Abstract
Nanotechnology in diabetes research has facilitated the development of novel glucose measurement and insulin delivery modalities which hold the potential to dramatically improve quality of life for diabetics. Recent progress in the field of diabetes research at its interface with nanotechnology is our focus. In particular, we examine glucose sensors with nanoscale components including metal nanoparticles and carbon nanostructures. The addition of nanoscale components commonly increases glucose sensor sensitivity, temporal response, and can lead to sensors which facilitate continuous in vivo glucose monitoring. Additionally, we survey nanoscale approaches to “closed-loop” insulin delivery strategies which automatically release insulin in response to fluctuating blood glucose levels. “Closing the loop” between blood glucose level (BGL) measurements and insulin administration by removing the requirement of patient action holds the potential to dramatically improve the health and quality of life of diabetics. Advantages and limitations of current strategies, as well as future opportunities and challenges are also discussed.
Keywords: Nanotechnology, Glucose Sensors, Insulin Delivery, Nanomedicine, Diabetes, Nanomaterials
Introduction
Diabetes is a metabolic disease characterized by chronically elevated blood glucose levels (BGLs) and an inability to maintain BGL homeostasis1, 2. Individuals with type 1 diabetes cannot produce insulin as a result of autoimmune destruction of the insulin producing cells within the pancreas, known as beta cells3. Type 2 diabetes is characterized by insulin resistance, or a deficiency in cellular response to insulin in the bloodstream2. In both cases, the loss of homeostasis-regulation mechanisms can lead to chronically high and low blood glucose levels known as hyperglycaemia or hypoglycemiea3. Chronic hyperglycaemia can lead to a variety of symptoms including cardiovascular and neurological complications4, while hypoglycaemia can lead to lack of energy, unconsciousness, and death5. Diabetes has grown to become one of largest public health challenges globally, affecting 25.8 million in the United States and 382 million worldwide; and this number is expected to grow to 592 million by 20355, 6. Furthermore, diabetes is expected to become the seventh largest cause of death worldwide by 20304. The current standard of care for type 1 and advanced type 2 diabetics involves daily subcutaneous insulin injections, and frequent finger pricks to draw blood for the measurement of BGLs5. Daily insulin injections are painful and lead to patient noncompliance, and can lead to dangerous insulin overdoses5. Additionally, periodic measurement of blood glucose may not detect large fluctuations in BGLs which occur between points of measurement. Therefore, systems which improve blood glucose monitoring, or “close the loop” between glucose measurement and insulin delivery, are highly desirable.
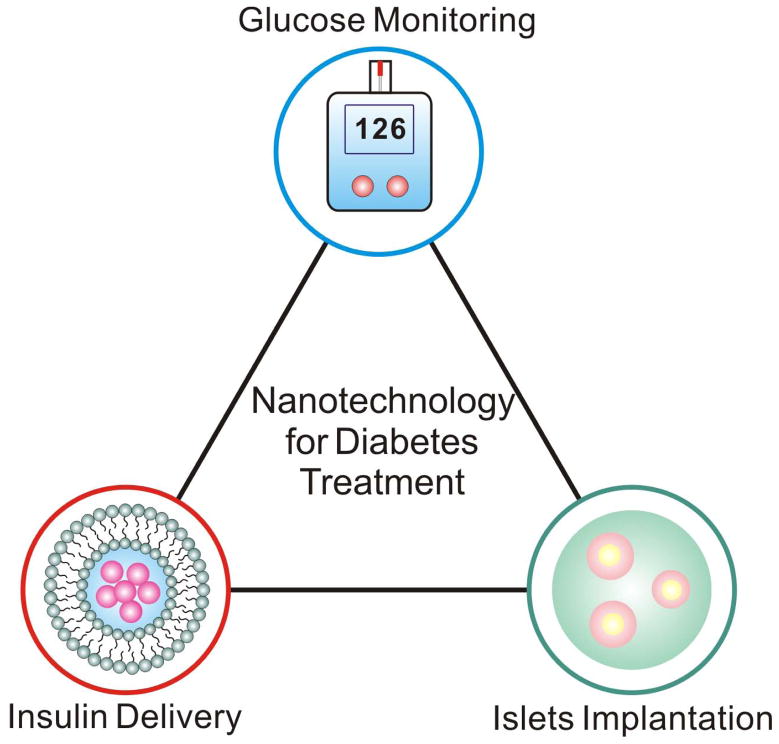
The application of nanotechnology to medicine holds many possible advantages, such as access to small and clinically relevant areas of cells and analysis of small volumes of analytes. Additionally, the emergence of quantum effects leads to interesting and useful physical properties; for example, nanoscale carbon is stronger than steel, highly malleable, fluorescent, and exhibits excellent electrical conductivity7. Figure 1 displays typical research themes using nanotechnology for diabetes treatment. For example, the improved glucose sensor technology has an immediate and significant impact on the health of diabetics, as improved sensing will lead to more accurate insulin dosing and diabetes management. Indeed, advances in nanomedicine have already facilitated novel sensors which are capable of more frequent and convenient blood glucose measurements6, 8–12. Nanomedicine has also enabled more robust insulin delivery systems that can detect fluctuations in BGLs and automatically modulate the rate of insulin release to maintain normoglycemiea6, 13. Such systems represent a tremendous advancement over contemporary standards of care. The clinical application of these technologies will allow diabetics to manage their disease more effectively and improve their health and quality of life.
Figure 1.
Schematic of research themes using nanotechnology for diabetes treatment.
Nanotechnology Enabled Glucose Sensing
Accurate and frequent glucose measurements are the basis of contemporary diabetes management. However, it is commonly acknowledged that contemporary clinical glucose measurement systems are a nuisance to the patient as a result of frequent and painful needle sticks, and the current standard of intermittent testing can miss dangerous fluctuations in blood glucose concentration7, 14. Therefore, one of the most significant challenges in diabetes research is the development of glucose sensors which achieve accurate glucose measurements painlessly and frequently, with the goal of continuous glucose measurement. A wide variety of glucose sensing modalities have been reported in the last two decades, and commonly employ Concanavalin A (Con A), phenylboronic acid (PBA), or most commonly, Glucose Oxidase (GOx) as a sensor for detecting glucose in solution. Common interfering species in blood and other bodily fluids include Uric and Acetic acids, as well as other carbohydrates such as Fructose, Lactose, and Sucrose6, 11, 15. Specificity for measuring only Glucose in solution requires the use of glucose specific enzymes, or the use of chemical or physical barriers with other detection modalities16.
Broadly speaking, glucose measurement systems can be classified into two categories: electrical and optical. A common mechanism of glucose detection involves using hydrogen peroxide (H2O2) or a similar reduced species as a chemical intermediary which drives the reduction of another species. This in turn generates a measurable signal such as an increase or shift in flourescence6, 8, 11 or a change in current through an electrode of an amperometric sensor17–19. Advantages of hydrogen peroxide based detection schemes include relatively straightforward sensor operation and characterization via amperometric techniques, but disadvantages include the degenerative effects of hydrogen peroxide on the sensor, the relatively high electrical potential required to catalyse hydrogen peroxide, and the possibility of sensor interference20–22. As a result of the importance of quantifying the concentration of hydrogen peroxide in solution to other fields, many electrical-based H2O2 sensors have been developed or adapted for glucose measurement. A summary of nanotechnology enabled glucose sensing technologies is included in Table 1.
Table 1.
Summary of reported glucose measurement systems.
| Type | Detection Principle | Response Time | Detection Limit | Reference Number |
|---|---|---|---|---|
| Optical | Nanotube Near-IR emission | ~1 min | 34.7 μM | 23 |
|
| ||||
| Nanotube Fluorescence Enhancement | ~ 1min | 2.5 mM | 28 | |
| Fluorescence Enhancement and AuNP Growth | 30–60 min | 0.01 mM (QDs); 0.1 mM (AuNPs) | 8 | |
| Graphene Catalytic Activity | 1h | 1 μM | 37 | |
| Nanotube Fluorescence Enhancement | ~1 min | 5 mM | 29 | |
| Raman spectroscopy | ~10 min | 0.5 μM | 6 | |
| Hydrogel Mediated Bragg Diffraction | 5 min | 90 μM | 11 | |
| Protein FRET signal | ~1 min | 25 μM | 9 | |
|
| ||||
| Electrical | Nanotube conductance modulation | ~20 s | 0.1 mM | 24 |
| Hydrogen Peroxide catalysis via Nanotubes | <20 s | 0.08 mM | 25 | |
| Hydrogen Peroxide catalysis | <5 s | 1.5 μM | 18 | |
| Hydrogen Peroxide catalysis via AuNPs | <30 s | 180 μM | 17 | |
| Nanomaterial enhanced conductance modulation | <20 s | 0.5 μM; 5 μM | 27 | |
| Nanomaterial enhanced conductance modulation | <20 s | 0.56 mM; 0.26 mM | 30 | |
| Enzyme-free glucose catalysis | <20 s | <25 nm | 22 | |
| Nanoparticle catalysis of Hydrogen Peroxide | 3 s | 0.7 μM | 32 | |
| Enzyme-free glucose catalysis | <20 s | 1 mM | 21 | |
|
| ||||
| Magnetic | Shift in magnetic resonance of a membrane | <1 min | -- | 34 |
Electrochemical Glucose Measurement
The excellent conductivity and catalytic ability of nanoscale carbon structures has led to their use in a variety of glucose sensing modalities23–29. Additionally, electrically coupling glucose oxidase to nanoscale carbon structures modulates the electrical resistance of the structures24. The first to publish research on using a single carbon nanotube as a biosensor was Besteman, et al., who immobilized GOx on a semiconductor carbon nanotube via 1-pyrenebutanoic acid succinimidyl ester, and found that the conductivity of the nanotube was correlated to the activity of the immobilized GOx’s response to the addition of glucose24. The addition of 0.1 mM glucose solution resulted in an approximate 10% increase in conductivity of the GOx-nanotube complex24. The system also exhibited a strong sensitivity to the pH of the surrounding solution24.
