Abstract
Objectives
The Late-Life Function and Disability Instrument (LLFDI) assesses two key dimensions of disability: frequency of and limitations in performance of major life tasks. The aim of this study was to determine and compare the predictive validity and responsiveness of the LLFDI frequency and limitation dimensions.
Design
Secondary analysis of 2-year follow-up data from the Boston Rehabilitative Impairment Study of the Elderly (RISE).
Setting
Primary care.
Participants
Community-dwelling older adults (age ≥ 65 years) (n=430) at risk for mobility decline.
Measurements
The LLFDI frequency and limitation dimensions, self-rated health, hospitalizations and emergency room (ER) visits over 2 years. Responsiveness measures included effect size (ES) estimates and minimal detectable change (MDC) scores.
Results
The LLFDI frequency dimension predicted low self-rated health (OR =0.51, P <.001), hospitalizations (OR =0.68, P <.001), and ER visits (OR =0.73, P =0.003) over 2 years while the limitation dimension did not. The absolute ES was 0.63 for the frequency dimension and 0.81 for the limitation dimension. The proportion of subjects with a decline ≥ the MDC was 10.6% for the frequency dimension and 14.2% for the limitation dimension. For patients who improved ≥ the MDC, the proportion was 1.7% for the frequency dimension and 15.3% for the limitation dimension.
Conclusion
Frequency of participation in major life roles was a better predictor of adverse outcomes than perceived limitations; however, limitations appeared to be more responsive to meaningful change. These results can be used to guide the selection of the most appropriate metric for measuring disability in geriatric research.
Keywords: Disability, community-dwelling older adults, minimal detectable change, responsiveness, validity
INTRODUCTION
Although disability is recognized as a critical outcome in geriatric research, it remains difficult to measure. There is considerable variability in the definition of disability; some studies define disability in terms of discrete functional deficits such as limitations in mobility or performance of basic activities of daily living, while others use broader indicators such as performance of socially defined life activities. This lack of conceptual clarity limits progress in both understanding the disablement process and in demonstrating important changes in disability after interventions.
In 2002, the Late-Life Function and Disability Instrument (LLFDI) was developed to address these limitations.1, 2 The LLFDI is a patient-reported outcome measure that assesses functional limitations and disability based on an explicit theoretical framework.3 Consistent with Nagi's disablement model, disability within the LLFDI refers to a person's performance of socially defined life tasks within his or her environment. This definition is also consistent with the concept of participation restrictions in the World Health Organization's International Classification of Functioning, Disability, and Health (ICF).4 One of the unique features of the LLFDI is that it considers two important dimensions of disability: frequency of performance of life activities and limitations in a person's capability to perform each activity.
Over the last decade, the LLFDI has been used in studies involving more than 17,000 older adults with extensive evidence supporting its construct validity.5 A recent systematic review5 confirmed the sensitivity to change of the disability limitation dimension, but there was limited data on the frequency dimension and no studies on predictive validity of either disability dimension. Therefore, the objective of this study was to determine and compare the predictive validity and responsiveness of the LLFDI disability frequency and limitation dimensions in a cohort of older primary care patients. We hypothesized that both dimensions would show similarly high predictive validity for adverse outcomes and responsiveness to change over 2 years.
METHODS
Participants
We performed a secondary analysis of baseline and 2-year follow-up data from the Boston Rehabilitative Impairment Study of the Elderly (Boston RISE), a longitudinal cohort study of 430 older adults at risk for mobility decline.
Methods for Boston RISE were approved by the relevant Institutional Review Boards and study details have been published previously.6 Participants were recruited from primary care practices in the Greater Boston Area. Inclusion criteria comprised: age ≥65 years, ability to speak and understand English, self-reported difficulty or task modification with walking ½ mile and/or climbing 1 flight of stairs, no planned major surgery and expectation of living in the area for ≥2 years. Exclusion criteria were: significant visual impairment, uncontrolled hypertension, lower-extremity amputation, supplemental oxygen use, myocardial infarction or major surgery in the previous 6 months, Mini Mental State Exam score <18 and Short Physical Performance Battery score <4. Baseline assessments were conducted over 2 visits and repeated annually for 2 years. Study staff contacted participants by phone every 3 months to track health care utilization.
