Abstract
Although the sea otter (Enhydra lutris) is a complete aquatic species, spending its entire life in the ocean, it has been considered morphologically to be a semi-aquatic animal. This study aimed to clarify the unique hindlimb morphology and functional adaptations of E. lutris in comparison to other Mustelidae species. We compared muscle mass and bone measurements of five Mustelidae species: the sea otter, Eurasian river otter (Lutra lutra), American mink (Neovison vison), Japanese weasel (Mustela itatsi) and Siberian weasel (M. sibirica). In comparison with the other 4 species, E. lutris possessed significantly larger gluteus, popliteus and peroneus muscles, but smaller adductor and ischiopubic muscles. The popliteus muscle may act as a medial rotator of the crus, and the peroneus muscle may act as an abductor of the fifth toe and/or the pronator of the foot. The bundles of the gluteus superficialis muscle of E. lutris were fused with those of the tensor fasciae latae muscle and gluteofemoralis muscles, and they may play a role in femur abduction. These results suggest that E. lutris uses the abducted femur, medially rotated crus, eversion of the ankle and abducted fifth digit or extended interdigital web as a powerful propulsion generator. Therefore, we conclude that E. lutris is a complete aquatic animal, possessing differences in the proportions of the hindlimb muscles compared with those in other semi-aquatic and terrestrial mustelids.
Keywords: hindlimb, muscle, mustelidae, sea otter, swimming locomotion
The purpose of this study is to clarify the sea otter (Enhydra lutris) as a complete aquatic species, based on its unique hindlimb morphology and functional adaptations. Previous investigators have morphologically described the muscles and skeleton of E. lutris appendages [2, 15,16,17], but there have been no comparative morphological studies between E. lutris and other closely related species in the Mustelidae family, except for one study on bone density [13]. Enhydra lutris has often been morphologically compared with pinnipeds and described as intermediate between terrestrial species and pinnipeds [17, 35, 36, 38]. However, to clarify the evolutionary adaptation of E. lutris from a terrestrial habitat to a complete aquatic lifestyle, its appendages should be functionally and morphologically compared with less aquatic species to which it is phylogenetically closely-related [34].
Such closely related species in the Mustelidae family include the Japanese weasel (Mustela itatsi), the Siberian weasel (M. sibirica), the American mink (Neovison vison) and the Eurasian river otter (Lutra lutra). These species and E. lutris show different levels of dependence on terrestrial and semi-aquatic ecology. M. itatsi and M. sibirica are closely related species, which diverged each other about 1.6 million years ago [30]. M. itatsi preys on fish in rivers [18], whereas M. sibirica does not [32, 37]. Further, N. vison and L. lutra depend on fish for 5–70% [1, 6, 7] and for 80% over [4] of their diets, respectively. Enhydra lutris feeds on sea urchins, octopus, clams and fish [20, 24]. These findings demonstrate that although M. itatsi, N. vison, L. lutra and E. lutris all hunt underwater for aquatic prey, they show differences in the degree of aquatic resources use.
The hindlimb structures act as the main propulsion generator during submerged swimming in highly aquatic Mustelidae species [8, 11, 12, 39, 40]. Therefore, by comparing the hindlimb bones and muscles of E. lutris with the four other Mustelidae species that show different levels of dependence on terrestrial and semi-aquatic ecology, we can investigate the functional and morphological gradations from a terrestrial habitat to an aquatic lifestyle [13]. Also, examining such closely related species might minimize any phylogenetic influences on hindlimb morphology.
MATERIALS AND METHODS
Specimens: Hindlimb bones were measured in 83 individuals among the five species, and also, 26 individuals were dissected in order to weigh the hindlimb muscle mass. The specimens used in this study are listed in Supplementary Table 1. We used only male specimens of M. itatsi and M. sibirica and omitted female specimens, because of their strong sexual dimorphism. In particular, it was pointed out that the feeding ecology of the female of M. itatsi differed from that of the males [19]. There is a possibility that the female of M. itatsi does not use the aquatic resources. Both male and female specimens of the other three species were used, since their both sexes also depend on the aquatic habitat [3, 20, 26].
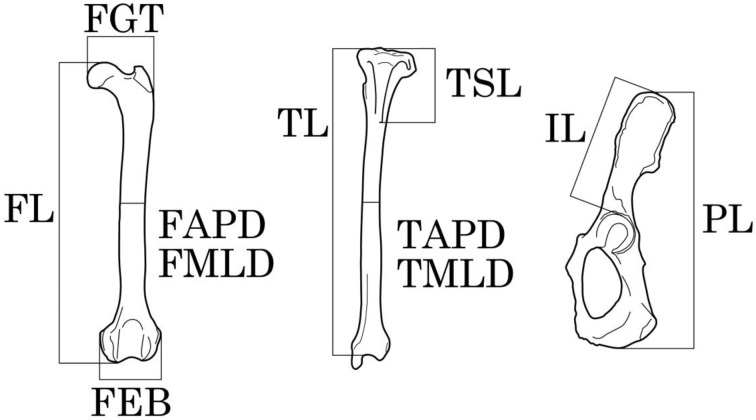
Muscle mass and skeletal length measurements: Muscle mass was recorded since it is considered to be proportional to the maximum power generated by the muscle [5]. The hindlimb muscle nomenclature used in the present study and equivalent names used in previous studies are listed in Supplementary Table 2 [9, 14, 16, 21]. All carcasses had been frozen at −20°C until dissection. During dissection, the left-side hindlimb muscles were exposed. Some of the muscles were fused and could not be divided; so 28 muscle groups were used for the measurements (Table 1). The presemimembranosus muscle was not used in the analysis, because it was absent in some specimens of N. vison. The hindlimb muscles were removed from the carcasses. Then, adipose and connective tissues were removed before the muscles were weighed to the nearest 0.001 g using an electronic balance (UX420H, Shimadzu Corp., Kyoto, Japan). Eleven osteological characteristics were measured to the nearest 0.1 mm using calipers (Fig. 1 and Table 2). The measurements were using definitions from a previous study [29], except for FGT, IL and PL. The FGT measurement was defined as the breadth of the femoral head to the greater trochanter of the femur, in order to obtain clear examination of lever arm length of the gluteus superficialis muscle in the lateral direction. The IL and PL pelvis measurements were taken in addition to the existing measurements described by Samuels et al. [29].
