Abstract
Background
Hepatitis C infection (HCV) is associated with chronic inflammation; yet studies show greater IL-6 but lower CRP levels. We determined whether liver fibrosis severity and HCV replication affect the ability of IL-6 to stimulate production of CRP from the liver.
Methods
We used multivariable generalized linear regression to examine the association of HIV, HCV and transient elastography-measured liver stiffness (LS) with IL-6 and CRP in participants (164 HIV-monoinfected; 10 HCV-monoinfected; 73 HIV/HCV-coinfected; 59 neither infection) of the Women's Interagency HIV Study. Significant fibrosis was defined as LS>7.1 kiloPascals.
Results
IL-6 was positively correlated with CRP levels in all women, but CRP levels were lower in HCV-infected women (with and without HIV infection) at all levels of IL-6. HCV-infected women with fibrosis had nearly 2.7-fold higher IL-6 levels compared to controls (95% Confidence Interval [CI]:146%, 447%); HCV-infected women without fibrosis had IL-6 levels that were similar to controls. By contrast, CRP was 28% lower in HCV-infected women with fibrosis (95% CI:-55%, 15%) and 47% lower in HCV-infected women without fibrosis (95% CI:-68%,-12%). Among the HCV-infected women, higher HCV RNA levels were associated with 9% lower CRP levels per doubling (95% CI: -18%, 0%).
Conclusion
Liver fibrosis severity is associated with greater IL-6 levels, but the stimulatory effect of IL-6 on CRP appears to be blunted by HCV replication rather than by liver fibrosis severity. Investigation of the potential CRP rebound after HCV RNA eradication and persistent liver fibrosis on organ injury is needed.
Keywords: HIV, HCV, transient elastography, CRP, IL-6
Background
Coinfection with Hepatitis C virus (HCV) is associated with increased vascular disease in HIV-infected adults;[15, 2] inflammation is postulated as a key underlying mechanism.[3-5] C-reactive protein (CRP) is a widely used clinical marker of inflammation. The inflammatory marker interleukin-6 (IL-6) is a potent stimulator of CRP production in the liver and has also been associated with vascular disease and death,[6-8] but is generally not tested on a clinical basis. Because the liver is the primary source of CRP, its value as a marker of vascular risk and death has been questioned in the setting of HCV and HIV/HCV coinfection.
Studies show that HIV/HCV coinfection is associated with higher IL-6 but lower CRP levels compared to those with neither HIV nor HCV infection.[9, 10] Liver fibrosis severity and HCV replication are postulated mechanisms for the attenuated CRP level. The paucity of data describing the effect of liver fibrosis severity on IL-6 and CRP levels likely reflects the difficulties of pursuing liver biopsy in large numbers of patients. In addition, few studies have examined the association of HCV replication with IL-6 and CRP levels. While HCV replication is thought to exploit pathways involved in low density lipoprotein (LDL) metabolism,[11, 12] the effects of altered lipid metabolism on IL-6 and CRP levels is unclear. An understanding of the relationship between HCV infection, liver fibrosis severity, and inflammation is of clinical importance (because liver fibrosis may remain even after successful HCV treatment) and could shed light on potential mechanisms by which chronic HCV infection leads to extrahepatic injury.
We characterize levels of IL-6 and CRP in the setting of HIV and HCV infection, and determine whether liver fibrosis severity affects levels of inflammation. We used ultrasound-based transient elastography (TE) to assess liver fibrosis severity in a cohort of women with HCV infection (in the presence or absence of HIV), HIV monoinfection and neither HIV nor HCV infection. In HCV-infected women, we also investigated whether HCV viremia and/or alterations in lipid parameters affect IL-6 and CRP levels. We hypothesized that liver fibrosis severity and HCV replication would blunt CRP levels despite increased IL-6 levels resulting from chronic HIV/HCV infection.
Methods
Study Population
The Women's Interagency HIV Study (WIHS) is a prospective cohort that investigates the progression of HIV in women with and at risk for HIV. A total of 4,137 women (3,067 HIV-infected; 1,070 HIV-uninfected) were enrolled from six consortia across the U.S. (Bronx, NY; Brooklyn, NY; Chicago, IL; Los Angeles, CA; San Francisco, CA; and Washington DC) in either 1994-95 (n=2,623), 2001-02 (n=1,143), or 2011-2012 (n=371). Baseline socio-demographic characteristics and HIV risk factors were similar between HIV-infected and uninfected women.[13, 14] An institutional review board approved study protocols and consent forms, and each study participant gave written informed consent.
Every six months, participants complete a comprehensive physical examination and an interviewer-administered questionnaire, which collects information on demographics, disease characteristics, and specific antiretroviral therapy use, and provide biological specimens.
