Abstract
Background
Community viral load (CVL) estimates vary based on analytic methods. We extended the CVL concept and used data from the Veterans Affairs Healthcare System (VA) to determine trends in the healthcare system viral load (HSVL), sensitivity to varying definitions of the clinical population, and assumptions regarding missing data.
Methods
We included HIV-infected patients in the Veterans Aging Cohort Study, 2000-2010, with >1 documented CD4 count, HIV-1 RNA or antiretroviral prescription (N=37,318). We created 6-month intervals including patients with ≥1 visit in the past 2 years. We assessed temporal trends in clinical population size, patient clinical status and mean HSVL and explored the impact of varying definitions of the clinical population and assumptions about missing viral load.
Results
The clinical population size varied by definition, increasing from 16,000–19,000 patients in 2000 to 23,000–26,000 in 2010. The proportion of patients with suppressed HIV-1 RNA increased over time. Over 20% of patients had no viral load measured in a given interval or prior two years. Among patients with a current HIV-1 RNA, mean HSVL decreased from 97,800 in 2000 to 2,000 copies/mL in 2010. When current HIV-1 RNA data were unavailable and the HSVL was recalculated using the last available HIV-1 RNA, HSVL decreased from 322,300 to 9,900 copies/mL. HSVL was underestimated when using only current data in each interval.
Conclusion
The CVL concept can be applied to a healthcare system, providing a measure of healthcare quality. Like CVL, HSVL estimates depend on definitions of the clinical population and assumptions about missing data.
Keywords: HIV, community viral load, population surveillance, quality of health care, epidemiologic methods
INTRODUCTION
Community viral load (CVL) is a population-based measure of the concentration of plasma HIV-1 RNA in HIV-infected individuals within a particular community(1). The National HIV/AIDS Strategy has identified the use of CVL as a key step towards identifying communities with greater HIV disease burden to inform the development of targeted interventions and resource allocation(2). CVL is calculated by summing viral load measurements from all HIV-infected individuals within a defined time interval and dividing by the total number of HIV-infected individuals in that community(1). CVL is a measure of both transmission potential and effectiveness of disease management. Regions with high HIV-associated morbidity and mortality have begun to measure CVL and assess its relationship with factors including geographic distribution of HIV prevalence, HIV incidence in the general population(3-5) and among particular risk groups(6, 7).
Application of CVL is not without challenges(8, 9). First, defining the population of interest may not be straightforward. For instance, while CVL calculations may include HIV-infected individuals in care but lacking available viral load data, there may be variability across locations and systems in how this subgroup is identified(1). Another challenge is the need to account for missing data(10), as a substantial proportion of patients with HIV are not successfully linked to or retained in care(11), even within systems that provide universal access to care(12). Recent CVL analyses had complete viral load data on only 52% to 74% of HIV-infected individuals when using surveillance registries(13-16). While multiple imputation techniques may be used(14), these can lead to important underestimates of CVL. Third, as sensitivity of HIV viral load testing has improved, the lower limit of detection (LLD) has decreased, complicating accurate comparison of CVL over time(1).
The concept of CVL can also be extended to integrated healthcare systems which we will refer to as “healthcare system viral load” (HSVL). HSVL could complement reporting the proportion of patients achieving viral suppression and serve as a measure of the effectiveness of disease management within a healthcare system. There is a paucity of literature which applies the concept of CVL to a healthcare system, assesses the impact of missing data or considers the variability in viral load assays over time on such calculations.
The Veterans Health Administration (VA) serves as a model for studying HIV diagnosis and treatment(17) given its implementation of expanded HIV testing strategies(18-20), uniform access to antiretroviral medications, high quality of care(21) and the ability to conduct surveillance through electronic medical record extracts and the VA’s HIV Clinical Case Registry. We sought to examine the impact of varying definitions of the clinical population within a national integrated health care system, and of alternative methods of handling missing data, on temporal trends in HSVL over an 11-year period of widely available effective antiretroviral therapy.
METHODS
Study Design and Setting
We used data from the Veterans Aging Cohort Study (VACS), a cohort of HIV-infected patients and matched uninfected comparators followed since 1996(22). Our algorithm for identifying HIV-infected patients within VA is highly sensitive and specific (90% and 99%, respectively), allowing us to effectively and accurately identify this population(22). Demographic data, medical diagnoses (based on International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes)(23), laboratory results and pharmacy records are extracted from electronic medical records. The study received approval from Human Investigations Committee at Yale University and the VA Connecticut Healthcare System and was granted a waiver of informed consent.