Table 1. Muscles and muscle groups examined in this study and their abbreviations.
| Abbreviations | Contained muscles |
|---|---|
| ILLPS | Iliopsoas |
| GSFCTFL | Gluteus superficialis, Gluteofemoralis (Caudofemoralis), Tensor fasciae latae |
| GMPI | Gluteus medius, Piriformis |
| GPAC | Gluteus profundus, Iliocapsularis |
| OE | Obturator externus |
| OIGE | Gemelli, Obturator internus |
| QF | Quadratus femoris |
| RF | Rectus femoris |
| V3 | Vastus lateralis, Vastus medialis, Vastus intermedius |
| BFSTTE | Biceps femoris, Tenuissimus, Semitendinosus |
| SMCR | Semimembranosus cranialis |
| SMCA | Semimembranosus caudalis |
| SAR | Sartorius |
| GLA | Gracilis |
| PEC | Pectineus |
| ADD | Adductor |
| TCREHL | Tibialis cranialis, Extensor hallucis longus |
| TCA | Tibialis caudalis |
| FI3 | Peroneus longus, Peroneus digiti quinti, Peroneus brevis |
| EDL | Extensor digitorum longus |
| GLAT | Gastrocnemius caput latelaris |
| GMED | Gastrocnemius caput medialis |
| PLA | Plantaris |
| FHL | Flexor hallucis longus |
| FDL | Flexor digitorum longus |
| POP | Popliteus |
| PESB | Extensor digitorum brevis, Flexor digitorum brevis, Quadratus plantae, Lumbricales, Interosseus, Calcaneometatarsalis |
| SOL | Soleus |
Fig. 1.
Bone measurements used in this study. The abbreviations are listed in Table 2.
Table 2. Bone measurements used in the analysis.
| Abbreviation | Measurement definitions |
|---|---|
| FL* | Maximum length of the femur from the upper rim of the femoral head to the medial condyle parallel to the shaft. |
| FGT* | Mediolateral breadth from the femoral head to the greater trochanter. |
| FAPD | Anteroposterior diameter in the mid-shaft of the femur. |
| FMLD | Mediolateral diameter in the mid-shaft of the femur. |
| FEB* | Biepicondylar breadth of the femur. |
| TL* | Length of the tibia from the anterior rim of the medial condyle to the anterior rim of the talar trochlear articulation parallel to the shaft. |
| TSL* | Length from the anterior rim of the medial condyle to the distal extension of the tibial tuberosity (spine) parallel to the shaft. |
| TAPD | Anteroposterior diameter the mid-shaft of the tibia. |
| TMLD | Mediolateral diameter the mid-shaft of the tibia. |
| PL* | Maximum length of the pelvis from the posterior end of the ischium to the anterior end of the iliac wing. |
| IL* | Length from anterior rim of acetabulum to distal end of iliac wing. |
Definition of aquatic tendency: Aquatic tendency was defined using the dietary data of each species based on the weight ratios of fish remnants in total feces weight in the four species, except for E. lutris [4, 6, 18, 22, 37]. The percentages of consumed fish and aquatic tendencies were as follows; M. sibirica, 0.0%; M. itatsi, 17.7%; N. vison, 23.9%; and L. lutra, 81.1%. Since E. lutris lives their entire lives on the sea surface [23], we considered their aquatic tendency to be 100%.
We defined the terms “aquatic”, “semi-aquatic” and “terrestrial” as follows. Aquatic animal: species that possess the ability to live on and/or in the water, e.g. E. lutris. Semi-aquatic animal: species that feed on fish and show terrestrial adaptation, e.g. L. lutra, N. vison and M. itatsi. Terrestrial animal: species that are completely adapted to terrestrial habitats and never feed on fish, i.e. they do not possess the ability to hunt fish, e.g. M. sibirica.
Statistical analysis: Statistical tests were performed to examine the relationships between hindlimb morphological characteristics and ecological aquatic tendency in Mustelidae. The tests included interspecific pair-wise comparisons, principal components analysis (PCA) and partial Mantel tests. The muscle masses were divided by the geometric means (GM) calculated from obtained 27 masses of muscle groups, except for SOL, since E. lutris lacks SOL. The bone measurements were divided by the GM of femoral length (FL), tibial length (TL) and pelvic length (PL). Analysis of variance (ANOVA) was conducted to detect significant differences in the muscular and skeletal measurements between the five species. Homogeneity of variance was tested using the Bartlett’s test for post-hoc comparisons. Pair-wise comparisons were made using the Games–Howell method to reveal the interspecific statistical significance between the muscle and bone measurements. The relative positions of the species were visualized in morphological space using PCA and by drawing biplots with the first (PC1) and second (PC2) principal components. The variables used in the PCA were the scaled measurement values calculated from the respective GM values. Seven skeletal measurement variables were used in the PCAs to avoid deficient values; these were FL, FGT, FEB, TL, TSL, PL and IL (Table 2). Correlation function matrices were used for both PCAs.
The partial Mantel tests were performed to estimate the functional and morphological correlations between hindlimb morphology and the degree of the aquatic tendency in Mustelidae, after phylogenetic effects had been removed. The partial Mantel test clarifies the relationships between two matrices controlling another matrix [23]. We prepared Euclidean distance matrices of the five mustelids for morphology, ecology and phylogeny. The morphological distance matrices were prepared with PC1, PC2 and PC3 from the PCA results of the muscle and bone data. The ecological distance matrix was compiled from sine-transformations of the aquatic tendency ratios described earlier. The phylogenetic distance matrix was constructed of molecular data obtained from the sequence homology of the cytochrome b gene [25] (Table 3). All statistical analyses were performed using R version 3.0.1 [27].
Table 3. Extracted data of cytochrome b gene patristic distances for 1,140 bp in the 5 species that were applied to the partial Mantel test; from Marmi et al. [25].
| E. lutris | L. lutra | N. vison | M. itatsi | |
|---|---|---|---|---|
| L. lutra | 18.4 | |||
| N. vison | 24.4 | 24.4 | ||
| M. itatsi | 22.0 | 22.1 | 18.6 | |
| M. sibirica | 22.1 | 22.1 | 18.7 | 6.4 |
The accession numbers of National Center for Biotechnology Information for samples used in making the phylogenetical distance matrix from cytochrome b analyses by Marmi et al. [25] are as follows: M. sibirica, AB026108, AB051242, AB051243; M. itatsi, AB026104; N. vison, AB026109, AF057129; L. lutra, AF057124, X94923; E. lutris, AB051244, AF057120, X94924.
RESULTS
Muscle mass measurements: The mean values, standard errors and interspecific significant differences of the 28 muscle weight measurements that were scaled by geometric mean are shown in Table 4. In ANOVA, there were significant differences between species for the muscle groups, except for SMCR, SAR, GLA, TCREHL, PLA, FDL and PESB. Interspecific pair-wise comparison analyses also revealed significant differences in the relative mass of the muscle groups between species (Table 5). There were significant differences between E. lutris and at least one other species for 18 muscle groups (GSFCTFL, GMPI, OE, OIGE, QF, RF, V3, BFSTTE, SMCR, SMCA, GLA, ADD, FI3, GLAT, GMED, FHL, POP and SOL) (Table 4). Some muscle groups showed significant differences between E. lutris and all four other species. For example, the masses of GSFCTFL, GMPI, OE, FI3 and POP muscle groups were significantly larger in E. lutris than in the other four species, but those of RF, SMCA, ADD and SOL were significantly smaller. In the case of SOL, this is because it does not exist in E. lutris. Notably, in four muscle groups (GSFCTFL, RF, ADD and FI3), the only significant difference was between E. lutris and the other four species.
