Abstract
Defects in antigen presenting cell function have been implicated in glioma immunosuppression. We measured peripheral CCL22, a dendritic cell/macrophage derived T cell trafficking chemokine, in sera from 1,208 glioma cases and 976 controls to assess whether it might provide a biomarker of glioma risk, survival, and immune dysfunction. Cluster models were used to examine the relationship between CCL22 and glioma risk. Patient survival was assessed using Cox regression models. We also examined the relationship between CCL22 levels and CD4 cell counts, as well as allergy history and IgE levels. CCL22 levels were significantly lower among glioma cases compared to controls (Mean±SEM: 1.23±0.03 ng/mL in cases versus 1.60±0.03 ng/mL in controls, P<0.0001), and this difference remained significant even after controlling for other covariates in the cluster models (highest quartile versus lowest Odds Ratio=0.21, P<0.0001). CD4 cell counts were positively correlated with CCL22 in glioma cases (Spearman r2=0.51, P<0.01) and were significantly lower in cases compared with controls. Higher CCL22 levels were associated with longer survival in all cases combined and in GBM cases (hazard ratioallcases=0.81; 95% CI: 0.72–0.91, P=0.0003). CCL22 levels were not associated with IgE level or self-reported allergies. Circulating CCL22 levels are related to both glioma risk and survival duration independent of age, histology, grade and IDH mutation status. CCL22 should be considered a marker of immune status with potential prognostic value.
Keywords: glioblastoma, T cells, CCL22, cytokines, survival
Introduction
Gliomas are the most common form of primary brain tumor with an incidence rate of approximately 6.5 individuals diagnosed per 100,000 in the United States1. Traditional histopathological criteria, age, and some tumor markers are currently used to assess glioma patient prognosis2, 38, 9. Survival for patients diagnosed with glioblastomas (World Health Organization (WHO) grade IV) is poor with a median survival of 14.6 months4. The median survival of grade III astrocytoma patients is in the range of 2 to 3 years while those with grade III oligodendrogliomas have a median survival of 3 to 6 years4. Patients who are younger at diagnosis and who have IDH mutated tumors also have a more favorable survival4. The standard therapies for high grade glioma, including surgery, temozolomide (TMZ) chemotherapy and radiation, have been shown to improve survival, although modestly4. Important molecular subsets of glioma also are associated with heritable risk5–7. It has also been recognized for many years that glioma patients, particularly GBM patients, show peripheral immune defects affecting T cells10, 11. Lymphocyte counts (particularly CD4 cells) in glioma patients are reduced and T-cell function is also suppressed, with impaired proliferation in response to IL2 and nonspecific mitogens12. A study of patients with high-grade gliomas (grades III and IV) treated with radiation and TMZ showed that after 2 months of treatment about 40% of patients had CD4 blood counts less than 200 cells/µL13. These patients continued to have low CD4 counts for the full year of follow-up and exhibited early mortality from tumor progression13. Other studies also have reported that low CD4 counts were associated with poorer glioma survival times14, 15. More generally, CD4 lymphopenia has been shown to be a grave prognostic indicator in multiple types of cancers16–21. Although the etiology of depressed T cell immunity in glioma is complex; exposures to therapeutic steroids, chemotherapy and ionizing radiation as well as tumor generated immune suppression are thought to be involved12, 13. One important aspect of glioma related suppression of the immune response is the effect that tumors may have on professional antigen presenting cells (APC) and their crosstalk with T cells15, 22, 23. For example, circulating peripheral blood monocytes in patients with GBM have increased expression of the suppressive B7-H1 protein and promote T cell apoptosis24; this suppressive phenotype was induced by exposing monocytes to glioma cell lines and supernatants. Glioma cells exposed to direct contact with blood monocytes were shown in an earlier study to induce a suppressive phenotype25. A crucial link between macrophage/dendritic cells (DC) and T cell immunity is the macrophage-derived chemokine CCL2226. CCL22 is a CC type chemokine and a potent chemoattractant for CD4 and CD8 T cells, as well as for DCs expressing the CCL22 receptor CCR4. It is involved in chronic inflammation mediated by the continuous homing of DCs and lymphocytes27, 28. In this study, we hypothesized that the chemokine CCL22 may be a biomarker of macrophage/DC compartment of immune suppression and provide relevant information about anti-tumor responses elicited by the host that may be negatively impacted by tumor cells or immunosuppressive treatments. We show that reduced levels of serum CCL22 are common in glioma patients and associated with low CD4 cell counts and shorter survival times. This suggests CCL22 may be a useful marker of a suppressed immune function.
