Abstract
Structural variations are common in the human genome but their contributions to human diseases have been hard to define. Lupiáñez et al demonstrate that some structural variants can interrupt chromatin topology, resulting in ectopic enhancer-promoter interactions, altered spatiotemporal gene expression patterns and developmental disorders.
Structural variations, such as insertion, deletion, duplication, translocation, or inversion of DNA segments, are commonly associated with human diseases, ranging from autism to cancer (Stankiewicz and Lupski, 2010). However, determining how these variants contribute to human diseases remains one of the greatest challenges in genomics research. Typically, structural variations that affect the copy number of a gene are thought to act through gene dosage effects. However, if a structural variant occurs in a non-coding region of the genome, predicting the phenotypic consequences is very challenging. In this issue of Cell, Lupiáñez et al. illustrate a conceptual framework to interrogate the molecular mechanisms by which structural variants cause developmental defects in humans (Lupiáñez et al., 2015). The authors show that disruption of chromatin organization by inversion, duplication or deletion is the culprit of at least three related human genetic disorders.
In interphase nuclei, chromosomes occupy distinct volumes termed “chromosome territories”. Each chromosome folds into a complex and dynamic structure, the form of which has been the subject of intense investigation recently. A key feature of the mammalian chromatin organization that has emerged from genome-wide chromatin interaction assays is topologically associating domains (TADs), which partition each interphase chromosome into mega-sized segments that exhibit frequent intra-domain chromatin interactions but relatively rare inter-domain interactions (Sexton and Cavalli, 2015). TADs are remarkably conserved between different cell types, suggesting that they are stable during development and are not easily disrupted by transcriptional activities of the cell. Furthermore, the TADs from related species are highly similar, indicating a strong evolutionary pressure to preserve such chromatin organization(Dixon et al., 2012). These findings have led to the proposal that TADs are important for maintaining proper enhancer/promoter interactions and ensuring precise spatiotemporal gene expression patterns during animal development (Dixon et al., 2012; Nora et al., 2012). Supporting this prediction, several studies have shown that deletion or inversion of TAD boundaries can disrupt TADs organization and lead to altered gene expression in cultured cells and in animals (Andrey et al., 2013; Nora et al., 2012). However, little evidence to date had linked alterations of these genomic structures to human disease.
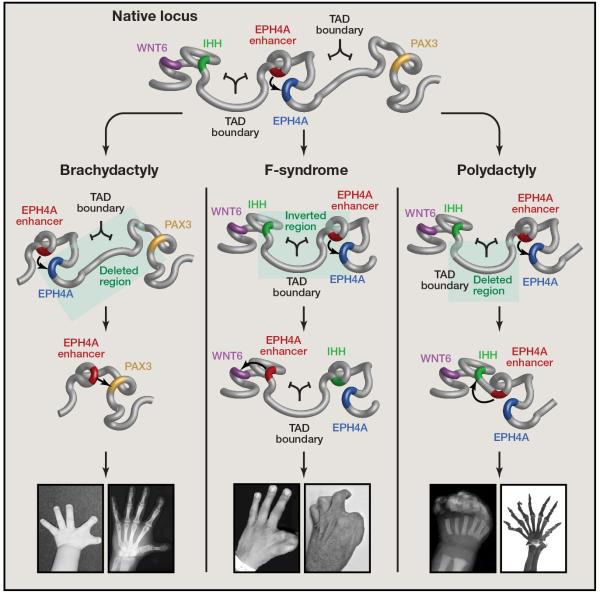
Lupiáñez et al. studied the structural variants underlying three congenital birth defects in humans (Lupiáñez et al., 2015). By genome sequencing or array CGH they precisely defined the nature and location of structural variants, and found that they all span a TAD boundary near the EPHA4 gene (Figure 1). The authors hypothesized that these structural variants could disrupt local chromatin organization and alter enhancer/promoter interactions, leading to ectopic expression of the adjacent genes, including WNT6, IHH and PAX3, all of which are implicated in vertebrate limb development (Geetha-Loganathan et al., 2005; Yang et al., 1998).
Figure 1. Structural variation and pathological rewiring of genetic regulatory interactions.
A genetic locus that includes genes and enhancers relevant to mammalian limb formation (top) has undergone deletions and inversions in humans causing altered promoter-enhancer interactions (middle) and three distinct malformation syndromes (bottom). Recapitulating these structural variations in mice indicates that disruption of the TAD boundary domain is a key component of rewired circuitry and a pathological phenotype.
