Abstract
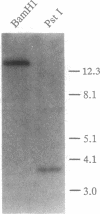
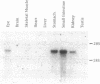
A DNA sequence encoding a G-protein-coupled receptor was isolated from a mouse genomic library. The predicted protein is similar in structure to the thrombin receptor and has a similar activation mechanism. When expressed in Xenopus laevis oocytes, the receptor was activated by low concentrations of trypsin (EC 3.4.21.4) and by a peptide (SLIGRL) derived from the receptor sequence, but was not activated by thrombin (EC 3.4.21.5). Trypsin failed to activate a mutant receptor in which the presumed cleavage site Arg-34-Ser-35 was changed to an Arg-Pro sequence. The agonist peptide (SLIGRL) activated equally well mutant and wild-type receptors. Northern blot analysis demonstrated receptor transcripts in highly vascularized tissues such as kidney, small intestine, and stomach. Because this, to our knowledge, is the second example, besides the thrombin receptor, of a proteolytically activated seven-transmembrane G-protein-coupled receptor, we have provisionally named it proteinase activated receptor 2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockaert J. G proteins and G-protein-coupled receptors: structure, function and interactions. Curr Opin Neurobiol. 1991 Jun;1(1):32–42. doi: 10.1016/0959-4388(91)90008-u. [DOI] [PubMed] [Google Scholar]
- Chang W. C., Shi G. Y., Chow Y. H., Chang L. C., Hau J. S., Lin M. T., Jen C. J., Wing L. Y., Wu H. L. Human plasmin induces a receptor-mediated arachidonate release coupled with G proteins in endothelial cells. Am J Physiol. 1993 Feb;264(2 Pt 1):C271–C281. doi: 10.1152/ajpcell.1993.264.2.C271. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen U. B., Vouret-Craviari V., Jallat S., Schlesinger Y., Pagès G., Pavirani A., Lecocq J. P., Pouysségur J., Van Obberghen-Schilling E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991 Aug 19;288(1-2):123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Scher W. The role of extracellular proteases in cell proliferation and differentiation. Lab Invest. 1987 Dec;57(6):607–633. [PubMed] [Google Scholar]
- Sundelin J. B., Provvedini D. M., Wahlestedt C. R., Laurell H., Pohl J. S., Peterson P. A. Molecular cloning of the murine substance K and substance P receptor genes. Eur J Biochem. 1992 Feb 1;203(3):625–631. doi: 10.1111/j.1432-1033.1992.tb16592.x. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V., Van Obberghen-Schilling E., Rasmussen U. B., Pavirani A., Lecocq J. P., Pouysségur J. Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell. 1992 Jan;3(1):95–102. doi: 10.1091/mbc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Wheaton V. I., Hung D. T., Charo I., Coughlin S. R. Domains specifying thrombin-receptor interaction. Nature. 1991 Oct 17;353(6345):674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Pixley R. A., Kucich U., Abrams W., Weinbaum G., Schapira M., Colman R. W. Purified plasma factor XIIa aggregates human neutrophils and causes degranulation. Blood. 1986 Jun;67(6):1731–1737. [PubMed] [Google Scholar]
- Wahlestedt C. Strategies to detect heterologously expressed tachykinin receptors in Xenopus Oocytes. Ann N Y Acad Sci. 1991;632:116–122. doi: 10.1111/j.1749-6632.1991.tb33100.x. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Fleischer D. T., Haviland D. L. Deficiency of the murine fifth complement component (C5). A 2-base pair gene deletion in a 5'-exon. J Biol Chem. 1990 Feb 15;265(5):2435–2440. [PubMed] [Google Scholar]
- Zhong C., Hayzer D. J., Corson M. A., Runge M. S. Molecular cloning of the rat vascular smooth muscle thrombin receptor. Evidence for in vitro regulation by basic fibroblast growth factor. J Biol Chem. 1992 Aug 25;267(24):16975–16979. [PubMed] [Google Scholar]