Material and methods
Study participants and interview
All cases were adults with newly diagnosed histologically confirmed glioma (International Classification of Disease for Oncology, morphology codes 9380–9481). Population-based cases residing in the six Bay Area counties were ascertained using the Cancer Prevention Institute of California’s early case ascertainment system during three recruitment periods: May 1997 to August 1999, November 2001 to September 2005, and September 2006 to September 2009. Clinic-based cases diagnosed between 2002–2006, 2006–2010, and 2009–2012 were recruited from the UCSF Neuro-oncology Clinic, regardless of place of residence. Cases enrolled in the 2009–2012 clinic-based recruitment were eligible to participate if they were 18 years or older at diagnosis, and cases recruited in the earlier years of the study were eligible to participate if they were 20 years and older at diagnosis.
Population-based controls from the same residential area as the population-based cases were identified using random digit dialing and were frequency matched to population-based cases on age group, gender and ethnicity. Clinic-based controls age 18 years and older were recruited from the UCSF phlebotomy clinic between 2010–2012 and frequency matched to clinic-based cases on age group, gender and ethnicity. Informed consent was obtained from all study participants and study methods were approved by the Committee on Human Research at the University of California, San Francisco.
Pathological material was retrieved, when possible, for all resected brain cancers and reviewed and classified by one of two neuropathologists (Kenneth Aldape, MD Anderson, Houston, TX and Tarik Tihan, UCSF, San Francisco, CA). Blood and serum samples were usually collected at the time of interview. Allergy history data were collected in tabular form as described in detail in our earlier report37, 38. All participants who provided a blood sample were administered an additional questionnaire at the time of blood draw about current and recent medications and treatments. IDH mutation was measured in cases with tumor tissue available as previously described9.
Measurement of serum CCL22 levels
The Luminex assay was developed using a standard sandwich capture format. Capture antibody to human CCL22 (DY336, Part 840498, R&D system, Minneapolis) was coupled to magnetic Luminex microspheres by using a two-step carbodiimide reaction. Serum samples were diluted 1:100 in sample diluent, which was a mixture of PBS, 10% (vol/vol) fetal bovine serum, and 2.5% (vol/vol) CBS-K (Millipore Corporation, Hayward, CA). The solution was then incubated at room temperature for 1 hour on a shaker. A standard curve was created by diluting human CCL22 (DY336, Part 840500, R&D system, Minneapolis) using the same sample diluent. The CCL22 standards or participant serum samples and coupled CCL22 microspheres were then incubated for 2 hours at room temperature on a shaker using a 96-well flat bottom plate (Bio-Rad Laboratories, Inc., Hercules, CA) and subsequently washed with the wash buffer (Bio-Rad Laboratories, Inc.). This step was followed by the addition of 25 µL of 1:200 diluted (45ng/mL) biotinylated anti-human CCL22 antibody (DY336, Part 840499, R&D system, Minneapolis) to each well and the incubation of the mixture at room temperature for 30 minutes on a shaker. The solution was then washed and treated with 50 µL of streptavidin-conjugated R-phycoerythrin 1:100 diluted stock (Bio-Rad Laboratories, Inc.). After a 10 minutes incubation and final wash, the microspheres were resuspended in 105 µL of assay buffer (Bio-Rad Laboratories, Inc.). The amount of CCL22 bound to the microspheres by this antibody sandwich technique was determined by the median fluorescence intensity (MFI) of the reporter molecules, phycoerythrin, using the Bio-plex 200 plate reader system. The MFI of the unknown serum sample was then converted into a picograms-per-milliliter value based on the known concentrations of the standard curve by using a five-parameter (5PL) regression formula. Each sample was run with a replicate. A single serum sample from a person without a brain tumor was repeated on some of the assay plates (13 out of 24). We performed standard addition experiments that yielded a recovery rate of 80%.