Measures
Late-Life Function and Disability Instrument (LLFDI)
The LLFDI is a patient-reported measure that assesses both functional limitations and disability. Questionnaire items for the LLFDI were developed and refined based on the Nagi3 and ICF4 disablement models, review of existing instruments, consultation with experts, and focus groups with older adults. Exploratory factor analysis and Rasch analysis were used to further refine the LLFDI and to develop the subscales. This investigation focused on the Disability component which assesses an older person's frequency of participation in 16 major life tasks and the person's limitation in his/her capability to perform each task. Frequency dimension questions are phrased, “How often do you” do a specific task with response options of “very often”, “often”, “once in a while”, “almost never” and “never”. Limitation dimension questions are phrased “To what extent do you feel limited in” doing the same task with response options of “not at all”, “a little”, “somewhat”, “a lot”, and “completely”. The frequency dimension is further broken down into a social role domain (includes social and community tasks such as keeping in touch with others and taking part in active recreation) and a personal role domain (includes personal tasks such as taking care of the home and personal health). Similarly, within the limitation dimension are the instrumental role domain (includes home or community activities such as taking care of the home and taking part in active recreation) and the management role domain (includes organizational tasks that require minimal mobility such as keeping in touch with others and taking care of personal health). For a full description of items please see Jette et al.2 Raw scores for each dimension and subscale are transformed to scaled scores (0–100) based on a Rasch model with higher scores indicating less disability. Evidence supports the LLFDI's construct validity, sensitivity to change and reliability among older adults.1, 2, 5
Self-rated health and self-reported walking difficulty
Self-rated health was determined using a 5-point Likert scale in response to the question “In general, how would you say your health is?”;7 a response of poor or fair was used to categorize those with low self-rated health at 2 years. Self-reported walking difficulty was determined in response to the question “Do you have difficulty walking a half mile?” with response options of yes or no.8 Both self-rated health and self-reported walking difficulty are predictive of mortality and health-care utilization.9-11
Short Physical Performance Battery (SPPB)
The SPPB is a performance-based test comprised of 3 components: standing balance, usual pace walking speed and a 5-repetition chair stand test. Scores from each component are added to create a summary score between 0-12, with higher scores indicating better performance. A 1-point change in SPPB score is predictive of mortality and nursing home admissions.12
Hospitalizations and emergency room visits
Hospitalizations (defined as an overnight hospital stay for any reason) and emergency room visits were recorded from standardized questions administered by telephone every 3 months and as part of the yearly in-person assessment.
Statistical Analyses
Predictive validity
Separate logistic regression models were constructed to assess each LLFDI disability scale as a predictor of: 1) low self-rated health at 2 years; 2) one or more hospitalizations over 2 years; and 3) one or more emergency room visits over 2 years. The increased odds of having an unfavorable outcome for a 1SD change in each scale were calculated. Models were adjusted for age and gender. Data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Responsiveness
In this study, we defined responsiveness as the degree to which a measure detects meaningful change. To determine meaningful change use of both distribution-based methods (i.e., statistical distributions of change and associated reliability) and anchor-based methods (i.e., external criterion of change reflecting a patient or clinician's perspective) is recommended.14 The following responsiveness metrics were calculated for each LLFDI Disability scale:
-
1)
Absolute effect sizes (ES) were computed as ES= abs((M2-M1))/Sb, where M2 is the mean score at year 2, M1 is the mean score at baseline, and Sb is the standard deviation at baseline. We used absolute ES to provide a metric of responsiveness independent of direction because some variability in the trajectories of change was anticipated (i.e., some participants might improve while others decline).Values of 0.20, 0.50, and 0.80 are considered small, moderate and large effect sizes, respectively.15
-
2)
The ES for each scale was also computed for subgroups of patients using external criteria to categorize those who either declined or improved in self-rated health, SPPB score, and self-reported walking difficulty.