From November 2010 through October 2012, 381 women from the San Francisco, Chicago, and Washington DC sites agreed to participate in a TE substudy and underwent TE using the Fibroscan® (Echosens, Paris, FR). The TE substudy and Fibroscan® procedure have been previously described [15]. Women who were excluded were older (52 vs 48 years), had greater BMI (28 vs 26 kg/m2), greater waist circumference (93 cm vs 80 cm), and reported drinking more alcohol (59% vs 52%). However, the excluded and included participants had similar prevalences of HIV (45% vs. 51%) and HCV (28% vs. 23%). A total of 306 women (164 HIV+/HCV-, 10 HIV-/HCV+, 73 HIV+/HCV+, 59 HIV-/HCV-) were included in the analysis. Sixteen women who were HCV seropositive but HCV RNA undetectable were included in the HIV+/HCV- group.
Measurement of IL-6 and CRP
IL-6 and CRP were measured from frozen sera that were stored at -70 degrees Celsius. IL-6 was measured by high sensitivity enzyme-linked immunosorbent assay (Quantikine HS Human IL-6 Immunoassay, R&D Systems) (lower limit of detection: 0.11pg/ml. The intra-assay coefficient of variation (CV) was 7.4% and the inter-assay CV ranged from 6.5% to 9.6%. CRP was measured using the BNII nephelometer from Dade Behring (Deerfield, IL), which utilizes a particle-enhanced immunonephelometric assay (lower limit of detection: 0.17mg/L). The inter-assay CV for CRP ranged from 1.1% to 4%.
Covariates
Key covariates included HIV infection and HCV infection status (defined by documentation of a positive HIV EIA confirmed by Western blot and a positive HCV EIA confirmed by detectable HCV RNA, respectively). Other candidate covariates included liver fibrosis severity estimated using TE-measured LS; demographic factors (age, race/ethnicity), menopausal status, lifestyle factors [history of injection drug use, alcohol use (none; light drinking 1-15 g/day; moderate drinking 15-30 g/day; heavy drinking >30 g/day); marijuana use (none, occasional, daily), smoking (none, current, past)], body composition and metabolic factors [waist circumference, body mass index (calculated from height and weight in kg/m2), diabetes mellitus (defined by a confirmed elevated fasting glucose, elevated hemoglobin A1C ≥6.5%, or self- report of anti-diabetes medications), insulin resistance estimated using the homeostasis model assessment, and use of anti-diabetes medications]. In analyses among HIV-infected women, current CD4 cell count, nadir CD4 cell count, current HIV RNA level, history of clinical AIDS, and current use of highly active antiretroviral therapy (HAART) and antiretroviral therapy (ART) by class were assessed. In analyses among HCV-infected women, HCV RNA level and HCV genotype were also assessed. We also additionally adjusted for fasting lipids (HDL, LDL, triglycerides, and total cholesterol), apoplipoprotein B (Apo B), and use of lipid lowering therapy. In exploratory analyses, we controlled for liver enzymes (ALT, AST). Multiple imputation using the Markov chain Monte Carlo method was used to impute missing covariates [16].
Statistical analysis
We compared demographic and clinical characteristics among four groups: HIV-monoinfected, HCV-monoinfected, HIV/HCV-coinfected, and those with neither infection using the Kruskal-Wallis test for continuous variables and Fisher's exact test for categorical variables.
We used Spearman coefficients to determine correlations between continuous parameters in four groups: HIV-monoinfected, HCV-infected with significant fibrosis, HCV-infected without significant fibrosis, and those with neither infection. A LS value >7.1kilopascals (kPa) was used to define significant fibrosis (which corresponds to fibrosis stage 2 or greater in validated studies of HCV-infected patients).[17] We also tested an alternative cutoff of LS >9.3kPa which has been validated in a study of US injection drug users to distinguish histologic stage 0 – 1 fibrosis from stage 2 or higher fibrosis [18].
IL-6 and CRP were found to be right-skewed and showed evidence of heteroscedasticity, even after log transformation. Use of log-linear ordinary least squares in such circumstances has been shown to lead to biased estimates. We therefore used generalized linear regression with a log link function and pseudo-maximum likelihood estimator, using a robust variance estimator to adjust for potential overdispersion in the models.[19-21] The relative percent difference was then estimated to evaluate the association of HIV and HCV infection (stratified by significant fibrosis) with IL-6 and CRP levels.
To determine whether HIV monoinfection and HCV infection were independently associated with IL-6 and CRP, multivariable models were sequentially adjusted for (1) demographic, (2) lifestyle factors, (3) metabolic parameters, and (4) liver fibrosis severity. Age and race/ethnicity were retained in all models. We then constructed separate models in participants with (1) HCV infection (with and without HIV) and (2) HIV infection alone in order to examine the associations of demographic, lifestyle, metabolic risk factors, and liver stiffness severity within each group. HIV-related factors were included in models of HIV-infected women, and HCV-related factors were included in models of HCV-infected women. A stepwise regression with p-value of <0.10 or less was used for entry and retention; the candidate variables were selected based upon their hypothesized associations with inflammation.
All analyses were conducted using the SAS system, version 9.3 (SAS Institute Inc., Cary, NC).