Study Population
HIV-infected patients from VACS, (identified using at least one inpatient or two outpatient HIV ICD-9-CM codes)(22) who were alive on January 1, 2000 and entered the cohort by September 30, 2009, were eligible for the current analysis. Those with at least one documented CD4 count, HIV-1 RNA test, or prescription for antiretroviral therapy (ART) during this period were considered in VA HIV care and constitute the study sample. We used these minimal criteria to ensure that identified individuals had interfaced with the VA for HIV care at least once.
The units of analysis were 6-month intervals (January to June, July to December) from 2000 to 2010. Patients were included in each interval if their earliest date of HIV care (ICD-9 code, CD4 count, HIV-1 RNA test, or ART prescription fill) occurred by the mid-point of the interval and they were alive at the start of that interval according to VA vital status records. The VA uses several sources to identify deaths with excellent ascertainment(24, 25).
Measures
Community Definitions
According to the Centers for Disease Control and Prevention, community viral load is the average viral load for individuals within a specific geographic region who are:
in care with undetectable virus, or
in care with detectable viral load, or
in care with no viral load measurement, or
diagnosed but not in care(1).
In the current analysis, we focused on patients receiving care within a specific healthcare system to determine the HSVL. We explored several definitions of the clinical population based on whether or not a patient had a visit within a VA facility. Visits included inpatient, outpatient or telephone; medical, psychiatric, substance abuse and laboratory encounters. Pharmacy fills were not counted as a visit because they can be done by mail. We created five increasingly restrictive definitions of HIV-infected clinical populations applied to each 6-month interval: 1) all individuals not known to be dead, regardless of whether they had a visit; 2) those with a visit in the 6-month interval or the previous two years; 3) those with a visit in the interval or the past year; 4) those with a clinic visit in the interval or the past 6 months; and 5) those with a clinic visit during the interval. We used definition 2 for our primary analyses. The CDC considers patients with at least two clinic visits (at least 60 days apart) in a given year to be retained in care(1). We chose to evaluate a more comprehensive assessment of the clinical population that included those not retained in care. Further, our group believed that patients should be considered part of the healthcare system for up to 2 years after last clinic visit.
Categorization of Patient Clinical Status
In each 6-month interval (described above), patients were assigned to one of eight mutually exclusive categories:
New to care, if earliest date of HIV care was in the interval or prior 3 months;
Dead, if they died during the interval;
Current HIV-1 RNA >10,000 copies/mL;
Current HIV-1 RNA 501 - 10,000 copies/mL;
Current HIV-1 RNA 51 - 500 copies/mL;
Current HIV-1 RNA ≤50 copies/mL;
Recent, no HIV-1 RNA in the given interval, but had one in past two years;
Missing, no HIV-1 RNA viral load available in the given interval or past two years.
If multiple HIV-1 RNA values were available, the one closest to the midpoint was used as this would be most representative of the interval.
Mean Healthcare System Viral Load
As recommended by CDC for CVL determinations, we calculated the geometric mean HSVL for each interval, by converting the HIV-1 RNA values to the log10 scale, calculating the mean of the logs, and then taking the antilog of the mean(1). We used mean, instead of median, as it more accurately captures variability in HIV control(14). As the proportion of patients with undetectable virus increases, the median simply becomes LLD of the assay in use at the time. Because HIV-1 RNA values are strongly right skewed, use of the arithmetic mean would be unduly influenced by extreme values and would violate assumptions of normality required for statistical inferences. HIV-1 RNA values reported as undetectable were set to the LLD, which varied by site and calendar year. In sensitivity analyses, we held the LLD constant at 500, 50 and 25 HIV-1 RNA copies/ml so we could see whether temporal trends might be attributed to changes in assay methods.
Assumptions about Missing Data
To assess the impact of missing data, we calculated mean HSVL using four different approaches: 1) only values measured in each 6-month interval, choosing the value closest to the midpoint if more than one; 2) additionally including the most recent value in the past two years, referred to as last value carried forward (LVCF), for those without a value in the given interval; 3) current values and LVCF, plus an assumed value of 10,000 copies/mL for those with no HIV-RNA result in the last 2 years; and 4) current values, LVCF, plus an assumed value of 100,000 copies/mL for those with no result.
Statistical Analysis
We assessed patient characteristics at the later of January 1, 2000 or cohort entry. Average annual change in size of the clinical population was calculated as the difference in number of patients between the end of 2010 and the start of 2000, divided by the starting value, divided by 10 intervals × 100. To test for trends in size of the clinical population and mean HSVL we used linear regression with an integer value for each interval ranging from 1 to 22. We compared HSVL between subgroups using a t-test of the mean log10 transformed HIV-1 RNA values.