In most electrochemical methods, glucose concentration measurements are achieved by measuring an enzyme’s activity either directly by coupling it to an electrode or indirectly by measuring changes in concentration of the reactants or products. An advantage of coupling this technique with nanoscale components is that nanostructures such as carbon nanotubes24, 26, 27, 29 or gold nanoparticles17, 18 are excellent catalysts for reducing H2O2. Furthermore, the high surface area to volume ratio of nanostructures increases the reaction kinetics. Lin and co-workers applied the technique of hydrogen peroxide reduction to electrochemically detect glucose25. GOx was directly immobilized on carbon nanotubes by forming amide linkages between GOx’s amine residues and carboxylic acid groups on the carbon nanotubes via 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and N-hydroxysulfo-succinimide25. At low operating voltages (−0.2 V), the H2O2 generated by GOx action could be efficiently catalysed by the nanotubes, resulting in current flow proportional to glucose concentration. A linear response from 0–30 mM was found, and a small limit of detection of 0.08 mM was reported. As opposed to large glucose sensing electrodes, the use of an array of many nanoscale electrodes increases the signal-to-noise ratio and the sensitivity of the sensor by increasing its functional surface area. Additionally, the use of small negative potentials can reduce the risk of interference from unwanted redox reactions which occur at higher potentials. It has also been demonstrated that combinations of nanoparticles work together synergistically to realize more efficient hydrogen peroxide catalyzation than can be realized with any single component. Shan et al. found that the combination of graphene, gold nanoparticles (AuNPs), and GOx within a nanocomposite material can cause an increase in the electrocatalyzation current of hydrogen peroxide and oxygen as a result of synergistic interactions between graphene and AuNPs17. They developed a glucose sensor based on this technology which could operate at low potential (−0.2 V) and was capable of accurately measuring human blood glucose concentrations from 2.5–7.5 mM17.
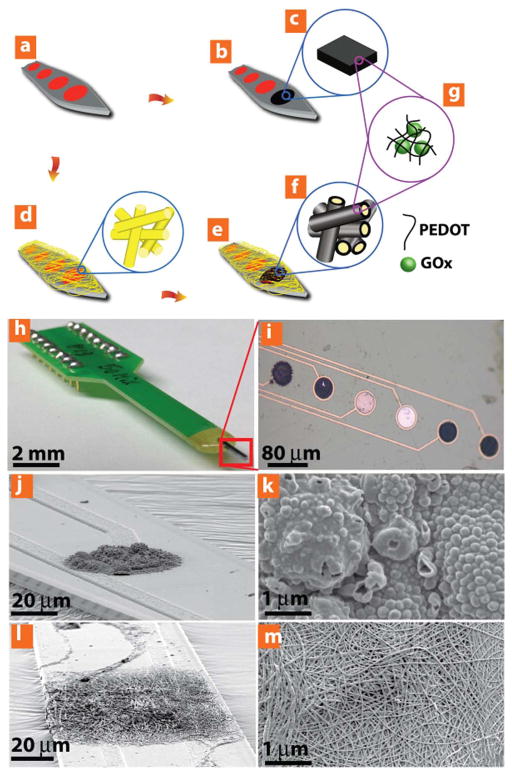
Yang and co-workers (Figure 2) compared similar micro- and nano-scale GOx electrodes, and found superior performance from the nanoscale sensors30. The micro scale electrode was based on a bulk conducting polymer film with entrapped GOx, and the nanoscale electrode was comprised of nanofibers with embedded GOx. Both were placed between microfabricated electrodes and were principally comprised of a conducting polymer, poly(3,4-ethylenedioxythiophene) (PEDOT), and were tested at potentials of +0.3 V and +0.7 V30. As a result of its larger surface area, the nanofiber sensor exhibited an improved signal-to-noise ratio and a higher density of GOx immobilization than the bulk film. Additionally, the conductivity of the nanofiber sensor was greater than the bulk film system, and a 433% increase in sensitivity was reported30. Further, the detection limit for the nanofiber sensor was 0.26 mM, compared to 0.56 mM for the bulk polymer. The nanofiber sensor also exhibited significantly improved sensitivity over 30 days compared to bulk hydrogel (83% loss of sensitivity compared to 28% loss of sensitivity).
Figure 2.
Yang, et al investigated how nanowires can affect the sensitivity of amperometric glucose sensors. In this experiment, two types of sensors were developed; the first was formed by immobilizing GOx within a bulk hydrogel, and the second was formed by immobilizing GOx on the surface of conductive nanowires. This figure depicts a schematic of the two types of glucose concentration sensors: (a–c) A bulk hydrogel glucose sensor with embedded GOx. (a,d–g) A nanowire glucose sensor with embedded GOx. (h,i) The glucose sensor apparatus. (j–k) The bulk hyrdogel-GOx sensing system. (i–m) The nanowire-GOx based glucose sensing system. The nanowire based sensor was more sensitive than the bulk film sensor, which was attributes to its larger surface area, increased GOx loading capacity, and lower electrical resistance. (Reprinted with permission from Ref 30. Copyright 2014 John Wiley & Sons)
As a result of the superior surface area and reducing ability of nanostructures, highly sensitive glucose sensors can be fabricated with carbon nanotubes. Tang, et al. fabricated an amperometric sensor using multi-walled carbon nanotubes (MWCNTs) and Platinum nanoparticles (Pt NPs)27. The addition of Pt NPs to platinum electrodes containing chitosan-immobilized GOx reduced the glucose detection limit by a factor of greater than 1000 to an exceptionally low limit of detection of 5 nM as compared to a non-Pt NP functionalized sensor27. This drastic reduction in detection limit was attributed to the Pt NP’s excellent ability to reduce hydrogen peroxide generated by the GOx reaction 27.
To prevent contamination of the glucose signal by interfering chemical species, some groups have shielded their sensors from such species. This shielding is particularly important for sensors which utilize non-enzymatic glucose transducers, such as boronic acids. Zeng et al. immobilized GOx onto gold nanoparticle electrodes (AuNPs) with a chitosan hydrogel and covered the matrix with a thin Nafion layer to prevent interfering species from entering the sensor18. The sensor exhibited excellent performance as a result of the inclusion of AuNPs and despite the Nafion layer. The GOx-generated hydrogen peroxide was catalysed by the gold nanoparticle electrodes and the resulting current was measured18. The sensor was 2.8 times more sensitive to glucose than a sensor fabricated with a flat gold electrode, and a linear detection range of 3.0 μm – 9.0 mM with a detection limit of 1.5 μm and a response time of < 5 seconds was reported18. Additionally, Uehara, et al. developed a sponge like nanoporous membrane with a pore diameter of 5–30 nm16. This membrane was used to selectively allow the passage of glucose, while blocking larger albumin molecules16. Applications of this membrane could include shielding a glucose sensor from interference from larger molecules or preventing aggregation of large molecules on its surface. Nunes and co-workers developed a membrane capable of serving the same purpose, but with pH responsive pore diameters; pore diameters from 4–17 nm could be achieved over a pH range of 4.2–731.
The ability to detect both increasing and decreasing glucose concentrations, known as reversibility, is another important characteristic of glucose sensors and a requirement for an implantable continuous glucose sensor. Zhai and co-workers developed a highly reversible sensor with minimal hysteresis by immobilizing GOx and Platinum Nanoparticles (Pt NPs) within a polyaniline hydrogel matrix, and used the matrix as a conductor to measure the electrical activity of both the electrocatalytic action of the GOx enzymatic reaction and the Pt NP mediated catalysis of hydrogen peroxide32.