Results
Population Characteristics
The characteristics of the 306 women included in this analysis, stratified by HIV and HCV status are shown in Table 1. Approximately two-thirds of all women were African-American with the largest proportion among the HCV-infected group. HCV-infected women were more likely than HCV-uninfected women to be older, menopausal and to report using tobacco, marijuana, or alcohol. BMI and waist circumference were not statistically different among the four groups, but HCV-infected women had lower ApoB and LDL than HCV-uninfected women. On the other hand, HIV-infected women appeared to have lower HDL and higher triglyceride levels than HIV-uninfected women. HCV-infected women had higher median LS values compared to HCV-uninfected women. Among the HIV-infected women, coinfection with HCV was associated with lower current and nadir CD4 cell counts, slightly higher HIV RNA levels, a history of AIDS, and a lower prevalence of HAART use. HIV/HCV-coinfected women had higher HCV RNA levels compared to HCV-monoinfected women. The HCV-monoinfected and HIV/HCV-coinfected women were combined in subsequent analysis, because there were only ten HCV-monoinfected women and these women had similar demographic, metabolic, and LS characteristics to the HIV/HCV-coinfected women.
Table 1. Demographic and clinical characteristics stratified by HIV and HCV infection status*.
| Parameter | HIV monoinfection (n = 164) |
HCV monoinfection (n = 10) |
HIV/HCV coinfection (n = 73) |
Controls (n = 59) |
p-value** |
|---|---|---|---|---|---|
| Demographic Factors | |||||
|
| |||||
| Age (years) | 46 (40, 52) | 55 (48, 58) | 53 (50, 57) | 42 (35, 49) | <0.0001 |
| Race | 0.27 | ||||
| Caucasian | 21% | 10% | 10% | 14% | |
| African-American | 61% | 90% | 77% | 66% | |
| Latino | 12 % | 0% | 11% | 12% | |
| Other | 6% | 0% | 3% | 9% | |
| Menopause | 37% | 70% | 77% | 31% | <0.0001 |
|
| |||||
| Behavioral factors | |||||
|
| |||||
| Current smoker | 35% | 80% | 67% | 48% | <0.0001 |
| Heavy alcohol use | 6% | 40% | 7% | 7% | 0.01 |
| Marijuana user | 23% | 60% | 30% | 31% | 0.06 |
|
| |||||
| Metabolic factors | |||||
|
| |||||
| BMI (kg/m2) | 25 (22, 29) | 26 (23, 30) | 26 (23, 29) | 27 (23, 29) | 0.43 |
| Waist circumference (cm) | 87 (79, 96) | 89 (82, 101) | 92 (81, 101) | 86 (81, 97) | 0.23 |
|
| |||||
| Lipid Profiles | |||||
|
| |||||
| Apo B (mg/dL) | 58 (46, 77) | 45 (38, 54) | 49 (41, 62) | 59 (46, 80) | 0.01 |
| LDL (mg/dL) | 99 (81, 122) | 73 (57, 97) | 79 (59, 105) | 97 (81, 129) | <0.0001 |
| HDL (mg/dL) | 56 (44, 68) | 64 (48, 85) | 53 (40, 70) | 62 (47, 78) | 0.19 |
| Total Cholesterol (mg/dL) | 178 (157, 204) | 167 (127, 183) | 160 (132, 184) | 182 (169, 209) | 0.0002 |
| Triglycerides (mg/dL) | 96 (70, 136) | 77 (72, 95) | 97 (83, 132) | 74 (51, 122) | 0.01 |
|
| |||||
| HCV-related factors | |||||
|
| |||||
| HCV-RNA (× 1000 IU/ml) | - | 799 (698, 7460) | 2315 (834, 3930) | - | <0.0001 |
| Liver Stiffness (kPa) | 4.3 (3.6, 5.4) | 6.0 (4.2, 9.7) | 7.1 (5.3, 10.4) | 4.4 (3.7, 5.3) | <0.0001 |
|
| |||||
| HIV-related factors | |||||
|
| |||||
| HIV-RNA (copies/ml) | 35 (20, 453) | - | 66 (20, 470) | - | 0.48 |
| CD4 cells/mm3) | 574 (360, 747) | - | 517 (326, 746) | - | <0.0001 |
| CD4 nadir (cells/mm3) | 228 (123, 331) | - | 185 (94, 293) | - | <0.0001 |
| History of AIDS | 33% | - | 49% | - | <0.0001 |
| HAART use | 84% | - | 81% | - | <0.0001 |
Data are presented as medians (IQR) or percentages
p-values are based on Wilcoxon rank sum test, chi-square test or Fisher's exact test.