RESULTS
Patient Characteristics
From 44,180 patients in VACS diagnosed with HIV infection by September 30, 2010, we excluded those who died before January 1, 2000 (n=3,522). Another 3,672 without documented CD4 count, HIV-1 RNA viral load, or ART prescription any time between 2000 and 2010 (suggesting they were not actively receiving HIV care within the VA) were also excluded. Our analytic sample included 37,318 patients who received HIV care within the VA (Table 1). Patients were predominantly male, with a median age of 47 years (interquartile range 41, 53) in the year 2000 or at cohort entry. Nearly half the patients were black, about a third were co-infected with hepatitis C virus, nearly half began VA HIV care prior to January 1, 2000, and most initiated ART during the study period. Thirty percent of patients had died by December 31, 2010.
Table 1.
Characteristics of 37,318 HIV-infected veterans in care between 2000 and 2010
| N | (%) | ||
|---|---|---|---|
| Age, median (IQR) |
47 | (41, 53) | |
| Sex | Male | 36,433 | (98) |
| Race | White | 13,965 | (37) |
| Black | 18,455 | (49) | |
| Hispanic | 2,681 | (7) | |
| Other/unknown | 2,217 | (6) | |
| Hepatitis C coinfection | 13,047 | (35) | |
| Year of first recorded HIV indicator in VA |
<1996 | 5,699 | (15) |
| 1996-1999 | 12,642 | (34) | |
| 2000-2004 | 10,182 | (27) | |
| 2005-2010 | 8,795 | (24) | |
| Initiated ART | 32,999 | (88) | |
| Died by December 31, 2010 | 11,298 | (30) |
Age as of January 1, 2000 or first HIV date, whichever is later First recorded HIV indicator = ICD-9 code, CD4, positive HIV-RNA or ART ART: antiretroviral therapy
Clinical Population Size based on Varying Definitions
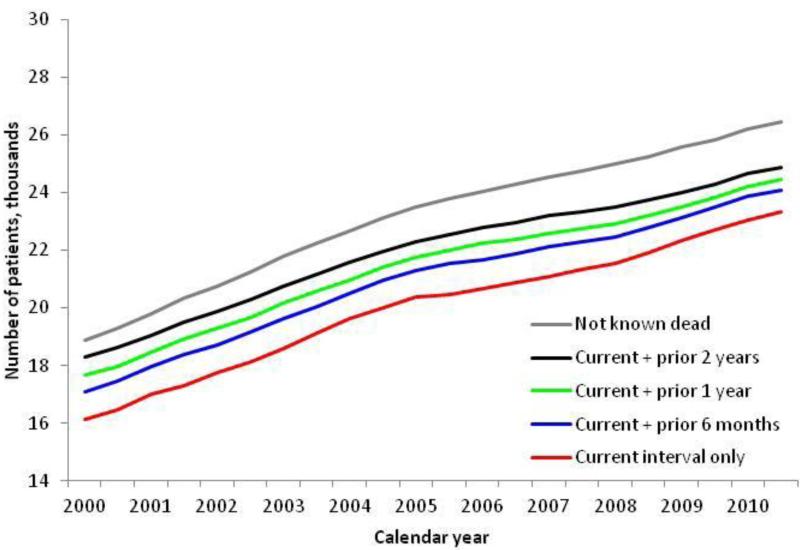
The size of the clinical population of HIV-infected patients varied based on the definition used (Figure 1). Regardless of definition, the size of the clinical population increased by 4% per year (p-trend < 0.001). Using the most inclusive definition, all patients not known dead, there were 18,900 patients at the start of 2000, increasing to 26,500 at the end of 2010. The proportion of those not known dead, without a current VA visit increased from 3% in 2000 to 6% at the end of 2010. Using the most restrictive definition, those who had a visit in the interval, there were 16,100 patients in the clinical population at the start of 2000, increasing to 21,400 in the second half of 2010. Among those with at least one visit in a given interval or prior two years, there were approximately 20,000 patients in each 6-month interval, increasing from 18,300 patients at the start of 2000 to 24,900 patients at the end of 2010. This definition was used for all subsequent analyses.
Figure 1.
Trends in number of HIV-infected veterans using varying definitions by 6-month interval, 2000 to 2010. Current indicates a VA inpatient or outpatient visit in the 6-month interval.