Table 4. Mean values, standard deviations and interspecific significant differences of measurements for geometric mean scaled muscle masses.
| Groups | Enhydra lutris | Lutra lutra | Neovison vison | Mustela itatsi | Mustela sibirica | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | SDa) | vs.b) | mean | SD | vs. | mean | SD | vs. | mean | SD | vs. | mean | SD | vs. | ||||||||||||||||
| ILLPS | 1.75 | 0.32 | 1.23 | 0.14 | I | S | 1.59 | 0.25 | 2.03 | 0.20 | L | 1.82 | 0.31 | L | ||||||||||||||||
| GSFCTFL | 3.16 | 0.32 | L | V | I | S | 1.75 | 0.09 | E | 1.64 | 0.20 | E | 1.73 | 0.11 | E | 1.82 | 0.19 | E | ||||||||||||
| GMPI | 4.07 | 0.38 | L | V | I | S | 2.23 | 0.14 | E | V | 1.79 | 0.14 | E | L | 1.93 | 0.17 | E | 1.98 | 0.28 | E | ||||||||||
| GPAC | 0.42 | 0.09 | 0.34 | 0.05 | S | 0.32 | 0.04 | S | 0.27 | 0.03 | S | 0.22 | 0.02 | L | V | I | ||||||||||||||
| OE | 0.99 | 0.15 | L | V | I | S | 0.56 | 0.05 | E | I | S | 0.50 | 0.04 | E | 0.45 | 0.06 | E | L | 0.41 | 0.05 | E | L | ||||||||
| OIGE | 0.23 | 0.06 | V | I | S | 0.23 | 0.01 | V | I | S | 0.41 | 0.08 | E | L | 0.41 | 0.04 | E | L | 0.38 | 0.06 | E | L | ||||||||
| QF | 0.08 | 0.03 | L | I | S | 0.18 | 0.01 | E | 0.14 | 0.04 | 0.18 | 0.03 | E | 0.24 | 0.05 | E | ||||||||||||||
| RF | 1.30 | 0.20 | L | V | I | S | 1.90 | 0.14 | E | 1.99 | 0.20 | E | 2.09 | 0.07 | E | 2.20 | 0.27 | E | ||||||||||||
| V3 | 2.13 | 0.41 | V | I | S | 2.97 | 0.35 | S | 3.35 | 0.08 | E | I | S | 3.61 | 0.09 | E | V | 3.93 | 0.26 | E | L | V | ||||||||
| BFSTTE | 5.02 | 0.46 | S | 5.62 | 0.34 | S | 5.53 | 0.47 | S | 5.98 | 0.57 | 6.66 | 0.40 | E | L | V | ||||||||||||||
| SMCR* | 1.95 | 0.14 | L | I | 2.62 | 0.25 | E | 2.31 | 0.31 | 2.30 | 0.19 | E | 2.55 | 0.34 | ||||||||||||||||
| SMCA | 1.16 | 0.11 | L | V | I | S | 2.48 | 0.18 | E | I | 2.26 | 0.16 | E | I | 3.13 | 0.22 | E | L | V | 3.14 | 0.52 | E | ||||||||
| SAR* | 2.10 | 0.39 | 1.70 | 0.15 | 1.84 | 0.25 | 2.12 | 0.32 | 2.11 | 0.31 | ||||||||||||||||||||
| GLA* | 1.03 | 0.19 | L | V | S | 1.54 | 0.22 | E | 1.81 | 0.29 | E | 1.41 | 0.08 | 1.56 | 0.18 | E | ||||||||||||||
| PEC | 0.44 | 0.06 | 0.35 | 0.06 | 0.33 | 0.04 | 0.27 | 0.12 | 0.35 | 0.05 | ||||||||||||||||||||
| ADD | 1.98 | 0.16 | L | V | I | S | 2.67 | 0.22 | E | 2.67 | 0.23 | E | 2.62 | 0.29 | E | 2.61 | 0.19 | E | ||||||||||||
| TCREHL* | 1.14 | 0.48 | 1.43 | 0.14 | 1.27 | 0.06 | 1.22 | 0.09 | 1.23 | 0.11 | ||||||||||||||||||||
| TCA | 0.61 | 0.32 | 0.33 | 0.05 | 0.30 | 0.04 | 0.25 | 0.06 | 0.26 | 0.08 | ||||||||||||||||||||
| FI3 | 1.29 | 0.09 | L | V | I | S | 0.77 | 0.11 | E | 0.82 | 0.06 | E | 0.77 | 0.04 | E | 0.70 | 0.05 | E | ||||||||||||
| EDL | 0.76 | 0.12 | 0.64 | 0.10 | 0.56 | 0.03 | 0.59 | 0.04 | 0.50 | 0.05 | ||||||||||||||||||||
| GLAT | 1.13 | 0.11 | V | S | 1.23 | 0.08 | 1.41 | 0.10 | E | 1.39 | 0.15 | 1.59 | 0.24 | E | ||||||||||||||||
| GMED | 2.39 | 0.13 | L | I | S | 2.05 | 0.10 | E | I | S | 1.68 | 0.49 | 1.71 | 0.09 | E | L | 1.76 | 0.15 | E | L | ||||||||||
| PLA* | 1.13 | 0.14 | 1.21 | 0.05 | 1.10 | 0.12 | 1.13 | 0.08 | 1.17 | 0.05 | ||||||||||||||||||||
| FHL | 0.54 | 0.05 | L | V | I | 0.91 | 0.06 | E | 1.03 | 0.12 | E | 0.91 | 0.07 | E | 0.91 | 0.31 | ||||||||||||||
| FDL* | 0.26 | 0.05 | 0.28 | 0.05 | 0.30 | 0.05 | 0.34 | 0.05 | 0.26 | 0.05 | ||||||||||||||||||||
| POP | 0.51 | 0.02 | L | V | I | S | 0.30 | 0.04 | E | 0.34 | 0.02 | E | S | 0.29 | 0.04 | E | 0.24 | 0.03 | E | V | ||||||||||
| PESB* | 1.01 | 0.16 | 1.10 | 0.20 | 1.04 | 0.11 | 0.89 | 0.21 | 0.88 | 0.24 | ||||||||||||||||||||
| SOL | 0.00 | 0.00 | L | V | I | S | 0.17 | 0.03 | E | 0.25 | 0.05 | E | S | 0.16 | 0.01 | E | 0.14 | 0.02 | E | V | ||||||||||
Muscle groups are defined in Table 1. Asterisks indicate no significant differences between the 5 species for that muscle group (P>0.05). a) SD, standard deviations. b) vs., species that showed significant differences in univariate ANOVA tests at the P<0.05 level using Games-Howell’s tests post hoc procedure (E, Enhydra lutris. L, Lutra lutra. V, Neovison vison. I, Mustela itatsi. S, Mustela sibirica.)