Flourescence-activated cell sorting (FACS) analysis of CD4 cells levels and IgE analysis
We measured CD4 cell levels on 47 clinic-based cases and 146 clinic-based controls recruited from the 2009–2012 clinic-based series of the study. The blood samples were directly stained with anti-human CD4 APC antibody (eBioscience, San Diego, cat #17-0048-41), anti-human CD45 PerCP-Cy5.5 antibody (eBioscience, San Diego, cat #45-0459-41), and anti-human CD3 FITC antibody (eBioscience, San Diego, cat #11-0038-41). CD45 and CD3 were added to provide reference for CD4 values. After staining the blood was incubated for 20 minutes in the dark at 4°C. Cal-Lyse Solution (Invitrogen, Camarillo, cat #GAS-010) was then added to lyse red blood cells and fix the cells. Flow cytometry counting beads were added for absolute quantification of CD4 cells. Flow cytometry was performed within 48 hours of blood draw on the FACSCalibur (Becton Dickinson, San Jose) flow cytometer using Cell-Quest (Becton Dickinson, San Jose) software. Analysis of flow data was done using Flowjo software (TreeStar Inc, Ashland).
IgE levels were assessed using Pharmacia Diagnostics UniCAP fluorescent “sandwich” assay as described previously29. IgE levels were determined using serum derived from the same blood draw as used for the CCL22 analysis. Total IgE was determined by measuring fluorescence against the standard curve with known quantity inputs as previously described30.
Statistical methods
Statistical analyses were conducted using SAS v9.3 (SAS Institute, Cary NC). Odds ratios for glioma cases versus controls were computed using a multivariable cluster analysis model (Proc Genmod) which controlled for variation within the CCL22 batches. Models were adjusted for age, gender, ethnicity (white/nonwhite), education (college education yes/no) and smoking history (ever/never). Analyses were conducted separately for all gliomas and by histological subtype (GBM versus non-GBM). For the case/control comparisons, CCL22 values were categorized into quartiles based on the distribution among controls. Total IgE was analyzed both as a log-transformed continuous variable and for comparison with earlier studies, as a categorical variable with groups defined based on clinically relevant cut points (IgE > 100 kU/ L = “elevated”, 25–100 kU/L = “borderline” and < 25 kU/L = “normal”). Cox proportional hazard models were run to evaluate the association between CCL22 levels and survival in glioma cases. Survival models controlled for age at diagnosis, race (white/non-white), gender, smoking history (ever/never), college degree, number of days between diagnosis and blood draw (continuous), Dexamethasone use at blood draw, and first course of treatment (Temozolomide or other chemotherapy use, radiation, and extent of surgery (biopsy only vs. any resection)). Survival was measured by calculating the total number of days between the date of diagnosis and date of death (if deceased) or last follow-up (if alive or lost to follow-up). Cases who were alive at the last follow-up or were lost to follow-up were treated as censored in the analysis. For the survival models, we compared the upper three quartiles of CCL22 to the lowest quartile since the survival experience for cases in the upper three quartiles was very similar (Supplemental Figure 1).
Results
Our study consisted of 1208 newly diagnosed glioma cases and 976 controls; cases and controls were comparable in their ethnicity and level of education (Table 1). However, cases were more likely than controls to be male, non-smokers, and less likely to report a history of allergies. Cases were also younger on average (median=51 years old) than controls (median=56 years old). Glioblastomas (GBM) were the most common histological subtype of brain tumor, followed by anaplastic astrocytomas, grade 2 astrocytomas, and oligodendrogliomas. More than three quarters of GBM cases (>82%) received the current standard of care treatment as a first course of treatment, which includes TMZ, radiation, and resection. Among GBM patients, 7% had IDH mutant tumors whereas for non-GBM patients, 74% of tumors were IDH mutant (Supplemental Table 1).
Table 1.