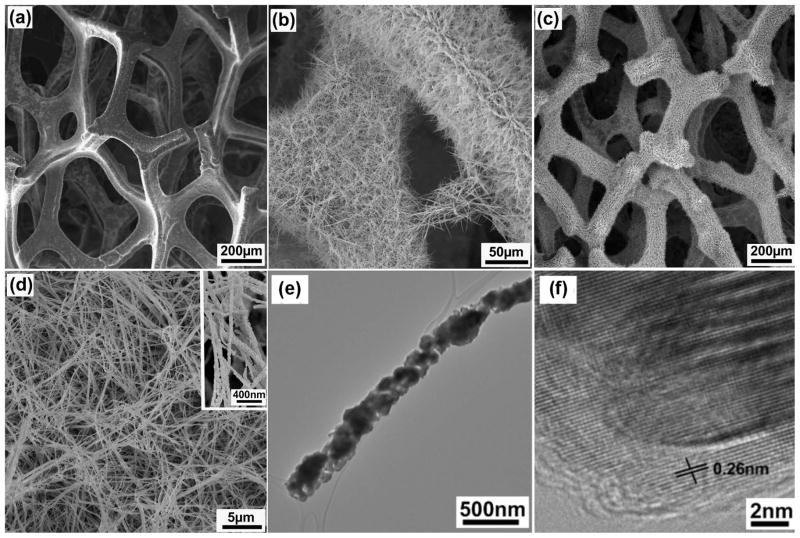
It is important to note that enzymes can be easily denatured, and enzymatic sensor lifetime may be an issue which precludes continuous in vivo glucose sensing22. Therefore, enzyme-free modalities have been developed. Dong, et al. reported growing Cobaltosic oxide nanowires on grapheme foam, which are capable of direct detection of glucose (Figure 3)22. Ding, et al. found that the cobalt nanowires serve as a catalyst for facilitating the direct oxidation of glucose to gluconolactone via the following equation33:
Figure 3.
The cobalt nanowires in this image can directly oxidize glucose without the use of enzymes, and thereby generate an amperometric signal proportional to the glucose concentration. An extremely small detection limit was reported (<25nM), and was attributed to the extremely large surface area of the sensor. This figure depicts scanning electron microscopy images of (A) Graphene foam; (B) Cobalt nanowires grown on the surface of graphene foam; (C) Graphene Foam functionalized with cobalt nanowires; (D) High magnification of cobalt nanowires; (E) Image of a single cobalt nanowire. (Reprinted with permission from Ref 22. Copyright 2012 American Chemical Society)
High sensitivity (3.39 mA mM−1 cm−2) with a low detection limit (<25 nM) was reported by Dong, et al. in glucose concentrations from 0–1 mM22. Additionally, this reaction was found to be specific to glucose, as the catalysis of uric and ascorbic acid was not catalysed by the nanowires22. Similarly, silicon nanoparticles can be used for direct enzyme-free detection of glucose20, 21. Wang and coworkers were among the first to report such a system, and immobilized Si29 nanoparticles on a Si substrate21. The electrical activity of the catalytic reaction was effectively transported to the substrate, and a linear detection range from 0–50 mM was reported. The system did not exhibit interference from ascorbic or uric acids21.
Another exciting option for glucose detection is the magnetic response technique reported by Sun and co-workers, who functionalized magnetic nanoparticles with Con A34. They found that the T2 relaxation time of the Con A functionalized nanoparticles was affected by the concentration of glucose due to the change in total particle mass, and reported a linear relationship between T2 relaxation time and glucose concentration (0–300 mg dL−1)34. The sensor was reported to exhibit excellent stability and is reversible, thereby making such a sensor a good candidate for continuous glucose monitoring. Furthermore, the toxicity of Con A has also been recently assessed, and has been found to be safe at low concentrations35.
Optical Glucose Measurements
Optical glucose measurements typically involve an increase in fluorescence intensity or a shift in the wavelength of the light produced by a fluorescent material coupled with a glucose sensitive material. The implantation of reversible optical sensors has led to the concept of a “smart tattoo” which continuously measures BGL by modulating the tattoo’s optical wavelength in response to glucose concentration36. Optical sensors have a number of advantages, including imperviousness to electromagnetic fields, capability to be miniaturized for single molecule detection schemes, and the ability to be used in continuous real time glucose monitoring11.
Carbon nanotubes exhibit properties which make them ideal for fluorescent biosensing, such as emission at a near-infrared wavelengths, which is a wavelength at which skin is particularly transparent23. Barone, et al. was among the first to use single walled nanotubes (SWNTs) for fluorescent glucose sensing, and demonstrated that SWNTs functionalized with Fe(CN)63− exhibit a change in fluorescence when the H2O2 generated by GOx action reduces Fe(CN)63− on functionalized SWNTs. This sensor was found to be sensitive to physiologically relevant glucose concentrations (1 mM – 8 mM) and was fully reversible23. Inherent fluorescence of carbon nanotubes was further investigated by Yum and co-workers (Figure 3), who tested 30 Boronic acids with SWNTs to find combinations which yield accurate and reversible changes in fluorescence signals in response to glucose concentration29. As glucose binds to several boronic acid functionalized SWNTs, the fluorescence signal increased. Two Boronic acids were found which optomized the desired properties: 4-cyanophenylboronic and 4-chlorophenylboronic acid, and a reversible sensor was realized by measuring the frequency shift of the fluorescence or the “turn on” response29. The sensor was sensitive to physiologically relevant glucose concentrations, and reacted quickly to changes in glucose (~1 min)29.
Additionally, it has been demonstrated that Glucose Binding Protein (GBP) bound to SWNTs can be used to mechanically actuate the SWNTs upon glucose binding, and thereby cause a quenching of the SWNT’s fluorescence in proportion to glucose concentration28. Such a sensor has been demonstrated by Yoon, et al, who bound GBP to SWNTs via amine coupling to lysine groups on the enzyme28. The group demonstrated a 5–10% decrease in fluorescence intensity in the presence of 20–40 mM of glucose, and a linear response was found from 2.5–10 mM. The system was fully reversible and generated stable responses for >60 h28. McNicholas, et al. investigated the chemical changes which occur as a result of immobilizing GBPs on SWNTs26. They found that GBP denatures at a lower temperature (4–5 °C) when bound to SWNTs, and were only found to be stable below 36 °C. This consideration should be taken into account when preparing glucose sensors with bound enzymes, especially when considering in vivo implantation for long term glucose monitoring.
Several optical sensor approaches utilizing metal nanoparticles have been developed. First, He and co-workers reported developing a highly sensitive glucose sensor by immobilizing GOx on Ag/Au nanoshells via poly(L-histidine)6. In this case, the hydrogen peroxide produced by the GOx enzymatic action caused the Ag coating to dissolve and expose increasingly large areas of Au on the surface of the nanoshells, thereby causing a red shift in surface plasmon resonance peaks6. This shift, sampled at a standard time interval, could detect glucose concentrations from 0.5 μM – 0.02 mM. Additionally, Bahshi and co-workers developed two optical techniques for detecting glucose8. The first used Glucose Dehydrogenase (GDH) to catalyse the production of NADH in the presence of glucose. The NADH caused a fluorescence increase in functionalized quantum dots (Hops Yellow Core/Shell EviDots (CdSe/ZnS QDs), which was used to quantify glucose concentrations. Alternatively, the growth of Au NPs can result from the production of NADH by GDH. Specifically, when AuCl4− ions are present in solution, they can be reduced by the NADH produced by GDH action, and lead to the growth of AuNPs. This causes an increase in fluorescence in the visible spectral region (peaks near 530 nm) as the AuNPs grow. Sampling time for both systems was on the order of 20–50 min, and glucose concentrations tested were on the order of 0–2.5 mM8.
Nanomaterials can also be used as a catalyst to cause fluorescence of materials in the presence of hydrogen peroxide. This has been exploited by Song and co-workers who demonstrated that carboxyl modified planar graphene can catalyse the oxidation of 3,3,5,5-tetramethylbenzidine (TMB) to produce a blue color when reacted with the hydrogen peroxide produced by GOx activity37. However, the catalysis of TMB by the carboxyl-graphene requires a pH of 4, which denatures the GOx enzyme. A two-step process was used to overcome this, and a highly sensitive glucose detection range from 1 μm–20 μm was reported. Additionally, the glucose concentration of blood could be accurately measured.
Yetisen and co-workers (Figure 5) developed an optical glucose sensor by fabricating a poly(acrylamide) (pAAm) hydrogel functionalized with 3-(acrylamido)phenylboronic acid (3-PBA) and Ag NPs11. As glucose entered the hydrogel and bound to the PBA, the hydrophobicity of the hydrogel increased, causing it to swell in proportion to glucose concentration11. The swelling modulated the distance between the embedded AgNPs, and caused a glucose-dependent shift in the frequency of refracted laser light. After optimization, a possible wavelength shift of ~350 nm across the visible spectrum was reported for glucose concentrations ranging from 0 mM–10 mM. Additionally, achieving a steady state response with this hydrogel took 50 min–1.5 h, but an analysis of the transient response was conducted, and by applying the results of this analysis the glucose concentration could be accurately estimated after only 5 min11. The sensor was reported to be more accurate than commercially available test strips, and could detect an exceptionally wide range of glucose concentrations (0.1 mM–375 mM)11. This sensor was designed to detect the glucose concentration of urine, which correlates with BGL11.
Figure 5.