Trends in Patient Clinical Status by 6-Month Intervals
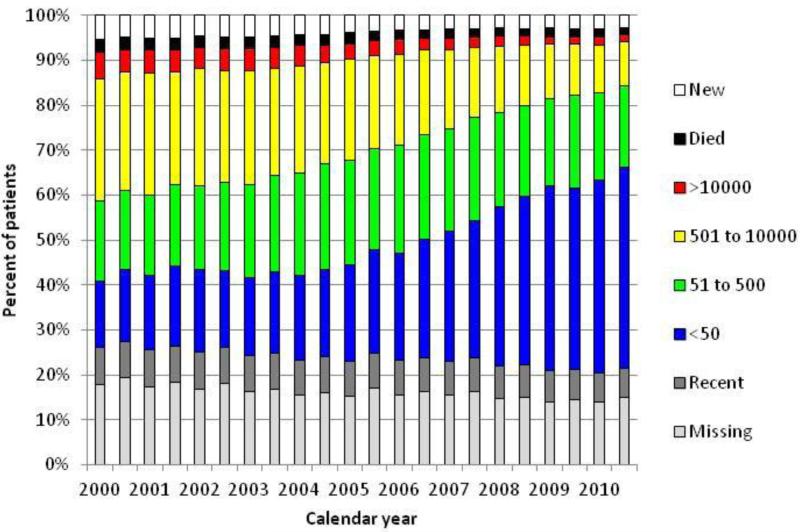
We found several significant temporal trends (p-trend <0.001). The proportion of patients who died, were new to care, had an HIV-1 RNA > 500 copies/mL or no current HIV-1 RNA all decreased (Figure 2). The proportion of deaths in a given interval decreased from 3% in 2000 to 2% in 2010. The proportion of patients new to care was 5% in 2000 decreasing and leveling off to approximately 3% by 2008. By the end of 2010, 45% of patients had HIV-1 RNA <50 copies/ml. A small proportion (6-8%) of patients had no HIV-1 RNA result in a given interval, but had one in the prior two years. Approximately 15% had no viral load in either a given interval or the prior two years (missing category). Combining these last two groups, we found that the proportion without a current HIV1-RNA decreased from 26% in 2000 to 22% in 2010.
Figure 2.
Trends in patient clinical status of HIV-infected veterans by 6-month interval, 2000 to 2010, for those with a VA visit in the current interval or prior two years.
Mean Healthcare System Viral Load
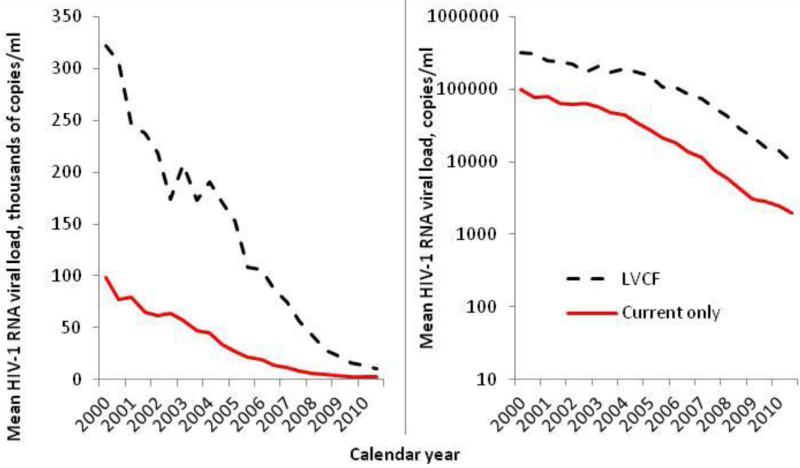
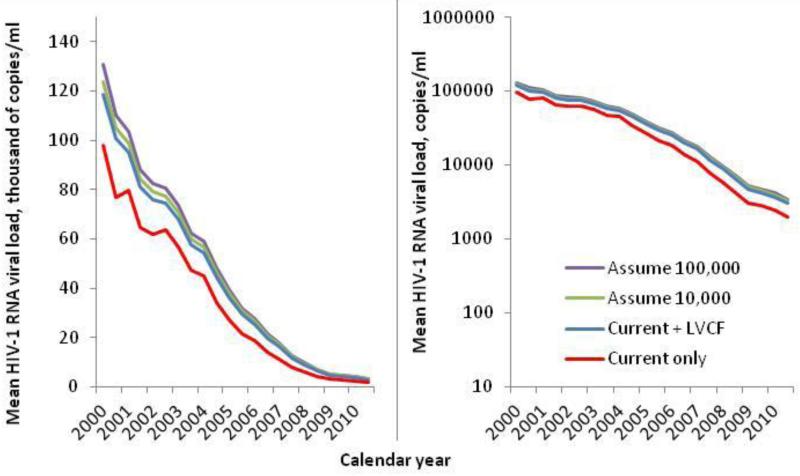
The mean HSVL decreased dramatically from 2000 to 2010. In patients with HIV-1 RNA values in a given interval, the mean HSVL was 97,800 copies/mL in the first half of 2000 dropping to 2,000 copies/mL at the end of 2010. In sensitivity analysis, temporal trends were also apparent when we held the LLD constant, but the slope of decline was lessened (data not shown). In patients without a viral load in a given interval (but who had a measurement in the prior 2 years), the mean HSVL estimated with LVCF decreased from 322,300 copies/mL to 9,900 copies/mL (Figure 3, p-trend <0.001 for both). In all intervals, the mean HSVL in patients with LVCF was at least 3 times higher than those with a current viral load value (p < 0.001). Combining current values and LVCF estimates, mean HSVL was 118,600 copies/mL in 2000 and 6,000 copies/mL in 2010 (Figure 4). When those without a viral load in the previous two years were assumed to have HIV-1 RNA of 10,000 or 100,000 copies/mL for a given interval, mean HSVL was higher still. Although HSVL decreased over time with all four approaches to missing data mean HSVL at the end of 2010 differed by method of accounting for missing HIV-RNA. Mean HSVL was 2,000 copies/mL including current values only; 3,000 copies/mL including LVCF; 3,300 copies/mL assuming 10,000 copies/mL for those with no result in the last two years; and 3,400 copies/mL assuming 100,000 copies/mL for those with no result.
Figure 3.
Trends in mean healthcare system viral load of HIV-infected veterans by 6-month interval, 2000 to 2010, comparing those with an HIV-1 RNA measurement in the current interval to those estimated with a value up to 2 years earlier, referred to as last value carried forward (LVCF). Left panel scale is thousands of copies/ml, right panel is copies/ml on a log10 scale.
Figure 4.
Temporal trends in mean healthcare system viral load of HIV-infected veterans by 6-month interval, 2000 to 2010, using available measurements and various estimates for missing data. Current only: actual HIV-1 RNA measurement in the current interval; LVCF: additionally includes last value carried forward, estimated with most recently measured value up to 2 years earlier; Assume 10,000: current, LVCF and an assumed value of 10,000 copies/ml for missing; Assume 100,000: current, LVCF and an assumed value of 100,000 copies/ml for missing. Left panel scale is thousands of copies/ml, right panel is copies/ml on a log10 scale.