Table 5. Factor loadings of muscle masses in PCA.
| Abbreviations | PC1 | PC2 | PC3 |
|---|---|---|---|
| ILLPS | 0.13 | 0.65 | –0.47 |
| GSFCTFL | –0.88 | 0.36 | 0.04 |
| GMPI | –0.92 | 0.22 | 0.16 |
| GPAC | –0.78 | –0.42 | –0.09 |
| OE | –0.96 | 0.01 | 0.02 |
| OIGE | 0.59 | 0.09 | –0.63 |
| QF | 0.75 | 0.13 | 0.27 |
| RF | 0.88 | 0.14 | 0.05 |
| V3 | 0.90 | 0.24 | –0.11 |
| BFSTTE | 0.68 | 0.46 | 0.39 |
| SMCR | 0.57 | –0.17 | 0.53 |
| SMCA | 0.87 | 0.21 | 0.04 |
| SAR | 0.08 | 0.72 | 0.15 |
| GLA | 0.64 | –0.22 | 0.08 |
| PEC | –0.52 | –0.03 | 0.04 |
| ADD | 0.73 | –0.07 | 0.18 |
| TCREHL | 0.11 | –0.76 | 0.25 |
| TCA | –0.70 | 0.34 | 0.05 |
| FI3 | –0.92 | 0.11 | –0.12 |
| EDL | –0.77 | –0.13 | 0.11 |
| GLAT | 0.68 | 0.22 | 0.10 |
| GMED | –0.71 | 0.03 | 0.39 |
| PLA | –0.01 | –0.45 | 0.35 |
| FHL | 0.64 | –0.41 | –0.21 |
| FDL | 0.22 | –0.24 | –0.54 |
| POP | –0.92 | 0.03 | –0.20 |
| PESB | –0.28 | –0.76 | –0.15 |
| SOL | 0.75 | –0.44 | –0.18 |
| EVa) | 13.28 | 3.70 | 2.04 |
| PVEb) | 47.44 | 13.22 | 7.30 |
| CPVEc) | 47.44 | 60.66 | 67.96 |
The abbreviations are defined in Table 1. a) EV, Eigen values. b) PVE, The percentage of the variation explained. c) CPVE, The cumulative percentage of the variation explained.
Not all muscle groups in E. lutris were significantly different to all four other species. For example, the GMED muscle group was significantly larger in E. lutris than in L. lutra, M. itatsi and M. sibirica, but not significantly different to that of N. vison. Some other muscle groups were smaller in E. lutris than in some of the other species; QF, SMCR, GLA and FHL muscle groups were significantly smaller than those of L. lutra; OIGE, V3, GLA, GLAT and FHL were significantly smaller than those of N. vison; OIGE, QF, V3, SMCR and FHL were significantly smaller than those of M. itatsi; and OIGE, QF, V3, BFSTTE, GLA and GLAT were significantly smaller than those of M. sibirica.
There were also significant differences between the muscle masses of the four other species. For example, the masses of 19 muscle groups in L. lutra were statistically significantly different from at least one other species (ILLPS, GSFCTFL, GMPI, GPAC, OE, OIGE, QF, RF, V3, BFSTTE, SMCR, SMCA, GLA, ADD, FI3, GMED, FHL, POP and SOL). The mass of GMPI in L. lutra was larger than in N. vison, but that of OIGE was smaller. Also, L. lutra had significantly larger OE and GMED muscle mass than M. itatsi, but smaller ILLPS, OIGE and SMCA muscle groups. Furthermore, in comparison to M. sibirica, L. lutra had larger GPAC, OE and GMED muscle groups, but smaller ILLPS, OIGE, V3 and BFSTTE muscle groups.
For N. vison, 16 muscle groups showed statistically significant differences from at least one other species (GSFCTFL, GMPI, GPAC, OE, OIGE, RF, V3, BFSTTE, SMCA, GLA, ADD, FI3, GMED, FHL, POP and SOL). V3 and SMCA were significantly smaller than those of M. itatsi; GPAC, POP and SOL were significantly larger than in M. sibirica; V3 and BFSTTE were significantly lower than those of M. sibirica.
M. itatsi and M. sibirica indicated statistically significant differences from at least one species in 17 muscle groups (ILLPS, GSFCTFL, GMPI, GPAC, OE, OIGE, QF, RF, V3, SMCR, SMCA, ADD, FI3, GMED, FHL, POP and SOL) and 18 muscle groups (ILLPS, GSFCTFL, GMPI, GPAC, OE, OIGE, QF, RF, V3, BFSTTE, SMCA, GLA, ADD, FI3, GLAT, GMED, POP and SOL), respectively. However, the only significant difference between M. itatsi and M. sibirica was for GPAC, which was significantly larger in M.itatsi.
No exclusive significant differences were observed between L. lutra and the three Mustelidae species, except for E. lutris in a comparison between E. lutris and the others. Although no significant differences were detected, L. lutra tended to have relatively larger distal hindlimb muscles (TCREHL, PLA and PESB) than those of the other species.
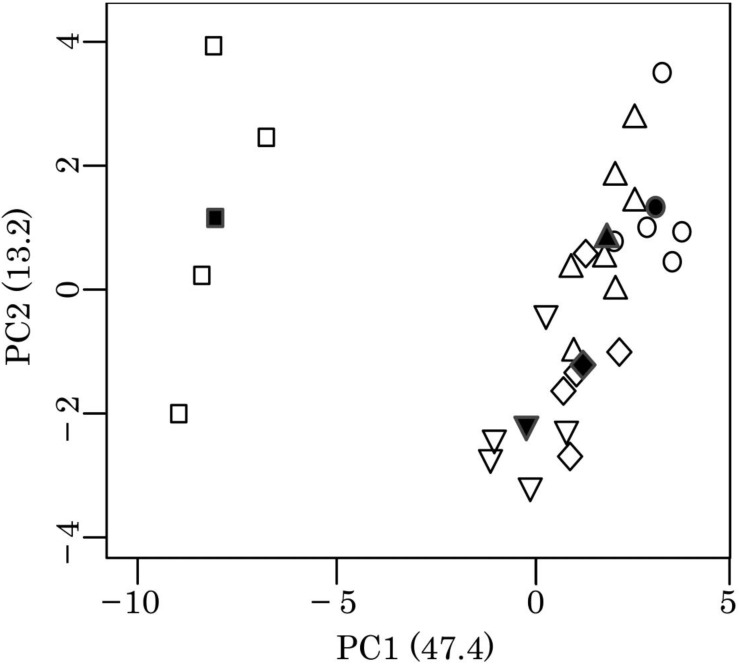
PC1 accounted for 47.4% of the variance and primarily separated E. lutris from the other species (Fig. 2 and Table 5). PC1 was negatively correlated with OE, GMPI, FI3, POP and GSFCTFL, but it was positively correlated with V3, RF and SMCA. PC2 accounted for 13.2% of variance in the dataset and primarily separated L. lutra and N. vison from the other species. PC2 was negatively correlated with PESB and TCREHL, but it was positively correlated with ILLPS and SAR.