To test this hypothesis, Lupiáñez et al used the CRISPR/Cas9 genome editing tool to create several mouse models that recapitulate the structural changes found in the human cases (Figure 1). Remarkably, mutant mice carrying these structural alterations accurately reproduce the human disease phenotypes of altered digits and limb malformation, confirming that the structural variations are indeed responsible for the developmental disorders. Analysis of gene expression profiles revealed that WNT6, IHH or PAX3 are ectopically expressed in e11.5 limb buds in the mouse models with corresponding structural changes. To further understand the mechanisms responsible for WNT6, IHH and PAX3 misexpression in these mutant mice, the authors carried out 4C-seq experiments, which can reveal the chromatin interactions between a bait sequence and the rest of the genome. The results confirmed that structural changes indeed resulted in reorganization of the local chromatin architecture, producing new interactions between a cluster of enhancers that is typically restricted to the EPHA4 gene, and the promoter of WNT, IHH or PAX3 in the respective mouse model. Finally, to show that the increased interactions were due to disruption of TAD boundaries, but not decreased linear genomic distances per se, the authors generated additional mutant mouse strains that contain essentially the same sized genomic deletions but with intact TAD boundaries. These mouse strains have normal limb and digits. These carefully designed experiments provided the strongest evidence yet that disruption of TADs by structural variants could cause developmental disorders in humans (Figure 1).
The demonstration that structural variations in the mouse genome could lead to developmental defects that mimic the human disorders is remarkable. Underlying the success of this approach are two properties of the chromatin organization in mammalian cells. First, the TAD structures are conserved between the mouse and the human genome. Thus, structural changes in syntenic sequences in the two genomes resulted in similar disruption of TADs in both species. Second, TADs are highly similar between different cell types in the body. Based on these observations, Lupiáñez et al. performed 4C-seq on patient fibroblasts and were able to show the same reorganization of chromatin architecture and abnormal interactions as they had observed in the mutant mouse limb buds. Hence, it is possible to use human fibroblasts to demonstrate alterations of chromatin topology present in human embryonic limb buds carrying structural variants, since the latter are nearly impossible to obtain for research.
Why are TADs conserved in different cell types and between different species? This is likely because TADs are defined by highly conserved boundary sequences and specific DNA binding factors that recognize unique DNA elements in these regions. One of the DNA binding proteins that are likely responsible for establishing TADs is the ubiquitously expressed CCCTC-binding factor (CTCF), binding sites of which are enriched at the TAD boundaries. CTCF is highly conserved in vertebrates and many metazoan species, with DNA binding specificity essentially unchanged during evolution (Ong and Corces, 2014). CTCF binding sites at a boundary in the HoxA locus are necessary for the separation of two TADs. Point mutations or small insertion/deletions that disrupt one of the CTCF binding sites can lead to increased expression of a gene adjacent to the boundary attributed to increased chromatin interactions (Narendra et al., 2015). While it is still unclear how exactly CTCF contributes to formation or maintenance of TAD boundaries, its ubiquitous expression pattern and the high degree of protein sequence conservation help explain the stable TAD structure in different cell types and species.
The newly reported findings demonstrate that inversions, deletions or other structural variations that affect TAD boundaries can change chromatin organization, rewire enhancer-promoter interactions, alter gene expression patterns and cause human diseases. As more and more structural variants are discovered in the human genome and linked to uncharacterized genetic disorders, consideration of their impact on chromatin topology will be essential for understanding their molecular mechanisms of pathogenesis.
References
- Andrey G, Montavon T, Mascrez B, Gonzalez F, Noordermeer D, Leleu M, Trono D, Spitz F, Duboule D. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science. 2013;340:1234167. doi: 10.1126/science.1234167. [DOI] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Prols F, Patel K, Scaal M, Huang R, Christ B. Ectodermal Wnt-6 promotes Myf5-dependent avian limb myogenesis. Dev Biol. 2005;288:221–233. doi: 10.1016/j.ydbio.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Lupiáñez DG, Kraft K, Heinrich V, Krawitz P, Brancati F, Klopocki E, Horn D, Kayserili H, Opitz JM, Laxova R, et al. Disruptions of Topological Chromatin Domains Cause Pathogenic Rewiring of Gene-Enhancer Interactions. Cell. 2015 doi: 10.1016/j.cell.2015.04.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra V, Rocha PP, An D, Raviram R, Skok JA, Mazzoni EO, Reinberg D. Transcription. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science. 2015;347:1017–1021. doi: 10.1126/science.1262088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CT, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet. 2014;15:234–246. doi: 10.1038/nrg3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton T, Cavalli G. The Role of Chromosome Domains in Shaping the Functional Genome. Cell. 2015;160:1049–1059. doi: 10.1016/j.cell.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- Yang Y, Guillot P, Boyd Y, Lyon MF, McMahon AP. Evidence that preaxial polydactyly in the Doublefoot mutant is due to ectopic Indian Hedgehog signaling. Development. 1998;125:3123–3132. doi: 10.1242/dev.125.16.3123. [DOI] [PubMed] [Google Scholar]