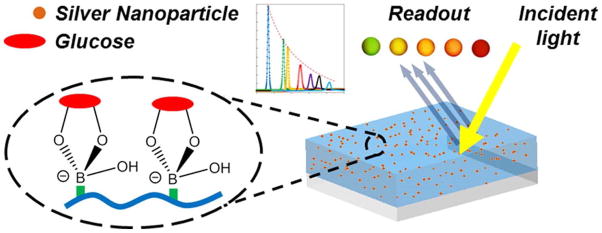
In this study, a boronic acid functionalized hydrogel was formed and included suspended silver nanoparticles. The functionalized hydrogel swelled and contracted in proportion to glucose concentration, as a result of the binding interaction between glucose and the boronic acid. As the hydrogel swells, the distance between the Au NPs modulates in response to glucose concentration, and thereby causes a shift in the wavelength of the refracted light. A wavelength shift of 350nm across the visible spectrum was reported for glucose concentrations ranging from 0mM–10mM. (Reprinted with permission from Ref 11. Copyright 2014 American Chemical Society)
Veetil and co-workers developed a mutant glucose binding protein (GBP) which contains the fluorescence resonance energy transfer (FRET) probes AcGFP1 and mCherry9. Upon glucose binding, the FRET pair is brought in proximity to each other, causing a change in the FRET signal. When the FRET pairs are separated, the intensity of 505 nm light is significant, but decreases sharply upon glucose binding9. The sensor could accurately detect glucose in the range of 25–800 μM9. Recently, this group reported expressing a similar mutant GBP protein within murine myoblast cells by transfecting the mutant GBP DNA into the cell’s DNA15. The mutant GBP protein was synthesized within the cells, and could be used to analyse the glucose concentration within the cells15. The resulting ability to see real time glucose metabolism within the cell is important for understanding diabetes pathology and may be useful in understanding possible intracellular therapies15. Furthermore, this approach may be especially useful for studying the pathology of type 2 diabetes15. Within the cell, elevated glucose concentrations were found close to the cell membrane, and low glucose concentrations were found close to the nucleus15.
A novel method for glucose detection developed by Stuart, et al. is the adaption of Surface Enhanced Raman Scattering (SERS) for in vivo glucose detection by coating the surface of Ag NPs with decanethiol and mecaptohexanol38. This facilitates the localization of glucose to the NP surface and leads to a detectable FRET signal based on glucose concentration38. Stuart, et al. demonstrated the system in a rat model, and found that the system was capable of detecting glucose concentration in vivo with accuracy comparable to a commercial glucose sensor12, 38.
Nanotechnology Enabled Closed-Loop Insulin Delivery
An attractive alternative to traditional insulin injections are “closed-loop” systems, or systems which continuously monitor blood glucose and release insulin in a self-regulated fashion. There are several benefits of such a system over the traditional open loop system, including tighter control of BGLs, which can reduce the complications characteristic of diabtes39, 40. Closed-loop systems may also lead to a decrease in the dosage of insulin, and reduce the number of hypoglycemic and hyperglycemic events. These advantages have led to considerable interest in developing closed-loop systems. The reported systems have demonstrated favourable results in terms of responsiveness and biocompatibility7, 41.
Microencapsulation of Islet Cells – Artificial Pancreas
Encapsulating the body’s own insulin producing β-cells within semi-permeable polymer matrices, such as alginate hydrogel, is an attractive option for closed-loop insulin delivery. This strategy calls for the encapsulation of β-cells within a semi-permeable membrane that shields the cells from host immune responses while allowing small molecules, nutrients, and insulin to passively diffuse across the membrane. Designing such a membrane is a complex challenge and is being approached from several perspectives. One of the most significant challenges to this approach is overcoming the host’s immune response to directly implanting foreign islet cells. Typically, the cells are rapidly engulfed by fibrotic overgrowths as a result of an immune response42.
Another technique for encapsulating islet cells is the Layer by Layer (LbL) deposition of a semi-permeable membrane onto the cells7, 43. A persistent challenge of using LbL deposition for islet cell encapsulation is avoiding the use of polyionic polymers, which are cytotoxic44, 45. Alternative binding strategies are needed to create LbL coatings on the cells that have increased stability and reduced cytotoxicity. Recently, Gattas-Asfura and co-workers reported a new LbL technique utilizing interpolymeric covalent linking to enhance the stability of the LbL coatings46. Alginate and poly(amidoamine) (PAMAM) dendrimers were functionalized with complementary Staudinger ligation groups: azide and methyl 2-(diphenylphosphino)terephthalate. Covalent linkages and electrostatic forces between the films increased the stability and thickness of the resultant bilayer. This bioorthoganal functionalization technique for interpolymeric linking also serves as a method for functionalizing the surface of the islet and can be modified to include secondary therapeutic agents such as immunosuppressant drugs or bioactive groups that enhance the nutrient supply47. However, the coatings still face the issue of cytotoxicity due to the inclusion of polyionic polymers.
A solution to the polyionic induced cytotoxicity was recently reported by Kozlovskaya and coworkers41. The group reported an LbL coating strategy that used hydrogen bonding between tannic acid (TA), a natural polyphenol with poly(N-vynilpyrrolidone) (PVNOP) which holds the polymeric films together. The interactions between TA and the cell membrane proteins resulted in a highly stable coating. The hydrogen bonded films maintained good stability for up to 7 days after the initial coating. Furthermore, the cells coated with the bilayers displayed no significant increase in cytotoxicity compared to un-coated cells. Significant challenges in terms of immunoisolation of the islet cells remain before islet cell transplantation can become a clinically viable option.
Glucose-Responsive Insulin Delivery
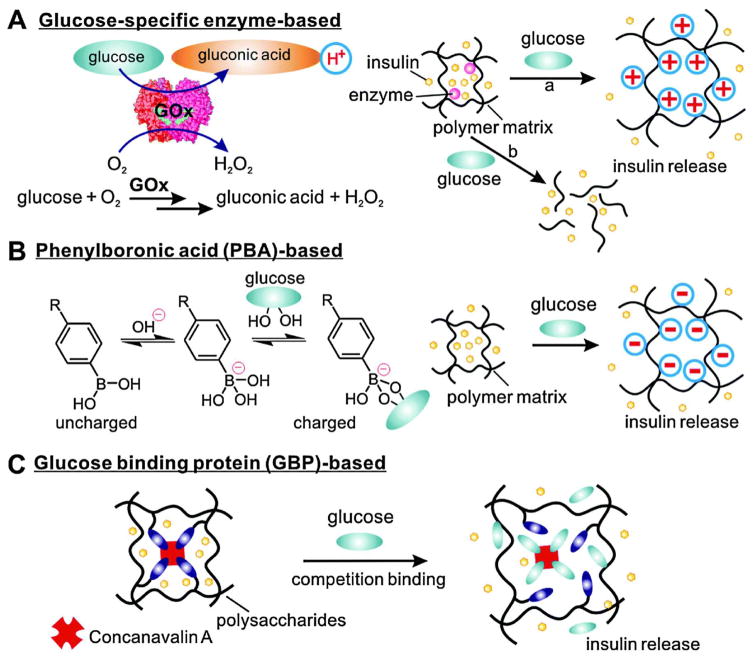
Synthetic closed-loop systems are often characterized by their method of glucose detection. Glucose can be detected through enzymatic methods using enzymes such as glucose oxidase (GOx), Binding of glucose to synthetic groups such as those in Phenylboronic acid (PBA), or binding to glucose binding proteins such as Concavalin A (Con A)13 as seen in Figure 6.
Figure 6.
Schematic of typical strategies for glucose responsive insulin release. (Reprinted with permission from Ref 5. Copyright 2014 Chemical Society Reviews)
Glucose Oxidase (GOx) Systems
Glucose oxidase is capable of enzymatically catalysing the oxidation of glucose to gluconic acid with high specificity. The rate of enzymatic production of gluconic acid is directly related to the local glucose concentration. A typical closed-loop system utilizing glucose oxidase is comprised of a pH sensitive polymer matrix containing both insulin and GOx. The matrix undergoes a physical change such as hydrolysis or volume phase transition as the pH of the system is altered by gluconic acid production, thereby releasing the insulin entrapped within the matrix in a glucose dependent fashion6, 13.