-
3)
To provide a value for measurement error in the same units as the LLFDI, the standard error of the measurement (SEM) was calculated as SEM = Sb*√(1-r), where Sb is the standard deviation at baseline and r is the test-retest reliability coefficient. Data for the reliability coefficients were obtained from previous work.2
-
4)
Minimal detectable change scores with 90% confidence were calculated as (MDC90) = SEM*1.645*sqrt2.16 The MDC90 corresponds to the smallest amount of change that can be considered true change that falls outside of measurement error. The percentages of patients who demonstrated a decline/improvement ≥ the MDC90 over 2 years were calculated for each scale.
RESULTS
At baseline, 430 Boston RISE participants had a mean age of 77 years and 68% were female. The sample was predominately white (82.6%) with an average of 4.0 (SD 1.9) chronic conditions and a mean baseline SPPB score of 8.7 (SD 2.3). The mean score on the LLFDI disability frequency dimension was 52.3 (SD 5.7) with no ceiling or floor effects; the mean score on the disability limitation dimension was 68.9 (SD 11.8) with no floor effects but 6.5% at the ceiling (data not shown in tables).
At 2 years, 360 participants remained in the study. Sample sizes for the individual analyses varied based on the methods and outcomes used. Of the 276 participants with data on patient-reported health status at 2 years, 56 (20.3%) reported low self-rated health. Quarterly phone call data were available on 427 participants for hospitalizations and 423 for emergency room visits; 164 (38.4%) reported one or more hospitalizations and 201 (47.5%) reported one or more emergency room visits over 2 years.
Predictive validity
The logistic regression models that show the odds of having an unfavorable outcome for every 1 SD increase in LLFDI score, adjusted for age and gender, are presented in Table 1. The LLFDI summary frequency dimension and personal and social role subscales showed high predictive validity for low self-rated health and hospitalizations. The summary frequency dimension and personal role subscale were also predictive of emergency visits over 2 years. The summary limitation dimension and subscales were generally not statistically significant predictors of adverse outcomes; with the exception of the management role subscale which predicted low self-rated health. There was also a trend for some predictive value with the instrumental role subscale for low self-rated health (P =.06), and with overall limitation and instrumental role subscales for hospitalizations (P =.08 and P =.06, respectively).
Table 1.
Logistic regression models* predicting adverse outcomes over 2 years
| Low self-reported health (n=276) | Hospitalizations (n=427) | Emergency room visits (n=423) | ||||
|---|---|---|---|---|---|---|
| LLFDI scale | OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value |
| Frequency | 0.51 (0.36-0.73) | <.001 | 0.68 (0.55-0.84) | <.001 | 0.73 (0.60-0.90) | 0.003 |
| Personal role | 0.66 (0.46-0.95) | 0.025 | 0.68 (0.54-0.85) | <.001 | 0.73 (0.59-0.90) | 0.003 |
| Social role | 0.53 (0.37-0.75) | <.001 | 0.82 (0.67-1.0) | 0.047 | 0.85 (0.70-1.03) | 0.096 |
| Limitation | 0.74 (0.53-1.04) | 0.082 | 0.83 (0.68-1.02) | 0.080 | 0.96 (0.79-1.17) | 0.682 |
| Management role | 0.73 (0.54-0.99) | 0.043 | 0.92 (0.75-1.12) | 0.407 | 0.97 (0.80-1.18) | 0.755 |
| Instrumental role | 0.72 (0.52-1.01) | 0.060 | 0.82 (0.67-1.0) | 0.057 | 0.95 (0.78-1.16) | 0.624 |
Models show the increased odds of having an adverse outcome for a 1 standard deviation increase in score on the Late-Life Function and Disability Instrument (LLFDI) adjusted for age and gender.
OR = odds ratio; CI = confidence interval
Responsiveness
Results of the responsiveness analysis are shown in Table 2. Larger absolute ES (0.75-0.83) were observed for the LLFDI limitation summary scale and subscales, whereas moderate ES (0.57-0.67) were noted for frequency dimension summary scale and subscales.
Table 2.