Fig. 2.
Plot of PC1 and PC2 scores of muscle masses for the five mustelid species. PC1; the
first principal component. PC2; the second principal component. Numbers in parentheses
represent the percentage of the variation explained by the component.  , Enhydra lutris.
, Enhydra lutris.
 , Lutra lutra.
, Lutra lutra.
 , Neovison
vison.
, Neovison
vison.  , Mustela
itatsi.
, Mustela
itatsi.  ,
Mustela sibirica. Filled symbols indicate mean values of each
species.
,
Mustela sibirica. Filled symbols indicate mean values of each
species.
Table 6 shows the results of the partial Mantel test using the ecological, phylogenetic and morphological matrices prepared from PCA scores of the muscular weights. The coefficient of correlation between morphology (PC1) and ecology was 0.70 (P<0.05).
Table 6. The P values of partial Mantel test of muscle masses.
| Moa) vs. Ecb) | Mo vs. Phc) | Ec vs. Ph | Mo vs Ec con.d) Ph | Mo vs Ph con. Ec | |
|---|---|---|---|---|---|
| PC1 | 0.74 | 0.37 | 0.45 | 0.70 | n.s.e) |
| PC2 | n.s. | n.s. | 0.45 | n.s. | n.s. |
| PC3 | n.s. | n.s. | 0.45 | n.s. | n.s. |
PC1 score indicates significance level. a) Mo, Morphological distance matrix. b) Ec, Ecological distance matrix. c) Ph, Phylogenetic distance matrix. d) con., conditioned on. e) n.s., not significant.
Bone length measurements: There were significant differences in FL and PL bone length measurements between the five species (Table 7). FL was shorter in species that possessed the higher aquatic tendency (E. lutris and L. lutra), being the shortest in E. lutris. In contrast, PL was longer in these species with more aquatic tendency and was longest in E. lutris. IL was significantly larger in E. lutris than in the other species.
Table 7. Mean values, standard deviations and interspecific significant differences of geometric mean scaled bones measurements.
| Items | Enhydra lutris | Lutra lutra | Neovison vison | Mustela itatsi | Mustela sibirica | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| na) | mean | SDb) | vs.c) | n | mean | SD | vs. | n | mean | SD | vs. | n | mean | SD | vs. | n | mean | SD | vs. | ||||||||||||||||
| FL | 13 | 0.81 | 0.01 | L | V | I | S | 10 | 0.92 | 0.01 | E | V | I | S | 10 | 1.01 | 0.01 | E | L | I | S | 26 | 1.05 | 0.01 | E | L | V | S | 24 | 1.09 | 0.01 | E | L | V | I |
| FGT | 13 | 0.31 | 0.01 | L | V | I | S | 10 | 0.28 | 0.01 | E | V | I | S | 10 | 0.26 | 0.00 | E | L | I | S | 23 | 0.23 | 0.01 | E | L | V | 24 | 0.23 | 0.01 | E | L | V | ||
| FAPD | 13 | 0.10 | 0.01 | V | I | S | 5 | 0.09 | 0.00 | V | I | 10 | 0.07 | 0.00 | E | L | I | S | 24 | 0.08 | 0.01 | E | L | V | 24 | 0.08 | 0.00 | E | V | ||||||
| FMLD | 13 | 0.14 | 0.01 | L | V | I | S | 5 | 0.10 | 0.01 | E | V | I | S | 10 | 0.08 | 0.01 | E | L | 24 | 0.08 | 0.00 | E | L | 24 | 0.08 | 0.00 | E | L | ||||||
| FEB | 13 | 0.24 | 0.01 | V | I | S | 10 | 0.23 | 0.01 | V | I | S | 10 | 0.20 | 0.01 | E | L | I | S | 23 | 0.19 | 0.01 | E | L | V | S | 24 | 0.18 | 0.01 | E | L | V | I | ||
| TL | 13 | 0.93 | 0.01 | L | V | I | S | 10 | 0.99 | 0.02 | E | V | I | S | 10 | 1.06 | 0.01 | E | L | S | 26 | 1.06 | 0.01 | E | L | S | 24 | 1.04 | 0.01 | E | L | V | I | ||
| TSL | 13 | 0.44 | 0.02 | I | S | 10 | 0.44 | 0.02 | V | I | S | 10 | 0.42 | 0.01 | L | I | S | 26 | 0.39 | 0.02 | E | L | V | S | 24 | 0.38 | 0.02 | E | L | V | I | ||||
| TAPD | 13 | 0.10 | 0.01 | V | I | S | 5 | 0.10 | 0.01 | V | I | S | 9 | 0.08 | 0.01 | E | L | 24 | 0.08 | 0.01 | E | L | 24 | 0.08 | 0.01 | E | L | ||||||||
| TMLD | 13 | 0.07 | 0.01 | V | I | S | 5 | 0.07 | 0.00 | V | I | S | 9 | 0.06 | 0.00 | E | L | 24 | 0.06 | 0.00 | E | L | 24 | 0.06 | 0.00 | E | L | ||||||||
| PL | 13 | 1.32 | 0.02 | L | V | I | S | 10 | 1.10 | 0.03 | E | V | I | S | 10 | 0.94 | 0.01 | E | L | I | S | 26 | 0.90 | 0.01 | E | L | V | S | 24 | 0.89 | 0.01 | E | L | V | I |
| IL | 13 | 0.59 | 0.03 | L | V | I | S | 10 | 0.49 | 0.02 | E | 10 | 0.49 | 0.01 | E | 26 | 0.48 | 0.01 | E | 23 | 0.48 | 0.01 | E | ||||||||||||
The abbreviations of measurements are defined in Table 2. a) n, sample size. b) SD, standard deviations. c) vs., species that showed significant differences in univariate ANOVA tests at the P<0.05 level using Games-Howell’s tests post hoc procedure (E, Enhydra lutris. L, Lutra lutra. V, Neovison vison. I, Mustela itatsi. S, Mustela sibirica)
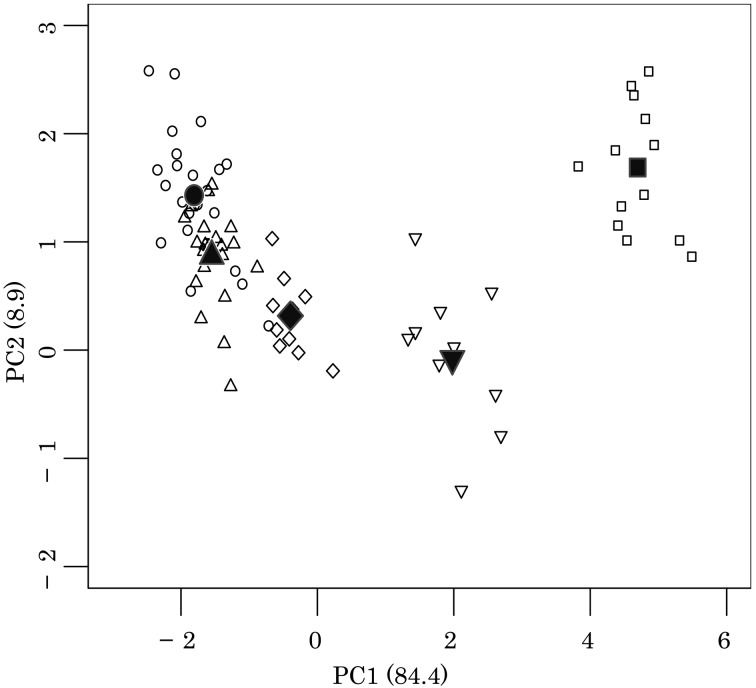
The percentage of variation explained by PC1 was 84.4%, which separated the samples into the following four plots on the basis of descending scores; E. lutris, L. lutra, N.vison and lastly both Mustela(Fig. 3 and Table 8). Both FL and TL correlated negatively with PC1, whereas FGT, FEB, TSL, PL and IL correlated positively. The percentage of variation explained by PC2 was 8.9%, and a separation of plots was found between E. lutris and both N. vison and L. lutra. M. itatsi and M. sibirica had intermediate scores and were not separated from other species. The factor loading of TSL correlated negatively with PC2.