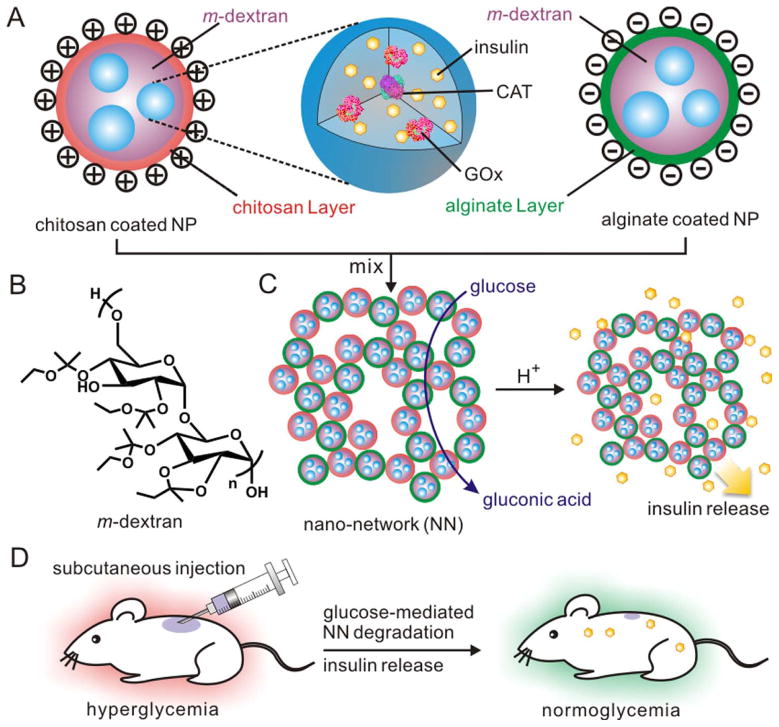
Gu and co-workers recently developed a glucose-responsive nano-network for insulin delivery using GOx as the glucose sensing element (Figure 7)48. A double emulsion method was used to prepare acetal modified dextran (m-dextran) nanoparticles which included insulin, GOx and catalase (CAT). The nanoparticles were coated with either positively charged chitosan or negatively charged alginate. The opposing surface charges caused the nanoparticles to assemble into a porous 3D nano-network. The conversion of glucose to gluconic acid triggered the hydrolysis of the acetal groups to ethanol and acetone which results in the phase transition of hydrophobic m-dextran to dextran, which is hydrophilic. This phase transition results in the release of the entrapped insulin in a glucose-responsive fashion. The nano-network architecture endowed the system with sufficient mechanical strength to overcome a burst release at the injection site. Furthermore, the porous structure led to the presence of microchannels which facilitated higher rates of glucose diffusion through the nano-network leading to near zero order enzyme kinetics. The system showed no significant insulin release at normal glucose levels (100 mg dL−1), but transitioned to fast insulin release under hyperglycemic conditions (400 mg dL−1). In in vivo studies, a single subcutaneous injection of the nano-network was capable of maintaining a diabetic mouse’s blood glucose levels in the normoglycemic range for 10 days48.
Figure 7.
Schematic of the glucose-responsive nano-network. (A) Acetal modified dextran nanoparticles containing insulin and GOx. The nanoparticles are coated with positively charged chitosan or negatively charged alginate. (B) Acetal modified dextran molecule. (C) Assembly of oppositely charged nanoparticles to form a nano-network and insulin release upon the enzymatic generation of gluconic acid under hyperglycaemic conditions. (D) Injection of the nano-network into a STZ-induced diabetic mouse model. (Reprinted with permission from Ref 48. Copyright 2013 American Chemical Society)
More recently, Tai and co-workers developed an enzyme based biomimetic polymersome for glucose-responsive insulin delivery49. Polymersome nanovesicles were comprised of a Polyethylene glycol-poly (Ser-Ketal) PEG-Poly (Ser-Ketal) amphiphilic diblock polymer, GOx, CAT, and Insulin. Under neutral conditions the polymersome forms a bilayer structure which contains the insulin and allows glucose and oxygen to diffuse through the membrane. The enzymatic production of gluconic acid in response to hyperglycaemic glucose concentrations results in the degradation of the polymer membrane by degrading the ser-ketal linkage into PEG-Poly(Serine), ethanol, and acetone. The degradation of the membrane causes a subsequent release of insulin. Alternating the nanovesicles between hyperglycaemic conditions (400 mg dL−1) and normoglycemic conditions (100 mg dL−1) resulted in a pulsatile release of insulin with maximal release at the hyperglycaemic state, and negligible release under normoglycemic conditions. The polymersome bilayer structure led to excellent mechanical stability of the system. No insulin release was observed from the vesicles without a glucose stimulus over a course of 1 month. In vivo studies revealed that insulin released from the polymersomes retained bioactivity and were effective for regulating the glucose levels of diabetic mice for up to 5 days.
Gordijo and co-workers developed a glucose-responsive nanocomposite membrane for insulin delivery10, 50. The composite was prepared by conjugating MnO2 nanoparticles and poly(N-isopropylacrylamide –co-methacrylic acid) poly(NIPAM-MAA) with GOx, insulin, catalase (CAT), bovine serum albumin (BSA) and gluteraldehyde. The resultant PNIPAM-MAA membrane is pH sensitive and is capable of shrinking under the acidic conditions induced by hyperglycaemia. The shrinking of the membrane leads to insulin release across the membrane by increasing the porosity of the hydrogel network. However, degradation of the enzyme is a significant challenge for glucose-responsive systems utilizing GOx. As H2O2 accumulates as a product of the enzymatic reaction, the activity of GOx is degraded. To address this, the CAT enzyme is often included to scavenge the H2O2 and catalyse its conversion to O210, 48, 51. However, CAT is not capable of scavenging all the H2O2, and is itself affected by an increase in H2O2 concentrations. The inclusion of MnO2 NPs works synergistically with CAT to scavenge more H2O2. The rigid MnO2 NPs increased the mechanical strength of the system as well the longevity of the enclosed enzyme. Inclusion of the MnO2 NPs and CAT led to a nanohybrid membrane that maintained excellent activity for 15 days, and maintained 65% of its initial activity after 30 days.
Most glucose-responsive insulin delivery systems utilize the degradation of a polymer matrix or a phase transition to mediate the release of insulin. However, attention has recently been directed to mesoporous silica nanoparticles (MSNs) as a result of their rigid structure and high surface area. Recently, Chen and co-workers developed a glucose dependent drug release system52. The system is comprised of MSNs loaded with rhodamine as a model drug. The MSNs are capped with GOx bound to N-glucosamine, a GOx inhibitor, which in turn is bound to the outside of the NP. As the glucose concentration increases, it competes with glucosamine to bind to the GOx. Glucose binding to GOx results in uncapping of the MSNs and leads to the release of the encapsulated drug. In vitro studies indicated that rhodamine release from the NP was glucose dependent, and that no release was observed in the absence of glucose. Furthermore, drug release was correlated with glucose concentration. In addition to Rhodamine, other drugs such as Insulin could be stored within MSNs and capped with a glucose responsive element52–54.
Glucose Binding Protein (GBP) Systems
Glucose binding proteins can bind to glucose or glucosyl moieties in glycopolymers5. The most common class of glucose binding proteins are lectins. Lectins are a family of proteins that can bind to carbohydrates, and have a significant role in cell signaling55. The most common lectin used for insulin delivery systems is Concanavalin A (Con A). It is the preferred GBP because of its high affinity for glucose. Con A can bind to gluconic acid modified insulin (G-insulin) and be included in hydrogels. Under hyperglycaemic conditions, competition between G-insulin and glucose leads to the release of G-insulin in a glucose dependent manner. Wu and co-workers recently developed a MSN system capped with Con A56. The system is comprised of Con A bound to a mannose moiety, which is used to cap the pores of the MSN which contain rhodamine as a model drug. The resulting system was both pH and glucose-responsive. Triggering of the system by pH occurs because Con A exists as a dimer or monomer at low pH, and assumes a tetrameric state at neutral pH. Competitive binding of glucose with the capped Con A led to a release of the drug loaded within the MSN. In the absence of a glucose or a pH trigger, no drug release occurred.
Phenylboronic Acid (PBA) Systems
A disadvantage of protein based glucose sensing is that environmental factors such as pH and temperature must be tightly controlled to prevent protein denaturation. The possibility of denaturation limits the glucose sensing applications and sensor lifetimes of protein based systems. This poses challenges for the long term measurement of blood glucose by such sensors. An attractive alternative is the use of Phenylboronic acid and its derivatives as the glucose sensing moiety in both BGL measurement and insulin delivery applications. The ability of Phenylboronic acid to reversibly bind to polyols has been well established57. This has allowed Boronic acid derivatives to serve as the detection element in glucose-responsive insulin delivery and BGL measurement applications.
Phenylboronic acid in its neutral form exists as a trigonal planar hydrophobic molecule but is deprotonated under slightly basic conditions (pKa> 8) where it exists as a tetrahedral hydrophilic molecule with a net negative charge. The equilibrium between the two states is pushed towards the tetrahedral state in the presence of diols and polyols such as those found in glucose 58. This equilibrium can be utilized to trigger the dissolution of amphiphilic nanocarriers and thus release insulin59–63. The equilibrium shift from the neutral to the charged form can also trigger the swelling of crosslinked hydrogel matrices.
Kataoka et al. were the first to report a synthetic glucose-responsive system for insulin delivery in 199858. The group synthesized a polymer matrix of poly (N-isopropylacrylamide) (PINPAAm) that included PBA as a glucose sensing moiety. Upon glucose complexation with the PBA, the hydrophilicity of the matrix increased, causing the matrix to swell and release insulin in a glucose-responsive fashion64. Since this initial work several other insulin delivery systems have been devised which utilize the PBA moiety for its glucose sensing properties48, 60, 65, 66.
One approach for glucose-responsive insulin delivery is the use of crosslinked polymer matrices with incorporated PBA. These matrices can reversibly swell when PBA forms a boronate ester with glucose. The ester formation leads to an accumulation of negative charge on the matrix, which results in repulsive forces that cause the cross-linked matrix to swell and release insulin. Recently, Wang and co-workers developed a co-polymer micelle comprised of poly (ethylene glycol)-block-poly-(acrylic acid-co-acryloamidophenylboronic acid) - (PEG-b-(PAA-co-PAAPBA)59. The micelle was capable of swelling in response to glucose concentration. The core of the micelle shifted from being hydrophobic to hydrophilic as the glucose formed a complex with the PBA moiety in the core of the micelle. The insulin stored within the micelle was then released in glucose dependent manner. In the absence of glucose the micelle was stable and no insulin release was detected.