Characteristics of Participants with sCCL22 Results (n=2184), UCSF Adult Glioma Study (1997–2012)
| Cases (n=1208) |
Controls (n=976) |
||||
|---|---|---|---|---|---|
| Outcome | Category | # | % | # | % |
| Histology | GBM | 681 | 56.4 | NA | |
| Non-GBM | 527 | 43.6 | |||
| IDH1 Status | Negative | 585 | 70.5 | NA | |
| Positive | 245 | 29.5 | |||
| Unknown/Not Done* | 378 | NA | |||
| 1p19q Co-Deletion Status (Oligodendroglial cases only) | No 1p/19q codeletion | 31 | 27.7 | NA | |
| 1p/19q codeletion | 81 | 72.3 | |||
| No 1p/19q data | 52 | NA | |||
| Gender | Male | 723 | 59.9 | 498 | 51.0 |
| Female | 485 | 40.1 | 478 | 49.0 | |
| Ethnicity | White | 999 | 82.7 | 743 | 76.1 |
| Non-White | 207 | 17.1 | 233 | 23.9 | |
| Unknown | 2 | 0.2 | 0 | 0.0 | |
| Recruitment Series (Year of diagnosis for cases, year of interview for controls) | 1997–2000 | 70 | 5.8 | 110 | 11.3 |
| 2001–2005 | 455 | 37.7 | 278 | 28.5 | |
| 2006–2010 | 546 | 45.2 | 374 | 38.3 | |
| 2009–2012 | 137 | 11.3 | 214 | 21.9 | |
| Used Temozolomide*** | Yes** | 860 | 71.2 | NA | |
| No | 334 | 27.6 | |||
| Unknown | 14 | 1.2 | |||
| Radiation Therapy*** | Yes | 949 | 78.6 | NA | |
| No | 254 | 21.0 | |||
| Unknown | 5 | 0.4 | |||
| Surgery*** | Biopsy Only | 156 | 12.9 | NA | |
| Resection | 1051 | 87.0 | |||
| Unknown | 1 | 0.1 | |||
| Any Allergy Reported | Yes | 882 | 73.0 | 779 | 79.8 |
| No | 320 | 26.5 | 190 | 19.5 | |
| Unknown | 6 | 0.5 | 7 | 0.7 | |
| Smoking History | Ever Smoker | 516 | 42.7 | 513 | 52.6 |
| Never Smoker | 685 | 56.7 | 462 | 47.3 | |
| Unknown | 7 | 0.6 | 1 | 0.1 | |
| Education Level | College Degree | 667 | 55.2 | 536 | 54.9 |
| No College Degree | 537 | 44.5 | 439 | 45.0 | |
| Unknown | 4 | 0.3 | 1 | 0.1 | |
| Age | Median | 51 | 56 | ||
| Days Between Dx and Blood Draw | Median | 96 | NA | ||
IDH has not been done yet or we do not have adequate tissue for IDH analysis.
includes recommended or likely used
NA=not applicable
Only temozolomide, radiation and surgery given during the first course of treatment are included.
Serum CCL22 levels are depressed in glioma cases compared to controls
The overall concentration of CCL22 was statistically significantly lower in glioma cases compared to controls (mean±SEM: 1.23±0.03 ng/mL versus 1.60±0.03 ng/mL respectively, P<0.0001, Table 2). The results from the cluster models showed that after controlling for covariates, CCL22 levels were statistically significantly lower in cases than controls (highest versus lowest quartile OR: 0.21, 95% CI: 0.14–0.30, P<0.0001). Results were consistent for both GBM and non-GBM cases, but the magnitude of the effect was greater for GBM cases. In particular, for GBM cases versus controls, the OR for the highest versus lowest quartile was 0.16 (95% CI=0.10–0.24, p<0.0001); while in the non-GBM comparison it was 0.30 (95% CI=0.20–0.41, p<0.001) (Table 2). However, further analyses among only the non-GBM cases revealed that the lowest CCL22 values were among the grade III astrocytomas. No overall difference in CCL22 concentrations was noted among grade II oligodendroglioma or oligoastrocytoma cases compared to controls (Supplemental Table 1).
Table 2.