Association of HIV monoinfection and HCV infection with IL-6 and CRP levels
IL-6 levels were highest in the HCV-infected women, while HIV-monoinfected women and uninfected controls had similar levels (median: 1.5 vs. 0.9 vs. 0.9pg/ml, respectively; p<0.0001; Figure 1). By contrast, CRP levels were lowest in the HCV-infected women, intermediate in the HIV-monoinfected women, and highest in the uninfected controls (median: 0.7 vs. 1.2 vs. 1.4mg/dL, respectively; p=0.03). We then performed multivariable analysis, which showed that HCV infection was associated with a 119% higher IL-6 level relative to controls (95% confidence interval [95%CI]:42%,237%) after adjustment for demographic, behavioral, and clinical risk factors. By contrast, HCV infection was associated with 43% lower CRP levels (95%CI:-62%,-14%) compared to controls after multivariable adjustment. HIV monoinfection was associated with a 15% higher IL-6 level (95%CI:-22%,70%), but 17% lower CRP level (95%CI:-40%,15%) relative to controls. In multivariable models that included both IL-6 and CRP, after controlling for levels of IL-6, HCV remained associated with 54% lower CRP in those with fibrosis (95%CI:-71%,-25%) and 47% lower CRP in those without fibrosis (95%CI:-66%,-16%), while HIV-monoinfected women had a 20% lower CRP (95%CI:-41%,9%) compared to controls.
Figure 1. Unadjusted and Adjusted Association of HIV monoinfection and HCV infection with IL-6 and CRP levels.
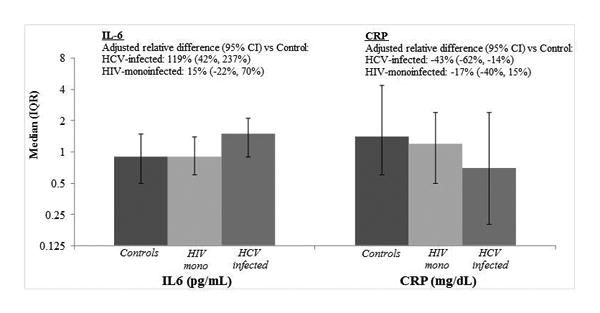
Association of HCV infection and liver fibrosis with IL-6 and CRP
In order to understand the contributions of HCV and liver fibrosis to IL-6 and CRP levels, we next stratified HCV-infected women according to the presence or absence of significant fibrosis (i.e. LS>7.1 kPa). Figure 2 shows the association of HCV infection, either with or without significant fibrosis, and HIV monoinfection with IL-6 and CRP levels relative to controls. HIV-monoinfected women and controls were not stratified by the presence or absence of significant fibrosis, because few met criteria for significant fibrosis [n=18 (11%) for HIV and n=6 (10%) for controls]. After multivariable adjustment, HCV-infected women with fibrosis had a 2.7-fold higher IL-6 level compared to controls (95%CI:146%,447%), while HCV-infected women without fibrosis and HIV-infected women had a 12% (95%CI:-31%,80%) and 7% (95%CI:-26%,57%) higher IL-6 level, respectively compared to controls, neither of which was statistically significant. By contrast, levels of CRP were 28% lower in HCV-infected women with fibrosis (95%CI:-55%,15%) and 47% lower in HCV-infected women without fibrosis (95%CI:-68%,-12%) compared to controls, while HIV-monoinfected women had 12% lower CRP (95%CI:-37%,21%) compared to controls. Results were similar when we defined significant fibrosis by LS >9.3kPa (data not shown).
Figure 2.
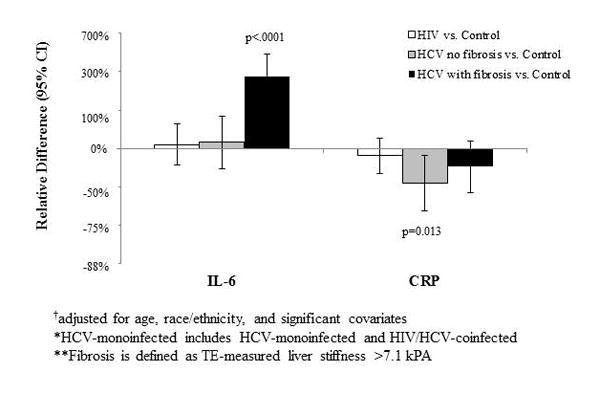
Multivariable-adjusted*† association of HIV monoinfection and HCV infection (by fibrosis status) with IL6 and CRP.
Relationship of IL-6 and CRP levels according to HIV and HCV infection status
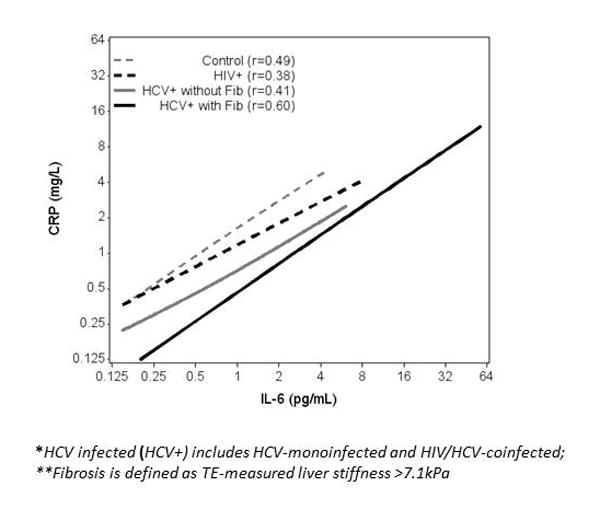
IL-6 was positively correlated with CRP, irrespective of HIV and HCV infection status with Spearman correlations ranging from r=0.4 to r=0.6 (Figure 3). However, the intercept was shifted downward in the HCV-infected women, so for any given IL-6 level, the CRP level was significantly lower than in the HIV-monoinfected and the control women (p<0.001). Among the HCV-infected women with fibrosis, CRP levels appeared to be lower at all levels of IL-6 compared to HCV-infected women without fibrosis, but the difference was not statistically significant (p=0.63). When the eighteen HIV-monoinfected women with significant fibrosis and the six control women with significant fibrosis were excluded, the Spearman correlations and p-values did not change.