One of the primary issues with using PBA in clinical systems is that the tetrahedral form requires a pH higher than those observed in most physiological systems. Yao and co-workers developed a polymeric glucose-responsive insulin delivery system that operates under neutral pH conditions60. Their system is comprised of an amphiphilic polymer made of Poly(ethylene glycol)-block-poly[(2-phenylboronic esters-1,3-dioxane-5-ethyl) methacrylate] (MPEG5000-block-PBDEMA). Electron donating groups were included within the polymer to increase its Lewis acidity and thereby reduce the pKa required to form the boronate ester. This co-polymer self assembles into micelles, and the system releases insulin upon the matrix’s transition from amphiphilic to hydrophilic.
Yang et al. devised a similar system utilizing a PBA sensing scheme at physiological pH using micelles67. The micelles were prepared by synthesizing a block co-polymer containing poly(ethylene glycol)-b-poly(aspartic acid co-aspartamidophenylboronic acid) PEG-b-P(Asp-coAspPBA) along with a poly(Aspartic acid co aspartglucosamine)-P(Asp-co-AGA) glycopolymer. The introduction of glycopolymers into the polymer structure of the micelle allowed it to remain relatively stable under normoglycemic conditions (1 g L−1) due to the competition between the glycopolymer and glucose binding to PBA. However, at elevated glucose concentrations (2 g L−1), the micelles disintegrated and rapidly released insulin. The system was stable at normal blood sugar levels, and this stability reduced the risk of a burst release of insulin, which is highly desirable in a self-regulating insulin delivery device.
Polymer based insulin delivery systems have great versatility. Modifying the structure of the polymer can be used to tune the insulin release behaviour and sensitivity. Kim and co-workers synthesized a PEG-polyboroxole block co-polymer polymersome which was capable of self-assembly into micelles or cylindrical polymersomes based on the polyboroxole block length68. The group reported that at neutral pH, binding of the boroxole group to monosaccharides such as glucose (0.5 M) and fructose (0.2 M) was capable of producing charged boronate groups in the core of the polymersome. This change in charge modified the core’s behavior from hydrophobic to hydrophilic, thereby causing the polymersome to dissociate and release embedded cargos such as insulin. The polymersomes did not dissolve in the absence of monosaccharaides, and instead maintained their morphology for up to 3 months. The boroxole group showed a stronger binding affinity for fructose over glucose, but the versatility this system shows promise for self-regulated insulin delivery platforms.
Several groups have attempted to create platforms which exhibit a step wise response to glucose concentration and can deliver multiple drugs. Zhao and co-workers used Mesoporous Silica Nanoparticles (MSNs) as a platform for glucose-responsive release of Insulin and cyclic adenosine monophosphate (cAMP) – a trigger for the production of insulin from pancreatic beta cells69. The MSNs were loaded with cyclic AMP and capped with PBA bound to gluconic acid modified insulin (G-insulin). The system used the preferential binding of glucose to PBA to release the G-insulin-PBA cap from the MSNs, thereby liberating both insulin (in the G-insulin form) and cAMP. Furthermore, MSNs have been shown to be internalized by cells through endocytosis; this facilitates the uptake of cAMP, which normally encounters difficulty in crossing the cell membrane. The system was well realized and showed almost no leaching of cAMP without a glucose or fructose trigger. 20 mM glucose was capable of releasing 2 μM of G-insulin at a MSN concentration of 2 mg mL−1. An ideal payload of insulin to lower blood glucose is about 200–350 pM 69. Furthermore, the release of cAMP was also glucose-responsive at a glucose concentration of 100 mM; 60% of the loaded cAMP was released from the MSN. 0.25 to 0.3 μM of MSN was capable of delivering a therapeutic dose of insulin in a glucose-responsive manner. The prospect of a dual delivery system is an exciting one, and has led to an increase in interest in MSNs for insulin delivery37, 52, 66, 70. However, the modification of insulin with gluconic acid raises questions about the bioavailability of the drug once released from the MSN.
Wu and coworkers designed a multifunctional hybrid nanogel system to serve as an insulin delivery device and glucose sensor (Figure 8)10, 62. The nanogel is comprised of poly(4-vynilphenylboronicacid-co-2-(diethylamino)ethyl acrylate) p[VPBA-DMAEA] polymer chains which serve as the glucose sensing moiety. The polymer is capable of crosslinking to form a nanogel which undergoes a phase transition in response to glucose concentration. The group included silver nanoparticles in the core of the nanogel that endows it with strong fluorescence. The wavelength of this flourescence changes as the nanogel swells and shrinks in proportion to glucose concentration. In addition to the sensing mechanism, the system is also capable of dispensing insulin in a glucose-responsive fashion by swelling in response to hyperglycaemic conditions. The system was capable of phase transitions at a physiologically relevant pH (7.4) and exhibited high sensitivity to glucose; a lower detection limit of 0.75 mM was reported. The nanogels could release 56–91% of the stored insulin at high glucose levels, and could quickly modulate between high and low insulin release rates in response to changing glucose concentrations. Insulin release at basal levels could be sustained for over 2 days.
Figure 8.
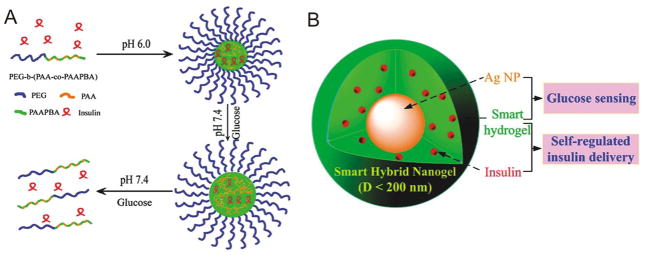
Glucose-responsive insulin delivery systems utilizing phenylboronic acid. (A) Insulin release via pH responsive micelles. (B) Multifunctional hydrogel for glucose-responsive insulin release and glucose concentration measurement. As the smart hydrogel swells in response to hyperglycemic conditions, the insulin is released and the fluorescence of the silver nanoparticles (AgNPs) is modulated. This fluorescence change can be detected and used to quantify the glucose concentration and the release of insulin. (Reprinted with permission from Ref 62. Copyright 2012 American Chemical Society)
Conclusion and Outlook
Significant advances in both glucose sensors and self-regulated insulin delivery systems have been facilitated by nanotechnology. Amperometric sensors utilizing nanotechnology now facilitate rapid, accurate, and highly sensitive glucose measurements in blood and other clinically relevant fluids, such as tears and urine. Additionally, recent advances in fluorescent glucose detection holds the potential to lead to continuous in vivo glucose measurement. The realization of sensors which do not require repeated finger pricks to draw blood for glucose testing is highly desirable, as alternatives which avoid the pain, tissue damage, and patient noncompliance associated with the legacy clinical standard are highly desirable5. Furthermore, the clinical realization of a continuous glucose sensor could lead to closed-loop systems which could utilize existing insulin pumps6. This could relieve the patient of the significant burden of continuously managing their diabetes, and may tremendously improve their long term health outcomes and well-being. Based on current developments in nanoscale glucose sensing, we can expect great clinical applications of this technology in the near future71.
Progress in closed-loop insulin delivery systems has been encouraging and shows tremendous promise for the treatment of diabetes. Recently, a Con-A based polymer approach developed by Zion, et al has been close to clinical trials, following his company’s 2010 acquisition by Merck72, 73. Current closed-loop systems are capable of releasing large amounts of insulin; however, clinical realization requires tight control of insulin release to reduce the risk of insulin overdose. Glucagon, a hormone which works antagonistically with insulin, could be co-delivered to reduce the risk of hypoglycaemia3. Additionally, because insulin is a growth factor, long term exposure to excess insulin can cause changes to the cell division process74.
Systems that offer remote control for the release of insulin are a promising development in dynamic insulin delivery. Stanley and co-workers developed nanoparticles coated with antibodies capable of binding to TRPV1 calcium channels on genetically engineered cells. The application of a 465 kHz RF signal causes localized heating of the channel via heating of the nanoparticles, and this heating causes subsequent passage of calcium into the cell, triggering the production of insulin within the cell75. The cells then release this insulin into the bloodstream75. Additionally, Di, et al. demonstrated insulin release via the bursting of nanocapsules by focused ultrasound (FUS)76. A nano-network48 comprised of PLGA nanoparticles containing insulin was used to form an insulin reservoir, which could be selectively degraded by the application of focused ultrasound and lead to insulin release in response to ultrasound application76. These remotely triggered strategies may lead to novel insulin delivery modalities which can reduce the pain associated with repeatedly injecting insulin and increase patient compliance,5 and may serve as an element of closed-loop glucose therapies. The best way of tackling the challenges ahead is by taking a multidisciplinary approach and incorporating knowledge of material science, physical chemistry, and pharmacology to develop more nuanced systems that are dynamic and stable.