Comparisons of sCCL22 Levels in Glioma Cases and Controls, UCSF Adult Glioma Study, 1997–2012
| Cases | Controls | ||||||||
| Value | Category | n | Mean ± SEM | n | Mean ± SEM | t-test p-value** | |||
| sCCL22 ng/mL | All | 1208 | 1.23 ± 0.03 | 976 | 1.60 ± 0.03 | <0.0001 | |||
| Value | Category | Value (Quartile for Controls) | n | % | n | % | OR* (95% CI) | OR p-value | Trend Test |
| sCCL22 ng/mL Values | All | 0.03–1.05 (Q1) | 607 | 50.2% | 245 | 25% | 1.00 | ||
| 1.05–1.40 (Q2) | 247 | 20.4% | 244 | 25% | 0.28 (0.25–0.31) | <0.0001 | |||
| 1.40–1.87 (Q3) | 187 | 15.5% | 243 | 25% | 0.24 (0.19–0.29) | <0.0001 | |||
| 1.87–15.13 (Q4) | 167 | 13.8% | 244 | 25% | 0.21 (0.14–0.30) | <0.0001 | P<0.0001 | ||
| GBM | 0.03–1.05 (Q1) | 404 | 59.3% | 245 | 25% | 1.00 | |||
| 1.05–1.40 (Q2) | 116 | 17.0% | 244 | 25% | 0.23 (0.19–0.27) | <0.0001 | |||
| 1.40–1.87 (Q3) | 87 | 12.8% | 243 | 25% | 0.18 (0.14–0.24) | <0.0001 | |||
| 1.87–15.13 (Q4) | 74 | 10.9% | 244 | 25% | 0.16 (0.10–0.24) | <0.0001 | P<0.0001 | ||
| Non-GBM | 0.03–1.05 (Q1) | 203 | 38.5% | 245 | 25% | 1.00 | |||
| 1.05–1.40 (Q2) | 131 | 24.9% | 244 | 25% | 0.38 (0.33–0.43) | <0.0001 | |||
| 1.40–1.87 (Q3) | 100 | 19.0% | 243 | 25% | 0.34 (0.27–0.41) | <0.0001 | |||
| 1.87–15.13 (Q4) | 93 | 17.6% | 244 | 25% | 0.30 (0.20–0.41) | 0.0009 | P<0.0001 | ||
| Log CCL22 | All | NA (continuous log CCL22) | 1208 | NA | 976 | NA | 0.24 (0.17–0.33) | <0.0001 | NA |
Cluster analysis was performed to control for variation within each batch. Models were adjusted for batch number, age (continuous), race (white/non-white), gender, education (college vs no college), and smoking (ever vs never).
T-test comparing sCCL22 means between cases and controls.
SE=Standard error
Low CCL22 levels are associated with shorter survival
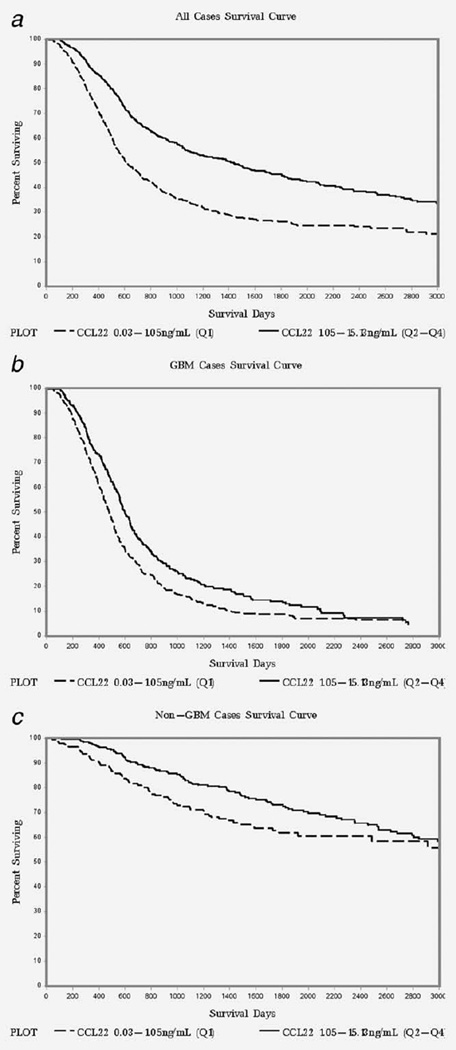
We found that lower CCL22 levels were associated with shorter median survival days among all cases (Table 3). Cox regression models using continuous log transformed CCL22 values showed a significantly lower risk of death as CCL22 levels increased in all cases as well as in the GBM only case group (HRallcases=0.81; 95% CI: 0.72–0.91, P<0.001) (Table 3). Cox regression models comparing the upper three quartiles of CCL22 to the lowest quartile in all cases also showed a significantly lower risk of death in cases with higher CCL22 levels (highest quartiles vs. lowest quartile HRallcases=0.80; 95% CI:0.69–0.93, P=0.003). However, the highest quartiles versus lowest quartile comparison did not show statistical significance in models where GBM and non-GBM cases were run separately. The Kaplan Meier (KM) curves comparing the survival of cases in the upper three quartiles (Q2–Q4) of CCL22 to those in the lowest quartile (Q1) are shown in Figure 1. The KM curves show that survival in the Q2–Q4 group (with higher CCL22 levels) is better than that in the Q1 group (with lower CCL22 levels) for all cases as well as the GBM and non-GBM case groups (Figure 1: A, B, and C).