Figure 3. Correlation of IL-6 with CRP in controls, HIV-monoinfected, and HCV-infected* with and without fibrosis**.
Factors associated with IL-6 and CRP levels among HCV-infected women
Table 2 shows factors associated with IL-6 and CRP in the HCV-infected women. In unadjusted analysis, higher HCV RNA level was associated with 20% lower IL-6 level (per HCV RNA doubling) and LS was associated with 31% higher IL-6 level per doubling, although the latter association did not reach significance. After multivariable adjustment, the association of HCV RNA weakened (16% lower) and was no longer statistically significant, while the association of greater LS with higher IL-6 levels strengthened (34% higher) and was significant. When examining the factors associated with CRP, in unadjusted analysis, greater waist circumference (per 10 cm increase) was associated with 4% higher CRP and greater LS was associated with 33% higher CRP levels, while per doubling of HCV RNA was associated with 7% lower CRP levels that did not reach significance. After multivariable adjustment, waist circumference remained strongly associated with 4% higher CRP levels, while LS weakened (21% lower) and was no longer significantly associated. By contrast, each doubling of HCV RNA level was significantly associated with 9% lower CRP levels. Additional adjustment for lipid factors did not show an association of LDL with IL6 (-28% per doubling; 95%CI:-48%, 29%) or CRP levels (-16% per doubling;95%CI:-49%,37%); greater HDL was associated with lower IL6 (-13% per doubling; 95%CI:-62%,98%) and lower CRP (-44%; 95%CI:-67%,-5%).
Table 2. Factors associated with IL-6 and CRP in the 83 HCV-infected women.
| All HCV-infected women | ||||
|---|---|---|---|---|
|
| ||||
| IL-6 | CRP | |||
|
| ||||
| Unadjusted | Adjusted* | Unadjusted | Adjusted** | |
| % difference (95%CI), p-value | % difference (95%CI), p-value | |||
| Demographic Factors | ||||
| Age (per decade) | 16% (-34%, 101%) p=0.61 |
23% (-18%, 84%) p=0.31 |
37% (-27%, 126%) p=0.22 |
3% (-38%, 73%) p=0.90 |
| Black (vs. Caucasian) | 99% (-49%, 674%) p=0.319 |
54% (-37%, 279%) p=0.343 |
39% (-52%, 301%) p=0.544 |
40% (-46%, 264%) p=0.495 |
| Hispanic (vs. Caucasian) | 36% (-76%, 669%) p=0.731 |
71% (-48%, 466%) p=0.374 |
11% (-72%, 351%) p=0.879 |
18% (-67%, 329%) p=0.797 |
| Metabolic Factors | ||||
| Waist Circumference (per 10cm) | 2% (-1%, 4%) p=0.14 |
0% (-2%, 3%) p=0.74 |
4% (2%, 6%) p<0.0001 |
4% (2%, 6%) p<0.0001 |
| HCV-Related Factors | ||||
| Liver Stiffness (per doubling) | 31% (-1%, 74%) p=0.06 |
34% (8%, 66%) p=0.009 |
33% (4%, 70%) p=0.02 |
21% (-6%, 57%) p=0.141 |
| HCV RNA (per doubling) |
-20% (-27%, -12%) p<0.0001 |
-16% (-30%, 1%) p=0.06 |
-7% (-26%, 3%) p=0.17 |
-9% (-18%, 0%) p=0.05 |
Outcomes are log-transformed; results are back-transformed to produce estimated relative differences in inflammatory marker attributable to each factor. Estimates are from multivariable Poisson regression models.
Factors associated with IL-6 and CRP levels among HIV-infected women
Among the HIV-monoinfected women, there was little association of any demographic, behavioral, metabolic, liver or HIV-related factor with IL-6 levels in unadjusted and adjusted analysis (Table 3). By contrast, in unadjusted analysis, current smoking was associated with 91% higher CRP levels and marijuana use was associated with 113% higher CRP levels. Among the HIV-related factors, having an undetectable HIV RNA was associated with 32% lower CRP levels and per doubling of CD4 cell count was associated with 39% higher CRP levels. After multivariable adjustment, having an undetectable HIV RNA was non-significantly associated with 17% lower CRP levels and per doubling of CD4 cell count was associated with 29% higher CRP levels. There was little association of liver stiffness with CRP levels (-3% per doubling; 95%CI:-32%, 37%).