Fig. 3.
Plot of PC1 and PC2 scores of bone measurements for the five mustelid species. PC1;
the first principal component. PC2; the second principal component. Numbers in
parentheses represent the percentage of the variation explained by the component.
 , Enhydra
lutris.
, Enhydra
lutris.  , Lutra
lutra.
, Lutra
lutra.  ,
Neovison vison.
,
Neovison vison.  , Mustela itatsi.
, Mustela itatsi.  , Mustela sibirica. Filled symbols indicate mean values of each
species.
, Mustela sibirica. Filled symbols indicate mean values of each
species.
Table 8. Factor loadings of bone measurements in PCA.
| Abbreviations | PC1 | PC2 | PC3 |
|---|---|---|---|
| FL | –0.98 | –0.02 | 0.00 |
| FGT | 0.96 | –0.06 | –0.13 |
| FEB | 0.94 | –0.17 | –0.23 |
| TL | –0.93 | –0.22 | 0.10 |
| TSL | 0.75 | –0.62 | 0.22 |
| PL | 0.99 | 0.13 | –0.01 |
| IL | 0.87 | 0.38 | 0.30 |
| EVa) | 5.91 | 0.62 | 0.22 |
| PVEb) | 84.39 | 8.88 | 3.10 |
| CPVEc) | 84.39 | 93.27 | 96.37 |
The abbreviations of bone measurements are defined in Table 2. a) EV, Eigen values. b) PVE, The percentage of the variation explained. c) CPVE, The cumulative percentage of the variation explained.
Table 9 shows the results of the partial Mantel tests of the ecological, phylogenetic and morphological matrices. The coefficient of correlation conditioned on the phylogeny between the morphology (PC1) and the ecology was 0.89 (P<0.05).
Table 9. The P values of partial Mantel test of bone measurements.
| Moa) vs. Ecb) | Mo vs. Phc) | Ec vs. Ph | Mo vs. Ec con.d) Ph | Mo vs. Ph con. Ec | |
|---|---|---|---|---|---|
| PC1 | 0.89 | 0.64 | 0.45 | 0.88 | 0.60 |
| PC2 | n.s.e) | n.s. | 0.45 | n.s. | n.s. |
| PC3 | n.s. | n.s. | 0.45 | n.s. | n.s. |
PC1 score indicates significance level in whole cases. a) Mo, Morphological distance matrix. b) Ec, Ecological distance matrix. c) Ph, Phylogenetic distance matrix. d) con., conditioned on. e) n.s., not significant.
DISCUSSION
Several studies have reported on the swimming motion of E. lutris [17, 36, 40], but no studies have described sequence data of the swimming motion and the movements of each hindlimb joint. Howell [17] studied the hindlimb skeleton of E. lutris and predicted that its submerged swimming motion differed from that of pinnipeds. He suggested that the swimming motion of E. lutris includes placing the feet horizontally to the rear with the soles up, one either side of the tail and oscillating them in the sagittal plane, in a motion similar to that of whale flukes. He suggested that the swimming motion of E. lutris is characterized by dorsoventral undulation of the trunk and by movements of both hindpaws in the dorsoventral direction, with the soles facing dorsally and/or caudally. However, Howell [17] did not comment on the movements of the hindlimb joints. Our results are consistent with the action predicted by Howell [17]. The GSFCTFL, GMPI, OE, FI3 and POP muscle groups are proportionately approximately two times larger in E. lutris than in the other species, but the RF, SMCA and ADD are significantly smaller in E. lutris than in the other species (Table 4). These results indicate that E. lutris utilizes GSFCTFL, GMPI, OE, FI3 and POP more powerfully than the other four species since muscle mass is proportional to work capacity [5]. Therefore, we suggest that during submerged swimming, the power-stroke phase of E. lutris comprises abduction of the femur by the GSFCTFL and GMPI muscles, medial rotation of the crus by the POP group, eversion movements of the ankle and also abduction of the fifth toe or extension of interdigital web by the FI3 group, in the FI3 group the peroneus brevis and the peroneus longus enable abduction of the fifth toe, and the peroneus digiti quinti extends the interdigital digit. The gluteus superficialis, gluteofemoralis and tensor fasciae latae muscles in the GSFCTFL group are fused in E. lutris [16, 17]. This muscle group covers the lateral side of the hip joint; it attaches the lateral femoral ridge of the inferior border of the greater trochanter to the upper border of the lateral femoral condyle [16]. Therefore, GSFCTFL may act as an abductor of the hip joint. In addition, it is pointed out that the piriformis muscle of E. lutris acts as an abductor on femur [16]. Movement of these three muscles at the hip, knee and ankle joints is not required for running on land. Therefore, the greater mass and power of these muscles in E. lutris suggests that these movements of the hindlimb are used for the swimming motion of this species.
The other mustelids are equipped with large muscles in the distal part of the hindlimbs (TCA, EDL, GMED and PESB), according to their aquatic tendency (Table 4). However, the evolutionary specializations observed in E. lutris were not observed in M. sibirica, M. itatsi, N. vison or L. lutra. The hindlimb morphology of terrestrial and semi-aquatic mustelids is regarded as a continuum. The North American river otter (Lontra canadensis) swims using dorsoventral movement of the trunk, whereas seals swim using lateral movement of the trunk [10]. It is possible that none of the five species examined in this study swim using lateral movement of the trunk. The swimming motion of M. itatsi and M. sibirica has not yet been studied, but Dunstone described the swimming motion of N. vison, which used all four limbs with either diagonally opposite legs simultaneously under the body [8]. We suspect that the swimming motion of both of these mustelids may be similar to that of N. vison, as the hindlimb muscle distribution morphologically resembles in these species. Terrestrial animals run on the ground with an anterior–posterior motion of the appendages under the trunk. Therefore, the results of our study suggest that the semi-aquatic animals investigated in this study swim with a motion similar to terrestrial running.