Table 3.
Relationship Between sCCL22 and Survival, Cox Regressions Stratified by Histology, UCSF Adult Glioma Study, 1997–2012
| Variable | Category | All Cases | ||||
| # | # Deceased | MS** | Hazard Ratio* | P-Value | ||
| Log CCL22 | NA | 1208 | 798 | 873 | 0.81 (0.72–0.91) | 0.0003 |
| sCCL22 value (ng/mL)*** | 0.03–1.05 (Q1) | 607 | 448 | 611 | Referent | NA |
| 1.05–15.13 (Q2 – Q4) | 601 | 350 | 1446 | 0.80 (0.69–0.93) | 0.003 | |
| Variable | Category | GBM Cases | ||||
| # | # Deceased | MS** | Hazard Ratio* | P-Value | ||
| Log CCL22 | NA | 681 | 614 | 520 | 0.79 (0.69–0.91) | 0.001 |
| sCCL22 value (ng/mL)*** | 0.03–1.05 (Q1) | 404 | 370 | 479 | Referent | NA |
| 1.05–15.13 (Q2 – Q4) | 277 | 244 | 598 | 0.85 (0.72–1.01) | 0.06 | |
| Variable | Category | Non-GBM Cases | ||||
| # | # Deceased | 75% survival** | Hazard Ratio* | P-Value | ||
| Log CCL22 | NA | 527 | 184 | 1380 | 0.90 (0.70–1.15) | 0.41 |
| sCCL22 value (ng/mL)*** | 0.03–1.05 (Q1) | 203 | 78 | 948 | Referent | NA |
| 1.05–15.13 (Q2 – Q4) | 324 | 106 | 1687 | 0.84 (0.61–1.15) | 0.28 | |
The following variables were controlled for in the models: age, gender, race (white/non-white), 1st course of treatment (radiation, surgery, temodar, other chemo), dexamethasone use at blood draw, smoking (ever/never), college degree (yes/no), blooddays (days between dx and blood draw).
MS=Median survival days. For non-GBM cases, median survival was not available due to high % censored observations so the cutoff where 75% of the cases were still alive was used instead of the median.
Quartiles were based on only sCCL22 values in controls.
Note: We did not include IDH status in the final survival models since it was not available for 378 cases. However, when we included IDH in the models we found similar results (Log CCL22 HR and 95% CI for all cases=0.88 (0.76–1.03), GBM cases=0.81 (0.68–0.95), and Non-GBM cases=1.11 (0.77–1.60)). We also did not include 1p/19q co-deletion status as we only had this on 112 of the 165 oligodendroglial cases. However, we ran the survival models for the 112 cases with and without 1p/19q in the model and found similar results (Without 1p/19q: HR=0.40 (0.13–1.26), With 1p/19q: HR=0.47 (0.15–1.46)).
Figure 1.
Kaplan-Meier survival curves stratified by CCL22 levels (Quartile 1 vs. Quartiles 2–4) in all glioma cases (a); GBM cases (b) and non-GBM cases (c), UCSF Adult Glioma Study 1997–2012.
We found that in GBM cases, the CCL22 levels were higher in cases treated with radiation before blood draw (p=0.04), but we did not find any significant differences in CCL22 levels when comparing cases who took chemotherapy prior to blood draw to those who did not (Supplemental Table 6). However, cases taking dexamethasone use at blood draw had significantly lower CCL22 levels than cases not taking dexamethasone at the time of blood draw. Dexamethasone use, along with first course of treatment, demographic, and other factors were adjusted for in the Cox regression models that show an association between CCL22 levels and overall survival in GBMs.