Table 3. Factors associated with IL-6 and CRP in the 164 HIV-monoinfected women.
| HIV-monoinfected Women | ||||
|---|---|---|---|---|
|
| ||||
| 1L-6 | CRP | |||
|
| ||||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| % difference (95%CI), p-value | % difference (95%CI), p-value | |||
| Demographic Factors | ||||
| Age (per decade) | 4% (-8%, 17%) p=0.54 |
4% (-8%, 18%) p=0.49 |
5% (-14%, 28%) p=0.64 |
6% (-13%, 29%) p=0.57 |
| Black (vs. Caucasian) | 1% (-24%, 34%) p=0.934 |
-1% (-26%, 34%) p=0.96 |
-5% (-39%, 49%) p=0.84 |
-16% (-46%, 30%) p=0.43 |
| Hispanic (vs. Caucasian) | -11% (-41%, 35%) p=0.585 |
-23% (-43%, 32%) p=0.52 |
-40% (-72%, 26%) p=0.65 |
-36% (-68%, 27%) p=0.20 |
| Behavioral Factors | ||||
| Current Smoking | 12% (-11%, 41%) p=0.34 |
14% (-11%, 46%) p=0.30 |
91% (34%, 172%) p<0.0001 |
62% (11%, 135%) p=0.01 |
| Marijuana Use | -16% (-37%, 11%) p=0.22 |
-22% (-42%, 5%) p=0.10 |
113% (48%, 207%) p<0.0001 |
86% (28%, 169%) p=0.001 |
| HIV-Related Factors | ||||
| Undetectable HIV RNA | 3% (-18%, 29%) p=0.81 |
-2% (-23%, 24%) p=0.84 |
-32% (-53%, 0%) p=0.05 |
-17% (-42%, 20%) p=0.33 |
| Current CD4 count (per doubling) | -4% (-9%, 1%) p=0.15 |
-5% (-11%, 1%) p=0.11 |
39% (12%, 73%) p=0.003 |
29% (5%, 58%) p=0.01 |
Outcomes are log-transformed; results are back-transformed to produce estimated relative differences in inflammatory marker attributable to each factor. Estimates are from multivariable Poisson regression models.
Discussion
In our study of women with HIV, HCV, and neither infection, we report several important findings that elucidate the relationship of HIV, HCV, and liver fibrosis severity with levels of IL-6 and CRP. We found that the expected positive correlation between IL-6 and CRP was preserved regardless of infection status. However, CRP levels were significantly lower for any given IL-6 level in HCV-infected women, suggesting attenuation of the stimulatory effect of IL-6 on CRP in the presence of HCV infection. We also found that HCV-infected women with significant liver fibrosis had 2.7-fold greater IL-6 levels compared to uninfected controls; but in the absence of significant fibrosis, IL-6 levels did not appear to be substantially elevated. By contrast, HCV-infected women had a lower CRP when compared to uninfected controls. Furthermore, among HCV-infected women, higher HCV RNA was associated with lower CRP levels. Taken together, our findings suggest that there is a strong association between HCV-related liver fibrosis and higher IL-6 levels, but HCV replication (and not liver fibrosis) likely blunts the stimulatory effect of IL-6 on CRP, resulting in relatively lower CRP levels.
Our finding that HIV/HCV-coinfected women had greater IL-6 and lower CRP levels is consistent with a study by Salter et al in HCV-infected (with and without HIV infection) and uninfected adults [9]. That study also examined the association of liver markers (albumin, AST, and ALT) with CRP, but not with IL-6. Our study extends the findings of that study by examining the complex inter-relationship of IL-6, CRP, and liver fibrosis severity (using direct measurement of liver stiffness by TE) in women with HIV monoinfection, HCV infection (with and without HIV infection) and those with neither infection.
First, we demonstrated preservation of the relationship of higher IL-6 with higher CRP in all groups. However, among the HCV-infected women, the IL-6 associated increase in CRP was blunted compared to those without HCV infection, suggesting an altered response to IL-6 stimulation that is specific to HCV infection. Second, we found that the association of HCV infection (in the absence or presence of HIV) with higher IL-6 levels is likely driven by the presence of liver fibrosis. Our findings are corroborated by studies [22, 23] of HIV-infected adults at risk for liver disease that showed that advanced fibrosis/cirrhosis measured by indirect serum fibrosis markers was associated with higher IL-6 levels. However, we showed that in the absence of fibrosis, there was little difference in IL-6 level between HCV-infected and control women, supporting a causal role of HCV-associated liver fibrosis with IL-6 levels.