Responsiveness metrics over 2 years of follow-up*
| Frequency | Personal role | Social role | Limitation | Management role | Instrumental role | |
|---|---|---|---|---|---|---|
| Absolute ES | 0.63 | 0.67 | 0.57 | 0.81 | 0.75 | 0.83 |
| Decline in SRH ES (n=72) | −0.28 | −0.09 | −0.27 | −0.45 | −0.19 | −0.51 |
| Improvement in SRH ES (n=51) | −0.02 | 0.01 | −0.01 | 0.37 | 0.01 | 0.39 |
| Decline in SPPB ES (n=136) | −0.49 | −0.25 | −0.41 | −0.19 | −0.12 | −0.23 |
| Improvement in SPPB ES (n=149) | −0.15 | −0.04 | −0.17 | 0.12 | 0.02 | 0.12 |
| Decline in walking ES (n=116) | −0.41 | −0.33 | −0.30 | −0.19 | −0.13 | −0.21 |
| Improvement in walking ES (n=109) | −0.26 | −0.14 | −0.22 | 0.11 | 0.07 | 0.08 |
| SEM | 3.20 | 8.29 | 4.33 | 4.99 | 9.72 | 5.31 |
| MDC90 | 7.43 | 19.29 | 10.07 | 11.62 | 22.61 | 12.35 |
| Percent changing ≥MDC90 | 12.23 | 11.94 | 12.78 | 29.45 | 9.17 | 31.95 |
n=360 except where otherwise indicated
ES = effect size = (M2-M1)/Sb, where M2 is the mean score at year 2, M1 is the mean score at baseline, and Sb is the standard deviation at baseline; SRH = self-rated health; SPPB = Short Physical Performance Battery; SEM = standard error of measurement = Sb*√(1-r), where Sb is the standard deviation at baseline and r is the test-retest reliability coefficient; MDC90 = minimal detectable change with 90% confidence = SEM*1.645*sqrt2.
When we sub-categorized participants based on improvement in self-rated health, SPPB score and self-reported walking ability over 2 years, the LLFDI limitation scales demonstrated larger positive ES in all three categories compared with the disability frequency scales (Table 2). Larger negative ES were also noted for the limitation scales among those who declined in self-rated health, whereas the frequency dimension scales showed larger negative ES among those who declined in the functional criteria (SPPB and self-reported walking ability).
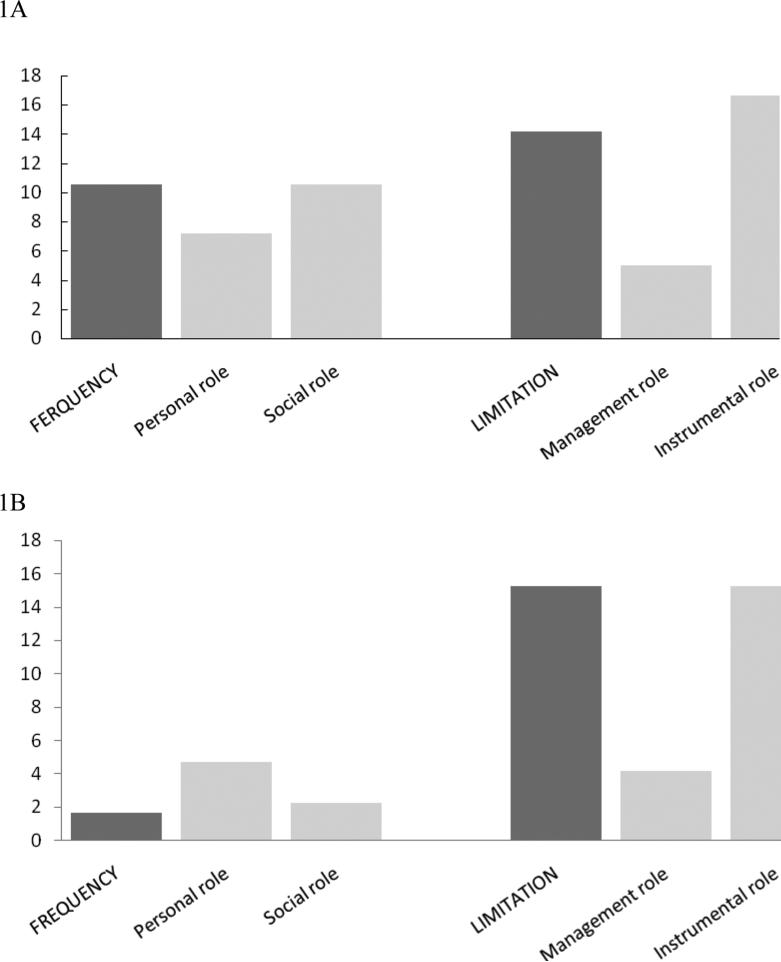
The percentages of participants demonstrating a change ≥ the MDC90 for each scale are shown in Table 2. Overall, the greatest proportion of participants with a meaningful change in disability in either direction was found for the limitation in instrumental role domain (32.0% changed ≥12.4 points). Figure 1 shows the proportion of participants with a decline (1A) or improvement (1B) on each LLFDI scale based on their individually determined MDC90 value in Table 2. The proportion of participants who declined ≥ the MDC90 was largest for the instrumental role and overall limitation domain (14.2-16.8%), followed by the overall frequency dimension and social role domain (both 10.56%) (Figure 1A). Improvement ≥ the MDC90 was highest for the overall limitation dimension and instrumental role domain (both 15.3%), with considerably less responsiveness for the other scales (all <5%) (Figure 1B).
