions of patients, it is important to realize that the entire course of the disease is hard to quantify owing to clinical variability.
Conclusion
We have shown grey matter volume and cortical thickness reduction in a brain network consisting of the cingulate, inferior and superior parietal and frontal brain areas. We assessed 2 parameters of grey matter integrity: grey matter volume and cortical thickness. We observed in both parameters comparable and circumscribed structural abnormalities in patients with anorexia nervosa in the frontoparietal–cingulate regions. Because cortical thickness and grey matter volume in the postcentral gyrus, the PCC and the precuneus showed high heritabilities,28 one can speculate about some genetic predisposition to observed structural abnormalities. Previous studies have documented a relative risk of 11.3 in first-degree relatives of patients with anorexia nervosa57 and a significant heritability of the disorder.58
An abnormal structure and function in the junction between the MCC and the PCC correlated with patients’ physical pain thresholds, their disease duration and their symptom severity and showed an abnormal BOLD signal in their thermal pain processing. We therefore suggest that heat pain application is a precise method for identifying the neural bases of body image distortion. The results of the study provide strong evidence for specific structural brain abnormalities in patients with the restrictive type of anorexia nervosa, which might be related to central clinical patterns in the disease.
Footnotes
Competing interests: None declared.
Contributors: K. Bär, S. Berger and G. Wagner designed the study. F. de la Cruz, S. berger, C. Schultz and G. Wagner acquired the data, which K. Bär, F. de la Cruz and G. Wagner analyzed. K. Bär, S. Berger and G. Wagner wrote the article, which all authors reviewed and approved for publication.
References
- 1.Bruch H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosom Med. 1962;24:187–94. doi: 10.1097/00006842-196203000-00009. [DOI] [PubMed] [Google Scholar]
- 2.van Kuyck K, Gerard N, Van Laere K, et al. Towards a neurocircuitry in anorexia nervosa: evidence from functional neuroimaging studies. J Psychiatr Res. 2009;43:1133–45. doi: 10.1016/j.jpsychires.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Delvenne V, Lotstra F, Goldman S, et al. Brain hypometabolism of glucose in anorexia nervosa: a PET scan study. Biol Psychiatry. 1995;37:161–9. doi: 10.1016/0006-3223(94)00189-A. [DOI] [PubMed] [Google Scholar]
- 4.Kojima S, Nagai N, Nakabeppu Y, et al. Comparison of regional cerebral blood flow in patients with anorexia nervosa before and after weight gain. Psychiatry Res. 2005;140:251–8. doi: 10.1016/j.pscychresns.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Uher R, Brammer MJ, Murphy T, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatry. 2003;54:934–42. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 6.Santel S, Baving L, Krauel K, et al. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114:138–48. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Friederich HC, Brooks S, Uher R, et al. Neural correlates of body dissatisfaction in anorexia nervosa. Neuropsychologia. 2010;48:2878–85. doi: 10.1016/j.neuropsychologia.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 8.McFadden KL, Tregellas JR, Shott ME, et al. Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci. 2014;39:178–88. doi: 10.1503/jpn.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano A, Shiga T, Kitagawa N, et al. Abnormal neuronal network in anorexia nervosa studied with I-123-IMP SPECT. Psychiatry Res. 2001;107:45–50. doi: 10.1016/s0925-4927(01)00093-2. [DOI] [PubMed] [Google Scholar]
- 10.Frank GK, Shott ME, Hagman JO, et al. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170:1152–60. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudio S, Quattrocchi CC. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci Biobehav Rev. 2012;36:1839–47. doi: 10.1016/j.neubiorev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Strigo IA, Matthews SC, Simmons AN, et al. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: evidence of interoceptive dysregulation. Int J Eat Disord. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 15.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 16.Nunn K, Frampton I, Gordon I, et al. The fault is not in her parents but in her insula — a neurobiological hypothesis of anorexia nervosa. Eur Eat Disord Rev. 2008;16:355–60. doi: 10.1002/erv.890. [DOI] [PubMed] [Google Scholar]
- 17.Bär KJ, Berger S, Schwier C, et al. Insular dysfunction and descending pain inhibition in anorexia nervosa. Acta Psychiatr Scand. 2013;127:269–78. doi: 10.1111/j.1600-0447.2012.01896.x. [DOI] [PubMed] [Google Scholar]
- 18.de Zwaan M, Biener D, Bach M, et al. Pain sensitivity, alexithymia, and depression in patients with eating disorders: Are they related? J Psychosom Res. 1996;41:65–70. doi: 10.1016/0022-3999(96)00088-8. [DOI] [PubMed] [Google Scholar]
- 19.Bär KJ, Boettger S, Wagner G, et al. Changes of pain perception, autonomic function, and endocrine parameters during treatment of anorectic adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:1068–76. doi: 10.1097/01.chi.0000227876.19909.48. [DOI] [PubMed] [Google Scholar]
- 20.Wöckel L, Hummel T, Zepf FD, et al. Changed taste perception in patients with eating disorders. Z Kinder Jugendpsychiatr Psychother. 2007;35:423–34. doi: 10.1024/1422-4917.35.6.423. [DOI] [PubMed] [Google Scholar]