By contrast, we found little role for liver fibrosis in the association of HCV with CRP. Salter et al also found little association of liver enzymes and markers of liver function with lower CRP [9], as did another small study of HIV/HCV-coinfected adults, but this latter study did not find a correlation of liver stiffness with either IL-6 or CRP [24]. Interestingly, in our study, HCV-infected women without fibrosis appeared to have a 47% lower CRP levels compared to the 28% lower CRP levels in those with fibrosis, suggesting that in the absence of fibrosis, the blunting effect of HCV infection on CRP production are not opposed by the stimulatory effect of IL-6 (observed in the presence of fibrosis). Our finding provides evidence for the role of HCV infection itself and not liver fibrosis on CRP levels.
We also found that HCV viremia was associated with lower CRP levels. These findings are consistent with the Salter et al study [9], although another study [25] did not find an expected increase in CRP level after reductions in HCV RNA level with HCV treatment, suggesting that other HCV-related factors may contribute to the lower CRP levels. The phenomenon of “immune tolerance” has been proposed as a mechanism by which HCV replication lowers CRP, [26] where the local host immune response becomes altered or refractory to continued HCV replication in hepatocytes. HCV replication may be associated with depressed IL-6 levels and in turn depressed stimulation of CRP production. While we found that HCV replication was associated with lower IL-6 levels in unadjusted analysis, we demonstrated that further adjustment for IL-6 in models of CRP in the HCV-infected women strengthened the association of HCV RNA with lower CRP levels, suggesting that the relationship of HCV RNA with lower CRP levels is independent of IL-6. We also examined whether HCV-associated alterations in lipid metabolism might play a role, as studies have shown that HCV is associated with lower LDL levels [27, 28]. We did not find an association of serum lipid and lipoproteins with CRP levels. Further study is needed to determine how HCV viral replication might inhibit CRP production.
Similar to findings in the general population [29, 30], we found that waist circumference, a marker of visceral obesity, was associated with higher CRP levels in HCV-infected women. Among the HIV-monoinfected women, current smoking and marijuana use were associated with higher CRP levels, as was a higher CD4 count. Smoking has been associated with higher CRP levels [10]; whether or not there is a direct association of marijuana with higher CRP levels is unclear. Although unexpected, the association of higher CD4 could represent a restoration to health, and thus restoration of fat stores and higher CRP levels.
Finally, consideration of our findings within the broader purview of studies of CRP and IL-6 in HIV and HCV infection is important. In our cohort of women, median CRP levels were 46–74% and 24% lower than those reported in other cohort studies of HIV-infected women [10], [31] and HIV/HCV-infected women [10], respectively. Our study was conducted in the era of potent antiretroviral therapy, which is reflected in high median CD4 counts (>500 cells/ml) and could explain the lower IL-6 and CRP levels than reported in other studies. We also observed that the median CRP level in control women was slightly higher than in HIV-infected women, while IL-6 levels were comparable between the HIV-monoinfected and control women. Uninfected women in the WIHS are enrolled based upon having similar high-risk behaviors as HIV-infected women.
Our study has several limitations. First, the cross-sectional design did not allow us to examine causal relationships. Second, we had few HCV-monoinfected women, but their demographic and metabolic characteristics were similar to the HIV/HCV-coinfected women. This allowed us to pool the two groups and increase our sample size of HCV-infected women. Third, our results may not generalize to men. Fourth, women with greater adiposity were more likely to be excluded, a factor which has been associated with both inflammation and liver fibrosis. This exclusion may have reduced our ability to detect associations by limiting the spectrum of inflammation and fibrosis. However, we were able to find differential and independent associations of HCV with inflammation. Future studies using the Fibroscan XL probe are needed in order to study liver fibrosis severity in those with obesity. Fifth, it is plausible that serum IL-6 and CRP levels may not accurately reflect the hepatic milieu (although peripheral IL-6 and CRP levels may be more relevant in terms of risk stratification). Finally, we recognize that the relationship of HCV and markers of inflammation is complex, given that some biomarkers are synthesized in the liver or may be more affected by HIV replication. A composite measure of multiple elevated biomarkers has been examined in the setting of HIV and HCV infection and found to be associated with HIV and HCV viremia [22]. Future study of other markers of inflammation is needed in order to understand the complex relationship of HCV infection with inflammation and activation.
In conclusion, we found that liver fibrosis severity is associated with greater IL-6 levels, but the stimulatory effect of IL-6 on CRP is blunted by HCV replication (and not liver fibrosis anti-HCV therapies. Such therapies have shown promise in eradicating HCV infection, but may not completely resolve liver fibrosis. Studies are needed in the new HCV treatment era to see if pre-existing liver fibrosis, IL-6 and the presumed rebound in CRP levels after HCV cure will be associated with increased vascular events, as well as other organ and tissue injury affected by chronic HCV infection.
Acknowledgments
Financial Support: The Women's Interagency HIV Study (WIHS) is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) (U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). The study was also supported by the UCSF Liver Center National Institute of Health [P30 DK026743] and by the National Institute of Allergy and Infectious Diseases [K24 AI 108516 and R01 AI 087176, which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, CA]. Data in this manuscript were collected by the WIHS.