Figure 1.
Comparison of percentages of participants demonstrating a decline (1A) or improvement (1B) ≥ the minimal detectable change (MDC90) over 2 years of follow-up on the Late-Life Function and Disability Instrument frequency and limitation dimensions and their subscales. For example, as shown in Figure 1B, 2% of participants improved ≥ the MDC90 of 7.4 points for the frequency dimension, compared to 15% of participants that improved ≥ the MDC90 of 11.6 points for the limitation dimension.
DISCUSSION
Our findings highlight the important distinction between frequency of and limitations in participation in major life activities for disability measurement. Choices about which component of multidimensional disability scales to use in geriatric research should be based on the objectives of the study and the best available evidence for a particular metric's psychometric properties.
To our knowledge this is the first study to evaluate the predictive validity of the disability component of the LLFDI. Our findings demonstrated that reported frequency of performing life activities, particularly personal tasks, had high predictive validity for low self-rated health, emergency room visits, and hospitalizations over 2 years. In contrast, reported limitation in performing major life roles had limited predictive value. This was an unexpected and noteworthy finding, especially since most generic disability measures are focused on what a patient “can do” and very few include questions regarding what a patient “does do”.17, 18 While there was a small ceiling effect with the limitation dimension (6.5% scored 100 at baseline), this alone is likely not enough to account for the contrasting results. As such, the frequency dimension of the LLFDI may prove a valuable indicator of disability for longitudinal investigations of the negative sequelae of the disablement process.
In a previous systematic review on the LLFDI,5 we noted that intervention studies (primarily exercise-based) more commonly evaluated the limitation dimension than the frequency dimension and that the former was associated with larger relative effect sizes. Therefore, our finding of larger overall ES estimates for the limitation dimension and subscales are in line with previous work. In addition, while both dimensions appeared to be responsive to decline in the external anchors of perceived health status and function, the limitation dimension, particularly the instrumental role, was also responsive to improvement. We found similar results when we considered the percentages of patients who improved/declined above the MDC90 values. Of note, previous reviews of studies examining the effect of exercise interventions for improving disability in older adults have shown conflicting results.19-21 It is possible that use of more responsive measures of disability would have resulted in more consistent conclusions; based on our findings, it appears that limitation in performing life tasks is more responsive to improvement than frequency of participation. This is perhaps not surprising, given that interventions that target changes in a person's capability are more easily achievable than changes in actual behavior.
This analysis should be interpreted in light of several limitations. Recall bias may have affected reporting of hospitalizations and emergency room visits. Our findings may not be generalizable to older adults residing outside the Boston area or those in other clinical settings. While we have presented MDC90 values for the LLFDI derived through distribution-based methods, we did not have a global measure of change in disability rated by the patient or clinician to use as a clinical anchor. This will be necessary to refine the estimation of the minimal clinically important difference for the LLFDI in future work.
In summary, we have shown that the frequency dimension of the LLFDI has high predictive validity for unfavorable outcomes and is responsive to decline over 2 years among community-dwelling older adults. We were unable to demonstrate the predictive validity of the limitation dimension; however, it appears this metric is responsive to both directions of change. These findings can be used to guide the selection of the most appropriate disability measure to address specific research questions in geriatric research.