Taylor [38] compared the roughness of iliac crest of E. lutris with that of harbor seal (Phoca vitulina) and L. canadensis quaritatively, and noticed more aquatic species held rougher crest. He suggested that the sartorius muscles (SAR), which originate from the crest, for adducting the femur were augmented in size with the increase in importance of the hindlimbs as a paddle from his observations. This aquatic adaptation tendency referred to by Taylor was not observed in any of the five species examined in our result, which showed no significant differences. Although, the mean SAR value in E. lutris (mean value 2.10) was proportionately larger than that of L. lutra (mean value 1.70), the mean values in other three mustelids: N. vison, 1.84; M. itats, 2.12 and M. sibirica, 2.11, were relatively larger than that in L. lutra. These data show that the aquatic tendency is not associated with the size increasing of SAR that was proposed by Taylor [38], at least not in Mustelidae.
Various studies have shown femoral shortening in aquatic or semi-aquatic animals [28, 29, 33, 35, 38]. Samuels et al. [29] reported that femoral shortening brought the paddling limb closer to the body and thus reduced induced drag during the recovery stroke when swimming. In our study, we also found femoral shortening (lower FL values) in E. lutris and L. lutra species which had higher aquatic tendency (Table 7). Additionally, it is interesting to note the significant difference in FL between M. itatsi (mean value 1.05) and M. sibirica (mean value 1.09), which hunts fishes but it does not well adapt to swimming, although both species diverged each other about 1.6 million years ago [30]. The shortening of femur enables the foot to close to the body axis [35]. It seems that the closing contributes to efficient swimming.
Smith and Savage [31] suggested that more aquatically adapted animals possess smaller gluteus and larger ischiopubic muscles, which include the semimembranosus, biceps femoris, semitendinosus and tenuissimus after comparing the iliac length in proportions to the pelvic bones among a marten (Martes sp.), a river otter (Lutra sp.) and a seal (Phoca sp.). However, our results do not support this suggestion since E. lutris had the largest relative mass of gluteus muscles, and it and L. lutra also had smaller relative mass of the semimembranosus, biceps femoris, semitendinosus and tenuissimus muscles (Table 4). In addition, the IL length value of E. lutris (mean value 0.59) was significantly much larger than that of the other four species (mean value; L. lutra and N. vison, 0.49; M. itatsi and M. sibirica, 0.48) (Table 7). There are morphological differences, since the body of seal moves bilaterally, but that of both E. lutris and L. lutra moves dorsoventrally when they swim [35]. The difference between the results of Smith and Savage and ours was caused by following reason: E. lutris developed their gluteal muscles and became to aquatic; however, seals adopt another development for aquatic.
In conclusion, our muscle and bone measurement analyses revealed that the hindlimb morphology of the mustelids has obvious functional and morphological relationships with their ecology rather than with their phylogeny (Tables 6 and 9). We could confirm that E. lutris had unique hindlimb morphological characteristics compared to the other Mustelidae. In our study, we defined the aquatic tendency of each species from ecological information. Based on this information, we defined E. lutris as having full aquatic tendency, whereas the other four species had semi-aquatic or fully terrestrial tendencies. Furthermore, the muscle and bone analyses indicated morphological differences associated with hip joint abductors between E. lutris and the four other species that were categorized as terrestrial or semi-aquatic species. Therefore, we suggest the hindlimb of E. lutris clearly reflects their complete aquatic lifestyle. This hindlimb structure has resulted in E. lutris acquiring a more abductable femur, as it evolved away from the other semi-aquatic mustelidae and their need to partly survive on land. The E. lutris abductable femur may enable the species to develop maneuverability when they swim by raising the center of mass near to the body axis, the same as the shortening of femur. We conclude that E. lutris is a complete aquatic animal, possessing differences in the proportions of the hindlimb muscles compared with those in other semi-aquatic and terrestrial mustelids. The semi-aquatic species share similar muscle proportions with terrestrial rather than with aquatic species.
Supplementary Material
Acknowledgments
We thank the following people for access to specimens: Shin-ichiro Kawada for access to specimens from the collections of The National Museum of Nature and Science, Tokyo; Hajime Taru for access to specimens from the collections of Kanagawa Prefectural Museum of Natural History; Darrin Lunde for access to specimens from the collections of the National Museum of Natural History, Smithsonian Institute; Sadao Ihara of Ohu University and the staff of the Oita Marine Palace Aquarium UMITAMAGO for providing specimens. Further thanks are due to Mikiko Abe of Osaka City University, and also Satoshi Kawaguchi and Sen-ichi Oda of Okayama University of Science for access to their private collections. Finally, we also thank Eun-Young Noh, Hanchan Park and Jinwoo Oh for preparation of specimens.