Serum CCL22 levels are associated with CD3 and CD4 cells in glioma cases
For the 47 cases and 146 controls with FACS analysis data, we compared the CCL22 levels with CD3 (pan-T cell marker), CD4 and CD45 cell counts as well as the ratio of CD3/CD45 and CD4/CD45. There were no observed differences in total CD45 cells (total leukocytes) among cases and controls. However, we observed a significant positive correlation between CCL22 levels and both CD3 and CD4 counts in cases (Table 4). The correlations between CCL22 and CD3 and CD4 were stronger in cases than in controls (p<0.01 in cases and p>=0.10 in controls for both CD3 and CD4). The mean absolute CD4 cell counts in glioma cases also were markedly lower than that in controls (659 cells/µL in all cases and 392 in GBM cases versus 813 in controls, p=0.04). Statistically significant case/control differences were also observed for the ratios of CD3/CD45 and CD4/CD45, with cases having lower ratios (p<0.01 for each).
Table 4.
CD3, CD4, and total leukocytes (CD45) cell counts (by flourescence-activated cell sorting) and correlations of these values with sCCL22 levels, UCSF Adult Glioma Study, 2010–2012
| Variable | Case Status | # | Median | Mean | St Dev |
All Cases vs All Controls T- test p-value** |
Correlation Coeff with sCCL22* |
P-value (Correlation) |
|---|---|---|---|---|---|---|---|---|
| CD3 (Tcells) | All Cases and Controls | 193 | 1225 | 1354 | 734 | 0.29 | <0.01 | |
| Absolute count/mL | Cases Only | 47 | 938 | 1232 | 1044 | 0.53 | <0.01 | |
| Non-GBM Cases Only | 21 | 1931 | 1758 | 1065 | 0.41 | 0.06 | ||
| GBM Cases Only | 26 | 511 | 808 | 825 | 0.50 | <0.01 | ||
| Controls Only | 146 | 1294 | 1392 | 601 | 0.19 | 0.13 | 0.13 | |
| CD4 (T-helper cells) | All Cases and Controls | 193 | 716 | 775 | 459 | 0.30 | <0.01 | |
| Absolute count/mL | Cases Only | 47 | 486 | 659 | 597 | 0.51 | <0.01 | |
| Non-GBM Cases Only | 21 | 758 | 989 | 708 | 0.36 | 0.11 | ||
| GBM Cases Only | 26 | 275 | 392 | 298 | 0.47 | 0.02 | ||
| Controls Only | 146 | 754 | 813 | 399 | 0.04 | 0.14 | 0.10 | |
| All Cases and Controls | 193 | 6949 | 7557 | 3252 | 0.08 | 0.29 | ||
| CD45 | Cases Only | 47 | 7827 | 8358 | 4634 | −0.03 | 0.85 | |
| Total leukocytes/mL | Non-GBM Cases Only | 21 | 8580 | 9301 | 4333 | −0.09 | 0.68 | |
| GBM Cases Only | 26 | 6312 | 7597 | 4812 | −0.18 | 0.38 | ||
| Controls Only | 146 | 6874 | 7299 | 2630 | 0.05 | 0.11 | 0.17 | |
| CD3/CD45 Ratio | All Cases and Controls | 193 | 0.19 | 0.19 | 0.08 | 0.21 | <0.01 | |
| Cases Only | 47 | 0.13 | 0.15 | 0.09 | 0.61 | <0.01 | ||
| Non-GBM Cases Only | 21 | 0.23 | 0.19 | 0.08 | 0.52 | 0.02 | ||
| GBM Cases Only | 26 | 0.10 | 0.11 | 0.08 | 0.53 | 0.01 | ||
| Controls Only | 146 | 0.20 | 0.20 | 0.08 | <0.01 | –0.004 | 0.96 | |
| CD4/CD45 Ratio | All Cases and Controls | 193 | 0.10 | 0.11 | 0.05 | 0.21 | <0.01 | |
| Cases Only | 47 | 0.07 | 0.08 | 0.05 | 0.57 | <0.01 | ||
| Non-GBM Cases Only | 21 | 0.12 | 0.11 | 0.05 | 0.48 | 0.03 | ||
| GBM Cases Only | 26 | 0.06 | 0.06 | 0.04 | 0.53 | <0.01 | ||
| Controls Only | 146 | 0.11 | 0.12 | 0.05 | <0.01 | 0.005 | 0.96 | |
Correlation between log transformed CCL22 and specified FACS variable (Spearman Rank Correlation).