DISCUSSION
This is the first study in a United States national sample to apply the concept of CVL to a healthcare system and examine trends and factors that may affect it. We extend the current literature by examining the impact of varying definitions of a clinical population and assumptions about missing data. We used data from the largest single provider of HIV care in the United States. The VA healthcare system’s electronic medical record allows for comprehensive monitoring of utilization of services and retention in care. We found, first, that a growing proportion of patients had a suppressed HIV-1 RNA viral load, translating into a dramatic decrease in mean HSVL from 2000 to 2010. Second, we identified a relatively large proportion of patients (over 20%) presumed in care who did not have viral load data in each 6-month interval, leading to a significant difference in the clinical population size depending on the definition used. Importantly, based on our use of varying definitions of the clinical population and different ways of handling missing HIV-1 RNA viral load, we demonstrated that the HSVL based on available data alone, without accounting for missing data, may underestimate the true level of viremia.
The observed decrease in mean HSVL is encouraging and consistent with other studies which have demonstrated a decreased CVL(5, 6, 13, 14). In part, this may reflect improvements in ART and simplification of regimens(26, 27) as 45% of patients had an undetectable viral load by 2010. It also likely captures the impact of adoption of policies and practices consistent with national efforts to improve detection of those unaware of their status through expanded HIV testing programs and timely linkage to care(18, 19). Decreased HSVL may also be affected by better implementation of HIV treatment guidelines and quality metrics(21), including access to ART to achieve viral load suppression. Further, VA-driven initiatives to understand and optimize care both across the spectrum of HIV care(28) and across settings(29, 30) may contribute to these trends. Recent reports indicate that while the number of HIV-infected patients in care within the VA is increasing, the proportion ever prescribed ART is also increasing. According to a VA report, in 2011, 93% of patients receiving care through the VA had ever been prescribed antiretroviral therapy. Among those prescribed antiretroviral therapy, 74% had evidence of viral suppression(31). In comparision, we found that by the end of 2010, only 45% of patients had viral suppression. The difference in the findings from the VA report and our findings is likely explained by the fact that we asssessed viral suppression among patients who had had at least one visit in the prior two years and regardless of whether or not they were receiving ART. In contrast, the VA report defined this metric as the proportion of patients who are “receiving HAART who have a viral load below the limit of quantification after at least 6 months of HAART.” As recent HIV clinical guidelines recommending ART for all HIV-infected patients(32) continue to be adopted, we expect the HSVL to decline further among patients retained in care.