- 21.Wager TD, Atlas LY, Lindquist MA, et al. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–97. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Eynde F, Suda M, Broadbent H, et al. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur Eat Disord Rev. 2012;20:94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- 23.Joos A, Kloppel S, Hartmann A, et al. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. 2010;182:146–51. doi: 10.1016/j.pscychresns.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Suchan B, Busch M, Schulte D, et al. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res. 2010;206:63–7. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Boghi A, Sterpone S, Sales S, et al. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res. 2011;192:154–9. doi: 10.1016/j.pscychresns.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Brooks SJ, Barker GJ, O’Daly OG, et al. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC Psychiatry. 2011;11:179. doi: 10.1186/1471-244X-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mühlau M, Gaser C, Ilg R, et al. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am J Psychiatry. 2007;164:1850–7. doi: 10.1176/appi.ajp.2007.06111861. [DOI] [PubMed] [Google Scholar]
- 28.Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner G, Schultz CC, Koch K, et al. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res. 2012;46:1449–55. doi: 10.1016/j.jpsychires.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Bär KJ, Brehm S, Boettger MK, et al. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Bär KJ, Wagner G, Koschke M, et al. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. 2007;62:1281–7. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 33.Ward BD. Simultaneous inference for FMRI data Milwaukee. Wisconsin: Medical College of Wisconsin; 2000. [Google Scholar]
- 34.Eggert LD, Sommer J, Jansen A, et al. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS ONE. 2012;7:e45081. doi: 10.1371/journal.pone.0045081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner G, Koch K, Schachtzabel C, et al. Self-referential processing influences functional activation during cognitive control: an fMRI study. Soc Cogn Affect Neurosci. 2013;8:828–37. doi: 10.1093/scan/nss074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr HM, Zimmermann J, Roder C, et al. Separating two components of body image in anorexia nervosa using fMRI. Psychol Med. 2010;40:1519–29. doi: 10.1017/S0033291709991826. [DOI] [PubMed] [Google Scholar]
- 39.Vocks S, Busch M, Gronemeyer D, et al. Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: an fMRI study. J Psychiatry Neurosci. 2010;35:163–76. doi: 10.1503/jpn.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaudio S, Nocchi F, Franchin T, et al. Gray matter decrease distribution in the early stages of Anorexia Nervosa restrictive type in adolescents. Psychiatry Res. 2011;191:24–30. doi: 10.1016/j.pscychresns.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 41.McAdams CJ, Krawczyk DC. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Soc Cogn Affect Neurosci. 2014;9:12–21. doi: 10.1093/scan/nss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooks S, Prince A, Stahl D, et al. A systematic review and meta-analysis of cognitive bias to food stimuli in people with disordered eating behaviour. Clin Psychol Rev. 2011;31:37–51. doi: 10.1016/j.cpr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–22. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leech R, Kamourieh S, Beckmann CF, et al. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kingston K, Szmukler G, Andrewes D, et al. Neuropsychological and structural brain changes in anorexia nervosa before and after refeeding. Psychol Med. 1996;26:15–28. doi: 10.1017/s0033291700033687. [DOI] [PubMed] [Google Scholar]
- 46.Lauer CJ, Gorzewski B, Gerlinghoff M, et al. Neuropsychological assessments before and after treatment in patients with anorexia nervosa and bulimia nervosa. J Psychiatr Res. 1999;33:129–38. doi: 10.1016/s0022-3956(98)00020-x. [DOI] [PubMed] [Google Scholar]
- 47.Szmukler GI, Andrewes D, Kingston K, et al. Neuropsychological impairment in anorexia nervosa: before and after refeeding. J Clin Exp Neuropsychol. 1992;14:347–52. doi: 10.1080/01688639208402834. [DOI] [PubMed] [Google Scholar]
- 48.Grunwald M, Ettrich C, Assmann B, et al. Deficits in haptic perception and right parietal theta power changes in patients with anorexia nervosa before and after weight gain. Int J Eat Disord. 2001;29:417–28. doi: 10.1002/eat.1038. [DOI] [PubMed] [Google Scholar]
- 49.Grunwald M, Ettrich C, Krause W, et al. Haptic perception in anorexia nervosa before and after weight gain. J Clin Exp Neuropsychol. 2001;23:520–9. doi: 10.1076/jcen.23.4.520.1229. [DOI] [PubMed] [Google Scholar]
- 50.Reed CL, Klatzky RL, Halgren E. What vs. where in touch: an fMRI study. Neuroimage. 2005;25:718–26. doi: 10.1016/j.neuroimage.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 51.Cowdrey FA, Filippini N, Park RJ, et al. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum Brain Mapp. 2014;35:483–91. doi: 10.1002/hbm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto R, Kitabayashi Y, Narumoto J, et al. Regional cerebral blood flow changes associated with interoceptive awareness in the recovery process of anorexia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1265–70. doi: 10.1016/j.pnpbp.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 53.Derbyshire SW, Jones AK, Creed F, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16:158–68. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- 54.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beissner F, Meissner K, Bar KJ, et al. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner A, Greer P, Bailer UF, et al. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry. 2006;59:291–3. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Strober M, Freeman R, Lampert C, et al. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry. 2000;157:393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- 58.Klump KL, Miller KB, Keel PK, et al. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol Med. 2001;31:737–40. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]