ACKNOWLEDGMENTS
Funding: This work was supported by the National Institute on Aging (R01 AG032052-03). Marla Beauchamp is supported by a fellowship from the Canadian Institutes of Health Research; Jonathan Bean by a National Institutes of Health K24 award (1K24HD070966-01); and Alan Jette in part by the National Institute on Disability and Rehabilitation Research (H133P120001).
Sponsor's Role: The sponsors had no role in any aspect of the study design, methods, data collection, analysis or manuscript preparation.
Footnotes
Author Contributions: Marla Beauchamp was responsible for the conception and design, data analysis, interpretation of data, and drafting and revising the manuscript. Jonathan Bean contributed to the conception and design, data interpretation and revision of the manuscript. Rachel Ward contributed to the data analysis and interpretation and revision of the manuscript. Laura Kurlinski contributed to data collection and interpretation and revision of the manuscript. Nancy Latham contributed to the data interpretation and revision of the manuscript. Alan Jette contributed to the conception and design, interpretation of data and revision of the manuscript.
Paper Presentation: An abstract of this work was selected for oral presentation at the American Congress of Rehabilitation Medicine annual conference, October 2014 in Toronto, Canada.
Conflicts of Interest: Alan M. Jette has stock holdings in CREcare, LLC, a small business created to disseminate outcome instruments such as the LLFDI. The authors have no other conflicts of interest to declare.
REFERENCES
- 1.Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- 2.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 3.Nagi S. Disability concepts revisited: implications for prevention. In: Pope A, Tarlov A, editors. Disability in America: Toward a National Agenda for Prevention. National Academy Press; Washington, DC: 1991. pp. 309–327. [Google Scholar]
- 4.World Health Organization . International Classification of Functioning, Disability and Health. WHO; ICF. Geneva: 2001. [Google Scholar]
- 5.Beauchamp MK, Schmidt CT, Pedersen MM, et al. Psychometric properties of the Late-Life Function and Disability Instrument: a systematic review. BMC Geriatr. 2014;14:12. doi: 10.1186/1471-2318-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Arch Phys Med Rehabil. 2013;94:347–355. doi: 10.1016/j.apmr.2012.08.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 8.Fried LP, Bandeen-Roche K, Chaves PH, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 9.DeSalvo KB, Bloser N, Reynolds K, et al. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSalvo KB, Fan VS, McDonell MB, et al. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40:1234–1246. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy SE, Kang Y, Studenski SA, et al. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.The prevention of falls in later life. A report from the Kellogg International Work Group on the Prevention of Falls by the Elderly. Dan Med Bull. 1987;34(Suppl 4):1–24. [PubMed] [Google Scholar]
- 14.Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates Inc; Hillsdale, New Jersey: 1988. [Google Scholar]
- 16.Haley SM, Fragala-Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther. 2006;86:735–743. [PubMed] [Google Scholar]
- 17.Harwood RH, Rogers A, Dickinson E, et al. Measuring handicap: the London Handicap Scale, a new outcome measure for chronic disease. Qual Health Care. 1994;3:11–16. doi: 10.1136/qshc.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin O, Ayuso-Mateos JL, Almansa J, et al. Validation of the “World Health Organization Disability Assessment Schedule, WHODAS-2” in patients with chronic diseases. Health Qual Life Outcomes. 2010;8:51. doi: 10.1186/1477-7525-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keysor JJ, Jette AM. Have we oversold the benefit of late-life exercise? J Gerontol A Biol Sci Med Sci. 2001;56:M412–423. doi: 10.1093/gerona/56.7.m412. [DOI] [PubMed] [Google Scholar]
- 20.Liu CJ, Latham N. Can progressive resistance strength training reduce physical disability in older adults? A meta-analysis study. Disabil Rehabil. 2011;33:87–97. doi: 10.3109/09638288.2010.487145. [DOI] [PubMed] [Google Scholar]
- 21.Keysor JJ, Brembs A. Exercise: necessary but not sufficient for improving function and preventing disability? Curr Opin Rheumatol. 2011;23:211–218. doi: 10.1097/BOR.0b013e3283432c41. [DOI] [PubMed] [Google Scholar]