REFERENCES
- 1.Bartoszewicz M., Zalewski A.2003. American mink, Mustela vison diet and predation on waterfowl in the Slonsk Reserve, Western Poland. Folia Zool. (Brno) 52: 225–238. [Google Scholar]
- 2.Berta A., Morgan G. S.1985. A new sea otter (Carnivora: Mustelidae) from the late Miocene and early Pliocene (Hemphillian) of North America. J. Paleo. 59: 809–819. [Google Scholar]
- 3.Birks J. D. S., Dunstone N.1985. Sex-related differences in the diet of the mink Mustela vison. Ecography 8: 245–252. doi: 10.1111/j.1600-0587.1985.tb01175.x [DOI] [Google Scholar]
- 4.Blanco-Garrido F., Prenda J., Narvaez M.2008. Eurasian otter (Lutra lutra) diet and prey selection in Mediterranean streams invaded by centrarchid fishes. Biol. Invasions 10: 641–648. doi: 10.1007/s10530-007-9158-1 [DOI] [Google Scholar]
- 5.Brand P. W., Beach R. B., Thompson D. E.1981. Relative tension and potential excursion of muscles in the forearm and hand. J. Hand. Surg. Am. 6: 209–219. doi: 10.1016/S0363-5023(81)80072-X [DOI] [PubMed] [Google Scholar]
- 6.Brzeziński M.2008. Food habits of the American mink Mustela vison in the Mazurian Lakeland, Northeastern Poland. Mamm. Biol. 73: 177–188. [Google Scholar]
- 7.Day M., Linn I.1972. Notes on the food of feral mink Mustela vison in England and Wales. J. Zool. 167: 463–473. doi: 10.1111/j.1469-7998.1972.tb01737.x [DOI] [Google Scholar]
- 8.Dunstone N.1980. Swimming and diving behavior of the mink (Mustela vison Schreber). Carnivore 2: 56–61. [Google Scholar]
- 9.Evans H. E.1993. Miller’s Anatomy of the Dog. 3rd ed., Saunders, Philadelphia. [Google Scholar]
- 10.Fish F. E.1994. Association of propulsive swimming mode with behavior in river otters (Lutra canadensis). J. Mammal. 75: 989–997. doi: 10.2307/1382481 [DOI] [Google Scholar]
- 11.Fish F. E.1996. Transitions from drag-based to lift-based propulsion in mammalian swimming. Am. Zool. 36: 628–641. [Google Scholar]
- 12.Fish F. E., Baudinette R. V.2008. Energetics of swimming by the ferret: Consequences of forelimb paddling. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150: 136–143. doi: 10.1016/j.cbpa.2006.06.019 [DOI] [PubMed] [Google Scholar]
- 13.Fish F. E., Stein B. R.1991. Functional correlates of differences in bone density among terrestrial and aquatic genera in the family Mustelidae (Mammalia). Zoomorphology 110: 339–345. doi: 10.1007/BF01668024 [DOI] [Google Scholar]
- 14.Fisher E. M.1942. The Osteology and Myology of the California River Otter, Stanford University Press, Redwood city. [Google Scholar]
- 15.Gambarjan P., Karapetjan W.1961. Besonderheiten im Bau des Seelöwen (Eumetopias californianus), der Baikalrobbe (Phoca sibirica) und des Seeotters (Enhydra lutris) in Anpassung an die Fortbewegung im Wasser. Zool. Jahrb. 79: 123–148(in Germany with English abstract). [Google Scholar]
- 16.Howard L. D.1975. Muscular anatomy of the hind limb of the sea otter (Enhydra lutris). Proc. Calif. Acad. Sci. 4th Series 40: 335–416. [Google Scholar]
- 17.Howell A. B.1930. Aquatic Mammals: Their Adaptations to Life in the Water, Dover, New York. [Google Scholar]
- 18.Kaneko Y., Shibuya M., Yamaguchi N., Fujii T., Okumura T., Matsubayashi K., Hioki Y.2009. Diet of Japanese weasels (Mustela itatsi) in a sub-urban landscape: Implications for year-round persistence of local populations. Mammal Study 34: 97–105. doi: 10.3106/041.034.0205 [DOI] [Google Scholar]
- 19.Kawada S.2001. Two species of mustelid carnivores caught by mole-traps. Special Public. Nagoya Soc. Mammal. 3: 39–40. [Google Scholar]
- 20.Kenyon K. W.1969. The Sea Otter in the Eastern Pacific Ocean, Dover, New York. [Google Scholar]
- 21.Klingener D.1979. Laboratory Anatomy of the Mink, William C Brown, Dubuque. [Google Scholar]
- 22.Kvitek R. G., Bowlby C. E., Staedler M.1993. Diet and foraging behavior of sea otters in southeast Alaska. Mar. Mamm. Sci. 9: 168–181. doi: 10.1111/j.1748-7692.1993.tb00441.x [DOI] [Google Scholar]
- 23.Legendre P., Legendre L.1998. Numerical Ecology, 2nd ed., Elsevier, Amsterdam. [Google Scholar]
- 24.Love J. A.1990. Sea Otters, Whittet Books, London. [Google Scholar]
- 25.Marmi J., Lopez-Giraldez J. F., Domingo-Roura X.2004. Phylogeny, evolutionary history and taxonomy of the Mustelidae based on sequences of the cytochrome b gene and a complex repetitive flanking region. Zool. Scr. 33: 481–499. doi: 10.1111/j.0300-3256.2004.00165.x [DOI] [Google Scholar]
- 26.Nowak R. M.1999. Walker’s Mammals of the World. 6th ed. Johns Hopkins University Press, Baltimore. [Google Scholar]
- 27.R Core Team. 2013. R: A language and Environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available from http://www.R-project.org.
- 28.Samuels J. X., Valkenburgh B. V.2008. Skeletal indicators of locomotor adaptations in living and extinct rodents. J. Morphol. 269: 1387–1411. doi: 10.1002/jmor.10662 [DOI] [PubMed] [Google Scholar]
- 29.Samuels J. X., Meachen J. A., Sakai S. A.2013. Postcranial morphology and the locomotor habits of living and extinct carnivorans. J. Morphol. 274: 121–146. doi: 10.1002/jmor.20077 [DOI] [PubMed] [Google Scholar]
- 30.Sato J. J., Wolsan M., Prevosti F. J., D’Elía G., Begg C., Begg K., Hosoda T., Campbell K. L., Suzuki H.2012. Evolutionary and biogeographic history of weasel-like carnivorans (Musteloidea). Mol. Phylogenet. Evol. 63: 745–757. doi: 10.1016/j.ympev.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 31.Smith J. M., Savage R. J. G.1956. Some locomotory adaptations in mammals. J. Linnean. Soc. London Zool. 42: 603–622. [Google Scholar]
- 32.Sasaki H., Ono Y.1994. Habitat use and selection of the Siberian weasel Mustela sibirica coreana during the non-mating season. J. Mammal. Soc. Jpn. 19: 21–32. [Google Scholar]
- 33.Stein B. R.1988. Morphology and allometry in several genera of semiaquatic rodents (Ondatra, Nectomys, and Oryzomys). J. Mammal. 69: 500–511. doi: 10.2307/1381341 [DOI] [Google Scholar]
- 34.Stein B. R.1989. Bone-density and adaptation in semiaquatic mammals. J. Mammal. 70: 467–476. doi: 10.2307/1381418 [DOI] [Google Scholar]
- 35.Tarasoff F. J.1972. Comparative aspects of the hind limbs of the river otter, sea otter, and seals. pp. 333–359. In: Functional Anatomy of Marine Mammals (Harrison, R. J. ed.), Academic Press, New York. [Google Scholar]
- 36.Tarasoff F. J., Bisaillo A., Pierard J., Whitt A. P.1972. Locomotory patterns and external morphology of the river otter, sea otter, and harp seal (Mammalia). Can. J. Zool. 50: 915–929. doi: 10.1139/z72-124 [DOI] [PubMed] [Google Scholar]
- 37.Tatara M., Doi T.1994. Comparative analyses on food habits of Japanese marten, Siberian weasel and leopard cat in the Tsushima islands, Japan. Ecol. Res. 9: 99–107. doi: 10.1007/BF02347247 [DOI] [Google Scholar]
- 38.Taylor W. P.1914. The problem of aquatic adaptation in the carnivora: as illustrated in the osteology and evolution of the sea-otter. Univ. Calif. Publ. Geol. 7: 465–495. [Google Scholar]
- 39.Williams T. M.1983. Locomotion in the North American mink, a semi-aquatic mammal. I. Swimming energetics and body drag. J. Exp. Biol. 103: 155–168. [DOI] [PubMed] [Google Scholar]
- 40.Williams T. M.1989. Swimming by sea otters − adaptations for low energetic cost locomotion. J. Comp. Physiol. A 164: 815–824. doi: 10.1007/BF00616753 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.