Our finding that as many as 20% of patients did not have HIV-1 RNA measurements according to guideline-recommended intervals(33), has important implications for clinical care and research. These data indicate that a significant proportion of HIV-infected Veterans are not in care. Our numbers may be an overestimate as we did not determine if patients were receiving care and viral load monitoring through other healthcare systems (i.e. Ryan White, private sector, prison system, etc). Yet our results are consistent with a recent meta-analysis that found up to 40% of patients may be not be retained in care(11). Regardless, our data support the need for clarification of which HIV-infected patients are at greatest risk for falling out of care. One such group may be those with a history of incarceration, as this population is known to receive fragmented healthcare and a history of incarceration is common among HIV-infected patients receiving care through the VA(34). Accordingly, developing processes and procedures for streamlining communication and care across institutions may have beneficial effects on the HSVL. Similarly, current efforts to decrease homelessness, experienced by approximately 5% of HIV-infected Veterans in a given time(35), may decrease the HSVL. As the quality of and access to electronically available data across clinics and healthcare systems increase, processes for identifying and reconnecting patients who have not been retained in care should be streamlined.
Our findings highlight the impact of varying the definition of the clinical population and the handling of missing HIV-1 RNA on determining HSVL. Given the quality of information available through the electronic medical record, we accounted for all HIV-infected patients who received care for HIV infection in the VA at least once during 2000-2010 and demonstrated how varying definitions of the clinical population may impact the HSVL assessments. While prior studies relied on data available through active surveillance, the proportion of patients with missing viral load data has ranged from 26%(14) to 52%(13). Surveillance data available through health departments are unlikely to be missing at random leading to underestimates of community viral load. This is supported by our finding that mean HIV-1 RNA using LVCF was significantly higher than viral loads from those with current data. Such differences may have important implications for understanding transmission risk in a particular community, as those lacking current data are likely to have higher viral load, with greater transmission potential, than those receiving routine care(36). While other studies have relied on multiple imputation(14), the limits of these techniques are well recognized, including violation of the assumption that missing data are missing at random(1). A recent analysis by Horberg and colleagues using data from Kaiser Permanente Medical Care Program reinforced the importance of the definition of the clinical population when evaluating outcomes. The proportion of patients with a suppressed viral load among those retained in care, was only 61%; however, when based on laboratory data alone, this rose to 80%(12).
Our analysis should be interpreted in the context of some limitations. First, we were unable to determine whether missing viral loads were due to failure to extract a value from the electronic record or if patients were receiving care outside the VA. In a prior analysis of a subset of HIV-infected patients, only 2% reported receiving their antiretroviral medications from external sources suggesting, that most received their care through the VA(37). Second, our cohort was defined by on the presence of an HIV diagnosis by ICD-9-CM code, which may be subject to underreporting. However, previous validation work has demonstrated high sensitivity and specificity of our method(22). We also required additional confirmation with relevant lab or pharmacy data to reduce false positives. Third, various definitions of CVL have been proposed and used across studies(3, 5, 7, 13, 14, 38). Although our study does not comprehensively test the application of each of these definitions to our calculations of HSVL, our main findings of the relative decrease in the HSVL over time and the variation in the HSVL based on handling of missing viral loads, are likely to remain accurate, regardless of the definition used(1). Fourth, we designed our analysis to be consistent with current guidelines; if we extended our intervals to allow for examining HIV viral load testing from 6 months intervals to one year, we may have found that more patients were retained in care. Finally, the mortality rate of patients in our cohort is higher than other cohorts. This may result from more complete ascertainment of mortality within the VA system the age and comorbidities of our patient population (39).