Footnotes
Conflict of interest: The authors do not have any commercial or other associations that might pose a conflict of interest.
Author Contributions: Shailja Shah: wrote 50% of the paper
Yifei Ma: performed the analysis, critical review of the manuscript
Rebecca Scherzer: oversaw the analysis, critical review of the manuscript
Greg Huhn: critical review of the manuscript
Audrey French: critical review of the manuscript
Michael Plankey: critical review of the manuscript
Marion Peters: critical review of the manuscript
Carl Grunfeld: critical review of the manuscript
Phyllis C. Tien: critical review of the manuscript, analysis, and wrote 50% of the paper
References
- 1.Freiberg MS, Chang CC, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4:425–432. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11:462–468. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 3.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. Aids. 2009;23:1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail. 2009;15:451–456. doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira CP, Kappel CR, Siqueira ER, Lima VM, Stefano JT, Michalczuk MT, et al. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol. 2013;164:221–226. doi: 10.1016/j.ijcard.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One. 2012;7:e34218. doi: 10.1371/journal.pone.0034218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 9.Salter ML, Lau B, Mehta SH, Go VF, Leng S, Kirk GD. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr. 2013;64:488–495. doi: 10.1097/QAI.0b013e3182a7ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arciello M, Petta S, Leoni V, Iannucci G, Labbadia G, Camma C, et al. Inverse correlation between plasma oxysterol and LDL-cholesterol levels in hepatitis C virus-infected patients. Dig Liver Dis. 2012;44:245–250. doi: 10.1016/j.dld.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacon MC, Von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clinical and diagnostic laboratory immunology. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The women's interagency HIV study. Epidemiology. 1998:117–125. [PubMed] [Google Scholar]
- 15.Bailony MR, Scherzer R, Huhn G, Plankey MW, Peters MG, Tien PC. Association of HIV infection, hepatitis C virus infection, and metabolic factors with liver stiffness measured by transient elastography. J Infect Dis. 2013;208:1776–1783. doi: 10.1093/infdis/jit357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilks WR, Richardson S, Spiegelhalter DJ. Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. [Google Scholar]
- 17.Vergara S, Macías J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D, et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clinical infectious diseases. 2007;45:969–974. doi: 10.1086/521857. [DOI] [PubMed] [Google Scholar]
- 18.Kirk GD, Astemborski J, Mehta SH, Spoler C, Fisher C, Allen D, et al. Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin Infect Dis. 2009;48:963–972. doi: 10.1086/597350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 20.Santos Silva J, Tenreyro S. The Log of Gravity. The Review of Economics and Staitistics. 2006;88:641–658. [Google Scholar]
- 21.Santos Silva J, Tenreyro S. On the Existence of the Maximum Likelihood Estimates in Poisson Regression. Economics Letters. 2010;107:310–312. [Google Scholar]
- 22.Armah KA, Quinn EK, Cheng DM, Tracy RP, Baker JV, Samet JH, et al. Human immunodeficiency virus, hepatitis C, and inflammatory biomarkers in individuals with alcohol problems: a cross-sectional study. BMC Infect Dis. 2013;13:399. doi: 10.1186/1471-2334-13-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuster D, Tsui JI, Cheng DM, Quinn EK, Armah KA, Nunes D, et al. Interleukin-6 is associated with noninvasive markers of liver fibrosis in HIV-infected patients with alcohol problems. AIDS Res Hum Retroviruses. 2013;29:1110–1116. doi: 10.1089/aid.2012.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charpentier C, Champenois K, Gervais A, Landman R, Joly V, Le Gac S, et al. Predictive value of liver enzymes and inflammatory biomarkers for the severity of liver fibrosis stage in HIV/HCV co-infected patients. PLoS One. 2013;8:e59205. doi: 10.1371/journal.pone.0059205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalabay L, Nemesanszky E, Csepregi A, Pusztay M, David K, Horvath G, et al. Paradoxical alteration of acute-phase protein levels in patients with chronic hepatitis C treated with IFN-alpha2b. Int Immunol. 2004;16:51–54. doi: 10.1093/intimm/dxh024. [DOI] [PubMed] [Google Scholar]
- 26.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 27.Floris-Moore M, Howard AA, Lo Y, Schoenbaum EE, Arnsten JH, Klein RS. Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS. 2007;21:479–491. doi: 10.1089/apc.2006.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheeler AL, Scherzer R, Lee D, Delaney JA, Bacchetti P, Shlipak MG, et al. HIV/hepatitis C virus coinfection ameliorates the atherogenic lipoprotein abnormalities of HIV infection. AIDS. 2014;28:49–58. doi: 10.1097/QAD.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009;32:1734–1736. doi: 10.2337/dc09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura H, Ito H, Egami Y, Kaji Y, Maruyama T, Koike G, et al. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res Clin Pract. 2008;79:330–336. doi: 10.1016/j.diabres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Dolan SE, Hadigan C, Killilea KM, Sullivan MP, Hemphill L, Lees RS, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]