Figure 4.
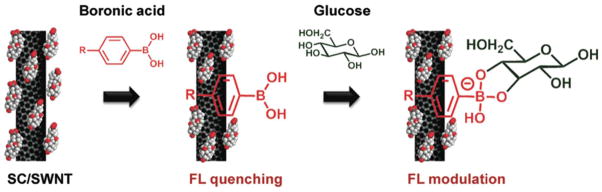
In this study, Yum, et al used fluorescent carbon nanotubes with boronic acid to fabricate an optical glucose sensor. As the boronic acid binds to the nanotubes, the fluorescence is quenched. As glucose binds to these boronic acids, the fluorescence returns. Two Boronic acids were found which optomized the desired properties: 4-cyanophenylboronic and 4-chlorophenylboronic acid. (Reprinted with permission from Ref 29. Copyright 2011 American Chemical Society)
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Rocco Michael DiSanto, Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, North Carolina 27695, United States.
Vinayak Subramanian, Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, North Carolina 27695, United States.
Zhen Gu, Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, North Carolina 27695, United States; Molecular Pharmaceutics Division, Center for Nanotechnology in Drug Delivery, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
References
- 1.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2004;27:s5–s10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 3.Ross SA, Gulve EA, Wang M. Chemistry and Biochemistry of Type 2 Diabetes. Chemical Reviews. 2004;104:1255–1282. doi: 10.1021/cr0204653. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR, Lester MA. Diabetes and cardiovascular disease. Diabetes care. 1999;22:C14–20. [PubMed] [Google Scholar]
- 5.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chemical Society Reviews. 2014;43:3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 6.Bratlie KM, York RL, Invernale MA, Langer R, Anderson DG. Materials for diabetes therapeutics. Advanced Healthcare Materials. 2012;1:267–284. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickup JC, Zhi Z-L, Khan F, Saxl T, Birch DJS. Nanomedicine and its potential in diabetes research and practice. Diabetes/Metabolism Research and Reviews. 2008;24:604–610. doi: 10.1002/dmrr.893. [DOI] [PubMed] [Google Scholar]
- 8.Bahshi L, Freeman R, Gill R, Willner I. Optical Detection of Glucose by Means of Metal Nanoparticles or Semiconductor Quantum Dots. Small. 2009;5:676–680. doi: 10.1002/smll.200801403. [DOI] [PubMed] [Google Scholar]
- 9.Veetil JV, Jin S, Ye K. A glucose sensor protein for continuous glucose monitoring. Biosensors and Bioelectronics. 2010;26:1650–1655. doi: 10.1016/j.bios.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordijo CR, Shuhendler AJ, Wu XY. Glucose-Responsive Bioinorganic Nanohybrid Membrane for Self-Regulated Insulin Release. Advanced Functional Materials. 2010;20:1404–1412. [Google Scholar]
- 11.Yetisen AK, Montelongo Y, da Cruz Vasconcellos F, Martinez-Hurtado JL, Neupane S, Butt H, Qasim MM, Blyth J, Burling K, Carmody JB, et al. Nano Letters. 2014. Reusable, Robust, and Accurate Laser-Generated Photonic Nanosensor. [DOI] [PubMed] [Google Scholar]
- 12.Chun AL. Nanosensors: Bring it on. Nature Nanotechnology. 2006:84. [Google Scholar]
- 13.Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. Journal of Controlled Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Chertok B, Webber MJ, Succi MD, Langer R. Drug Delivery Interfaces in the 21st Century: From Science Fiction Ideas to Viable Technologies. Molecular Pharmaceutics. 2013;10:3531–3543. doi: 10.1021/mp4003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veetil JV, Jin S, Ye K. Fluorescence lifetime imaging microscopy of intracellular glucose dynamics. Journal of Diabetes Science and Technology. 2012;6:1276–1285. doi: 10.1177/193229681200600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uehara H, Kakiage M, Sekiya M, Sakuma D, Yamonobe T, Takano N, Barraud A, Meurville E, Ryser P. Size-Selective Diffusion in Nanoporous but Flexible Membranes for Glucose Sensors. ACS Nano. 2009;3:924–932. doi: 10.1021/nn8008728. [DOI] [PubMed] [Google Scholar]
- 17.Shan C, Yang H, Han D, Zhang Q, Ivaska A, Niu L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosensors and Bioelectronics. 2010;25:1070–1074. doi: 10.1016/j.bios.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, Li X, Xing L, Liu X, Luo S, Wei W, Kong B, Li Y. Electrodeposition of chitosan–ionic liquid–glucose oxidase biocomposite onto nano-gold electrode for amperometric glucose sensing. Biosensors and Bioelectronics. 2009;24:2898–2903. doi: 10.1016/j.bios.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Wang L, Yu H, Wang J, Chen Z. Organization of glucose-responsive systems and their properties. Chemical reviews. 2011;111:7855–7875. doi: 10.1021/cr200027j. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu A. Glucose sensing: Silicon’s sweet spot. Nature Nanotechnology. 2007 [Google Scholar]
- 21.Wang G, Mantey K, Nayfeh MH, Yau S-T. Enhanced amperometric detection of glucose using Si29 particles. Applied Physics Letters. 2006;89 [Google Scholar]
- 22.Dong X-C, Xu H, Wang X-W, Huang Y-X, Chan-Park MB, Zhang H, Wang L-H, Huang W, Chen P. 3D Graphene–Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano. 2012;6:3206–3213. doi: 10.1021/nn300097q. [DOI] [PubMed] [Google Scholar]
- 23.Barone PW, Baik S, Heller DA, Strano MS. Near-infrared optical sensors based on single-walled carbon nanotubes. Nature Materials. 2005;4:86–92. doi: 10.1038/nmat1276. [DOI] [PubMed] [Google Scholar]
- 24.Besteman K, Lee J-O, Wiertz FGM, Heering HA, Dekker C. Enzyme-Coated Carbon Nanotubes as Single-Molecule Biosensors. Nano Letters. 2003;3:727–730. [Google Scholar]
- 25.Lin Y, Lu F, Tu Y, Ren Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Letters. 2003;4:191–195. [Google Scholar]
- 26.McNicholas TP, Yum K, Ahn JH, Mu B, Plettenburg O, Gooderman A, Natesan S, Strano MS. Structure and Function of Glucose Binding Protein-Single Walled Carbon Nanotube Complexes. Small. 2012 doi: 10.1002/smll.201200649. [DOI] [PubMed] [Google Scholar]
- 27.Tang H, Yan F, Lin P, Xu J, Chan HLW. Highly Sensitive Glucose Biosensors Based on Organic Electrochemical Transistors Using Platinum Gate Electrodes Modified with Enzyme and Nanomaterials. Advanced Functional Materials. 2011;21:2264–2272. [Google Scholar]
- 28.Yoon H, Ahn J-H, Barone PW, Yum K, Sharma R, Boghossian AA, Han J-H, Strano MS. Periplasmic Binding Proteins as Optical Modulators of Single-Walled Carbon Nanotube Fluorescence: Amplifying a Nanoscale Actuator. Angewandte Chemie. 2011;123:1868–1871. doi: 10.1002/anie.201006167. [DOI] [PubMed] [Google Scholar]
- 29.Yum K, Ahn J-H, McNicholas TP, Barone PW, Mu B, Kim J-H, Jain RM, Strano MS. Boronic Acid Library for Selective, Reversible Near-Infrared Fluorescence Quenching of Surfactant Suspended Single-Walled Carbon Nanotubes in Response to Glucose. ACS Nano. 2011;6:819–830. doi: 10.1021/nn204323f. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Kampstra KL, Abidian MR. High Performance Conducting Polymer Nanofiber Biosensors for Detection of Biomolecules. Advanced Materials. 2014:n/a–n/a. doi: 10.1002/adma.201400753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes SP, Behzad AR, Hooghan B, Sougrat R, Karunakaran M, Pradeep N, Vainio U, Peinemann K-V. Switchable pH-Responsive Polymeric Membranes Prepared via Block Copolymer Micelle Assembly. ACS Nano. 2011;5:3516–3522. doi: 10.1021/nn200484v. [DOI] [PubMed] [Google Scholar]
- 32.Zhai D, Liu B, Shi Y, Pan L, Wang Y, Li W, Zhang R, Yu G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano. 2013;7:3540–3546. doi: 10.1021/nn400482d. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Wang Y, Su L, Bellagamba M, Zhang H, Lei Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosensors and Bioelectronics. 2010;26:542–548. doi: 10.1016/j.bios.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Sun EY, Weissleder R, Josephson L. Continuous analyte sensing with magnetic nanoswitches. Small. 2006;2:1144–1147. doi: 10.1002/smll.200600204. [DOI] [PubMed] [Google Scholar]
- 35.Ballerstadt R, Evans C, McNichols R, Gowda A. Concanavalin A for in vivo glucose sensing: A biotoxicity review. Biosensors and Bioelectronics. 2006;22:275–284. doi: 10.1016/j.bios.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Brown JQ, McShane MJ. Modeling of spherical fluorescent glucose microsensor systems: design of enzymatic smart tattoos. Biosensors and Bioelectronics. 2006;21:1760–1769. doi: 10.1016/j.bios.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Advanced Materials. 2010;22:2206–2210. doi: 10.1002/adma.200903783. [DOI] [PubMed] [Google Scholar]
- 38.Stuart DA, Yuen JM, Shah N, Lyandres O, Yonzon CR, Glucksberg MR, Walsh JT, Van Duyne RP. In Vivo Glucose Measurement by Surface-Enhanced Raman Spectroscopy. Analytical Chemistry. 2006;78:7211–7215. doi: 10.1021/ac061238u. [DOI] [PubMed] [Google Scholar]