In conclusion, our study demonstrates the feasibility of adapting the concept of CVL to determine a HSVL for monitoring trends in retention in care and achievement of viral load suppression. Our data also highlight how the underlying assumptions regarding community definitions and missing data lead to important variability in level of viremia. As metrics for assessing HIV transmission risk and quality of care evolve, efforts to standardize how to define “community” and handle missing data will be essential to allow comparisons across settings and populations. We suggest that a more precise strategy to estimate CVL and/or HSVL will rely on calculating an estimated range rather than a single value. Future analyses will focus on understanding differences among subgroups, based on demographic and behavioral characteristics, and by geography to identify potential areas to target improved HIV care.
Acknowledgements
Disclosures: This work was presented as an oral presentation in earlier versions at the Veterans Aging Cohort Study Scientific Meeting, October 13th, 2011, Washington, D.C. and as a poster presentation at CROI, Seattle, Washington, March 2012.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Robert Wood Johnson Foundation Clinical Scholars Program, the Department of Veterans Affairs, or the National Institute on Health.
Sources of Support: This work was generously supported by funding from the Robert Wood Johnson Foundation Clinical Scholars Program, Department of Veteran Affairs, and National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (U10-AA13566), and National Institute on Drug Abuse (K12DA033312-01A1).
Footnotes
Conflicts of Interest: None declared.
REFERENCES
- 1.Centers for Disease Control and Prevention . Guidance on Community Viral Load: A Family of Measures, Definitions and Method for Calculation. 2011. [Google Scholar]
- 2.Office of National AIDS Policy . National HIV/AIDS Strategy for the United States. 2010. [Google Scholar]
- 3.Henard S, Jeanmaire E, Nguyen Y, Yazdanpanah Y, Cheret A, Hoen B, et al. Is total community viral load a robust predictive marker of the efficacy of the TasP strategy? J Acquir Immune Defic Syndr. 2012 Jun 22; doi: 10.1097/QAI.0b013e318263a111. [DOI] [PubMed] [Google Scholar]
- 4.Das MCP, Glenn-Mil S, Scheer S, McFarland W, Vittinghoff E, Colfax GN. Success of Test and Treat in San Francisco? Reduced Time to Virologic Suppression, Decreased Community Viral Load, and Fewer New HIV Infections, 2004 to 2009. CROI; Boston, MA: 2011. [Google Scholar]
- 5.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk GD, Galai N, Astemborski J, Linas B, Celentano D, Mehta SH, Vlahov D. Decline in Community Viral Load is Strongly Associated with Declining HIV Incidence among IDUs. CROI; Boston, MA: 2011. [Google Scholar]
- 7.Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia Q, Wiewel EW, Torian LV. Revisiting the methodology of measuring HIV community viral load. J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):e82–4. doi: 10.1097/QAI.0b013e318282d2a4. [DOI] [PubMed] [Google Scholar]
- 9.Miller WC, Powers KA, Smith MK, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis. 2013 May;13(5):459–64. doi: 10.1016/S1473-3099(12)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . Using Viral Load Data to Monitor HIV Burden and Treatment Outcomes in the United States. In: Division of HIV/AIDS Prevention, editor. National Center for HIV/AIDS VH, STD, and TB Prevention. 2012. [Google Scholar]
- 11.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010 Nov 13;24(17):2665–78. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 12.Taylor BS, Wilkin TJ, Shalev N, Hammer SM. CROI 2013: Advances in antiretroviral therapy. Top Antivir Med. 2013 Apr-May;21(2):75–89. [PMC free article] [PubMed] [Google Scholar]
- 13.Castel AD, Befus M, Willis S, Griffin A, West T, Hader S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS. 2012 Jan 28;26(3):345–53. doi: 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 14.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laraque FMH, Robertson M, Gortakowski H, Terzian A. Disparities in Community Viral Load among HIV Infected Persons in NYC. CROI; Boston, MA: 2011. [DOI] [PubMed] [Google Scholar]
- 16.Laraque F, Mavronicolas HA, Robertson MM, Gortakowski HW, Terzian AS. Disparities in community viral load among HIV-infected persons in New York City. AIDS. 2013 Aug 24;27(13):2129–39. doi: 10.1097/QAD.0b013e328360f619. [DOI] [PubMed] [Google Scholar]