- 39.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 40.Control D, Group CTR. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-term Complications in Insulin-dependent Diabetes Mellitus. RETINA. 1994;14:286–287. [Google Scholar]
- 41.Kozlovskaya V, Zavgorodnya O, Chen Y, Ellis K, Tse HM, Cui W, Thompson JA, Kharlampieva E. Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Advanced Functional Materials. 2012;22:3389–3398. doi: 10.1002/adfm.201200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, Ma M, Hollister-Lock J, Tang KM, Gu Z, et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials. 2013;34:5792–5801. doi: 10.1016/j.biomaterials.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JT, Cui W, Chaikof EL. Layer-by-Layer Assembly of a Conformal Nanothin PEG Coating for Intraportal Islet Transplantation. Nano Letters. 2008;8:1940–1948. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teramura Y, Kaneda Y, Totani T, Iwata H. Behavior of synthetic polymers immobilized on a cell membrane. Biomaterials. 2008;29:1345–1355. doi: 10.1016/j.biomaterials.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, et al. Cell Surface Engineering with Polyelectrolyte Multilayer Thin Films. Journal of the American Chemical Society. 2011;133:7054–7064. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- 46.Gattas-Asfura KM, Stabler CL. Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers. ACS Applied Material Interfaces. 2013;5:9964–9974. doi: 10.1021/am401981g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boudou T, Crouzier T, Ren K, Blin G, Picart C. Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications. Advanced Materials. 2010;22:441–467. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- 48.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, Cheng H, Langer RS, Anderson DG. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano. 2013;7:4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tai W, Mo R, Di J, Subramanian V, Gu X, Gu Z. Bio-Inspired Synthetic Nanovesicles for Glucose-Responsive Release of Insulin. Biomacromolecules. 2014 doi: 10.1021/bm500364a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordijo CR, Koulajian K, Shuhendler AJ, Bonifacio LD, Huang HY, Chiang S, Ozin GA, Giacca A, Wu XY. Nanotechnology-Enabled Closed Loop Insulin Delivery Device: In Vitro and In Vivo Evaluation of Glucose-Regulated Insulin Release for Diabetes Control. Advanced Functional Materials. 2011;21:73–82. [Google Scholar]
- 51.Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano. 2013;7:6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 52.Chen M, Huang C, He C, Zhu W, Xu Y, Lu Y. A glucose-responsive controlled release system using glucose oxidase-gated mesoporous silica nanocontainers. Chemical Communications. 2012;48:9522–9524. doi: 10.1039/c2cc34290a. [DOI] [PubMed] [Google Scholar]
- 53.Sun L, Zhang X, Wu Z, Zheng C, Li C. Oral glucose-and pH-sensitive nanocarriers for simulating insulin release in vivo. Polymer Chemistry. 2014;5:1999–2009. [Google Scholar]
- 54.Sanchez C, Belleville P, Popall M, Nicole L. Applications of advanced hybrid organic–inorganic nanomaterials: from laboratory to market. Chemical Society Reviews. 2011;40:696–753. doi: 10.1039/c0cs00136h. [DOI] [PubMed] [Google Scholar]
- 55.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature Reviews Immunology. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu S, Huang X, Du X. Glucose- and pH-responsive controlled release of cargo from protein-gated carbohydrate-functionalized mesoporous silica nanocontainers. Angewandte Chemie. 2013;52:5580–5584. doi: 10.1002/anie.201300958. [DOI] [PubMed] [Google Scholar]
- 57.Lorand JP, Edwards JO. Polyol Complexes and Structure of the Benzeneboronate Ion. The Journal of Organic Chemistry. 1959;24:769–774. [Google Scholar]
- 58.Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y. Totally synthetic polymer gels responding to external glucose concentration: Their preparation and application to on-off regulation of insulin release. Journal of the American Chemical Society. 1998;120:12694–12695. [Google Scholar]
- 59.Wang B, Ma R, Liu G, Li Y, Liu X, An Y, Shi L. Glucose-responsive micelles from self-assembly of poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) and the controlled release of insulin. Langmuir. 2009;25:12522–12528. doi: 10.1021/la901776a. [DOI] [PubMed] [Google Scholar]
- 60.Yao Y, Zhao L, Yang J, Yang J. Glucose-responsive vehicles containing phenylborate ester for controlled insulin release at neutral pH. Biomacromolecules. 2012;13:1837–1844. doi: 10.1021/bm3003286. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L, Ding J, Xiao C, He P, Tang Z, Pang X, Zhuang X, Chen X. Glucose-sensitive polypeptide micelles for self-regulated insulin release at physiological pH. Journal of Materials Chemistry. 2012;22:12319–12328. [Google Scholar]
- 62.Wu W, Chen S, Hu Y, Zhou S. A fluorescent responsive hybrid nanogel for closed-loop control of glucose. Journal of Diabetes Science and Technology. 2012;6:892–901. doi: 10.1177/193229681200600421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Z, Zhang X, Guo H, Li C, Yu D. An injectable and glucose-sensitive nanogel for controlled insulin release. Journal of Materials Chemistry. 2012;22:22788–22796. [Google Scholar]
- 64.Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y. On-off regulation of insulin-release by totally synthetic polymer gels responding to external glucose concentration. Abstracts of Papers of the American Chemical Society. 1999;217:U564–U564. [Google Scholar]
- 65.Ancla C, Lapeyre V, Gosse I, Catargi B, Ravaine V. Designed glucose-responsive microgels with selective shrinking behavior. Langmuir. 2011;27:12693–12701. doi: 10.1021/la202910k. [DOI] [PubMed] [Google Scholar]
- 66.Kumar BV, Salikolimi K, Eswaramoorthy M. Glucose- and pH-responsive charge-reversal surfaces. Langmuir. 2014;30:4540–4544. doi: 10.1021/la500407r. [DOI] [PubMed] [Google Scholar]
- 67.Park CW, Yang H-M, Lee HJ, Kim J-D. Core–shell nanogel of PEG–poly(aspartic acid) and its pH-responsive release of rh-insulin. Soft Matter. 2013;9:1781. [Google Scholar]
- 68.Kim H, Kang YJ, Kang S, Kim KT. Monosaccharide-responsive release of insulin from polymersomes of polyboroxole block copolymers at neutral pH. Journal of the American Chemical Society. 2012;134:4030–4033. doi: 10.1021/ja211728x. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Trewyn BG, Slowing II, Lin VSY. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. Journal of the American Chemical Society. 2009;131:8398–8400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 70.Zhao W, Zhang H, He Q, Li Y, Gu J, Li L, Li H, Shi J. A glucose-responsive controlled release of insulin system based on enzyme multilayers-coated mesoporous silica particles. Chemical Communications. 2011;47:9459–9461. doi: 10.1039/c1cc12740c. [DOI] [PubMed] [Google Scholar]
- 71.Scognamiglio V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosensors and Bioelectronics. 2013;47:12–25. doi: 10.1016/j.bios.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 72.Zion T, Zarur A, Ying J. cross-linked polymer encapsulating the therapeutic agent; degradation rate of the cross-linked polymer is correlated with a local concentration of an indicator, and the therapeutic agent is released as the cross-linked polymer degrades. 2004. [Google Scholar]
- 73.Zion TC, Tsang HH, Ying JY. DSpace@MIT. 2003. Glucose-Sensitive Nanoparticles for Controlled Insulin Delivery. [Google Scholar]
- 74.Brogden RN, Heel R. Human Insulin. Drugs. 1987;34:350–371. doi: 10.2165/00003495-198734030-00003. [DOI] [PubMed] [Google Scholar]
- 75.Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science. 2012;336:604–608. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z. Ultrasound-Triggered Regulation of Blood Glucose Levels Using Injectable Nano-Network. Advanced Healthcare Materials. 2014;3:811–816. doi: 10.1002/adhm.201300490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading/Resources
- Scognamiglio V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosensors and Bioelectronics. 2013;47:12–25. doi: 10.1016/j.bios.2013.02.043. [DOI] [PubMed] [Google Scholar]
- Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. Journal of Controlled Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Zhi Z-L, Khan F, Saxl T, Birch DJS. Nanomedicine and its potential in diabetes research and practice. Diabetes/Metabolism Research and Reviews. 2008;24:604–610. doi: 10.1002/dmrr.893. [DOI] [PubMed] [Google Scholar]
- Bratlie KM, York RL, Invernale MA, Langer R, Anderson DG. Materials for diabetes therapeutics. Advanced Healthcare Materials. 2012;1:267–284. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chemical Society Reviews. 2014;43:3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]