- 17.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011 Jan 15;52(Suppl 2):S238–46. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz MB, Rimland D. Effect of Expanded HIV Testing Programs on the Status of Newly Diagnosed HIV-Infected Patients in Two Veterans Health Administration Facilities: 1999-2009. J Acquir Immune Defic Syndr. 2011 Jun 1;57(2):e23–5. doi: 10.1097/QAI.0b013e31821a0600. [DOI] [PubMed] [Google Scholar]
- 19.Kan VL. HIV testing at a VA Medical Center in a high prevalence area after VHA policy change: things are looking up. J Acquir Immune Defic Syndr. 2010 Jul 1;54(3):e1–2. doi: 10.1097/QAI.0b013e3181e15c0c. [DOI] [PubMed] [Google Scholar]
- 20.Czarnogorski M, Halloran Cns J, Pedati C, Dursa EK, Durfee J, Martinello R, et al. Expanded HIV testing in the US Department of Veterans Affairs, 2009-2011. Am J Public Health. 2013 Dec;103(12):e40–5. doi: 10.2105/AJPH.2013.301376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backus LI, Boothroyd DB, Phillips BR, Belperio PS, Halloran JP, Valdiserri RO, et al. National quality forum performance measures for HIV/AIDS care: the Department of Veterans Affairs’ experience. Arch Intern Med. 2010 Jul 26;170(14):1239–46. doi: 10.1001/archinternmed.2010.234. [DOI] [PubMed] [Google Scholar]
- 22.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006 Aug;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare and Medicaid Services . ICD-9 Provider and Diagnostic Codes. 2011. [PubMed] [Google Scholar]
- 24.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990 Jan;131(1):160–8. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 25.Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995 Feb 1;141(3):242–50. doi: 10.1093/oxfordjournals.aje.a117426. [DOI] [PubMed] [Google Scholar]
- 26.McKinnell JA, Willig JH, Westfall AO, Nevin C, Allison JJ, Raper JL, et al. Antiretroviral prescribing patterns in treatment-naive patients in the United States. AIDS Patient Care STDS. 2010 Feb;24(2):79–85. doi: 10.1089/apc.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Althoff KN, Justice AC, Gange SJ, Deeks SG, Saag MS, Silverberg MJ, et al. Virologic and immunologic response to HAART, by age and regimen class. AIDS. 2010 Oct 23;24(16):2469–79. doi: 10.1097/QAD.0b013e32833e6d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry SR, Goetz MB, Asch SM. The Effect of Automated Telephone Appointment Reminders on HIV Primary Care No-Shows by Veterans. J Assoc Nurses AIDS Care. 2012 Mar 16; doi: 10.1016/j.jana.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Hoang T, Goetz MB, Yano EM, Rossman B, Anaya HD, Knapp H, et al. The impact of integrated HIV care on patient health outcomes. Med Care. 2009 May;47(5):560–7. doi: 10.1097/MLR.0b013e31819432a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saifu HN, Asch SM, Goetz MB, Smith JP, Graber CJ, Schaberg D, et al. Evaluation of human immunodeficiency virus and hepatitis C telemedicine clinics. Am J Manag Care. 2012 Apr;18(4):207–12. [PubMed] [Google Scholar]
- 31.Department of Veterans Affairs . The State of Care of Veterans with HIV/AIDS: 2011 Summary Report. 2012. [Google Scholar]
- 32.Panel on Antiretroviral Guidelines for Adults and Adolescents . In: Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. (OARAC) OoARAC, editor. 2013. [Google Scholar]
- 33.Panel on Antiretroviral Guidelines for Adults and Adolescents . In: Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. (OARAC) OoARAC, editor. 2012. [Google Scholar]
- 34.Wang EA, McGinnis K, Long JA,KM, Edelman EJ, Rimland D, Justice AC, Fiellin DA, editors. Incarceration and Health Outcomes in HIV-Infected Patients: The Impact of Substance Use, Antiretroviral adherence, and Primary Care Engagement, Poster Presentation. Society of General Internal Medicine; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghose T, Fiellin DA, Gordon AJ, Metraux S, Goetz MB, Blackstock O, et al. Hazardous drinking and its association with homelessness among veterans in care. Drug Alcohol Depend. 2013 Sep 1;132(1-2):202–6. doi: 10.1016/j.drugalcdep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen SM, Hu X, Sweeney P, Johnson AS, Hall HI. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 U.S. jurisdictions. J Acquir Immune Defic Syndr. 2014 Sep 18; doi: 10.1097/QAI.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 37.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006 Aug;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das M, Raymond HF, Chu P, Nieves-Rivera I, Pandori M, Louie B, et al. Measuring the unknown: calculating community viral load among HIV-infected MSM unaware of their HIV status in San Francisco from National HIV Behavioral Surveillance, 2004-2011. J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):e84–6. doi: 10.1097/QAI.0b013e31828ed2e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013 Feb 20;27(4):563–72. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]