Comparing mean FACS values between cases and controls.
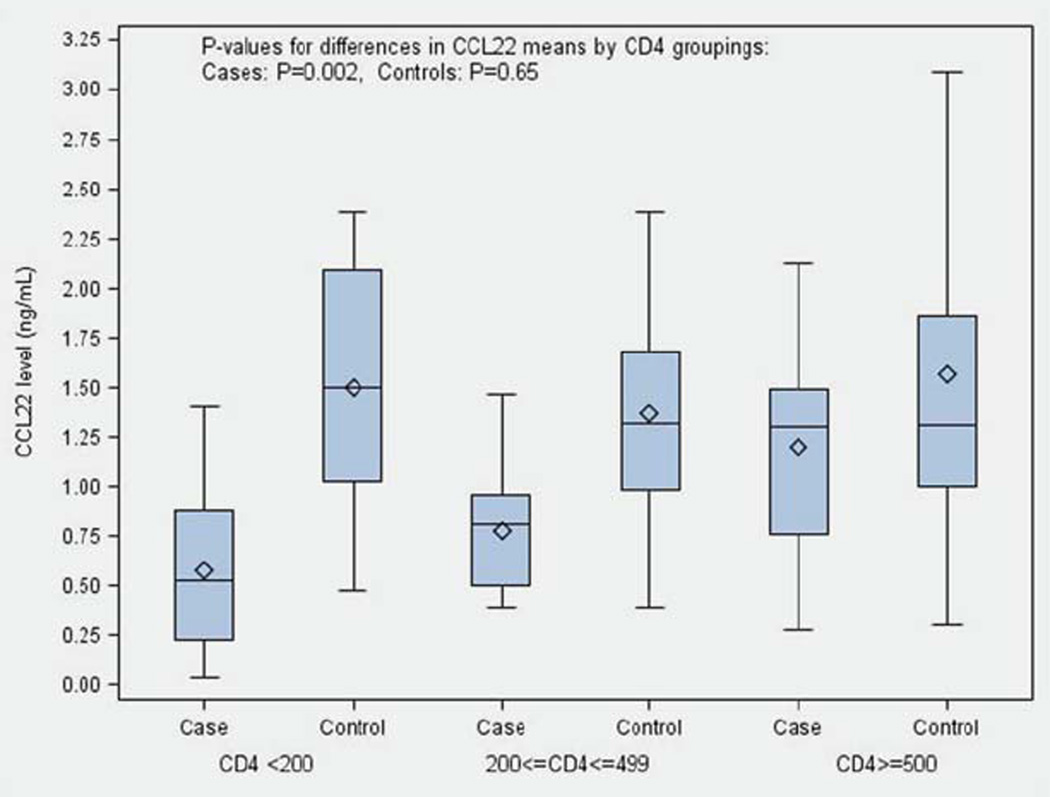
When we grouped the CD4 counts into three clinically meaningful categories (<200, 200–499, 500+ CD4 cells/µL), we found that in cases, the mean value of CCL22 increased as CD4 levels increased (Figure 2). Cases with the lowest levels of CD4 cells (<200) had a mean serum CCL22 level of 0.57 ng/mL, whereas cases with CD4 counts >=500 had a mean CCL22 level of 1.20 ng/mL (p=0.002 for difference in means between the CD4 groups). However, we did not observe differences in CCL22 levels among the three CD4 groups in controls (p=0.65 for difference in mean CCL22 levels across the CD4 groups). In addition, we found that the CD4 counts of cases recently treated with radiation or chemotherapy were significantly lower than cases who had not yet received treatment (P<0.001, Supplementary Table 4b).
Figure 2.
CCL22 levels by case/control status within CD4 groupings. Samples were divided into three groups according to CD4 counts/µL (<200, 200–499, >=500), CCL22 levels (ng/mL) were compared in cases and controls of each group, UCSF Adult Glioma Study 2010–2012. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
CCL22 and Allergies/IgE
We also examined whether CCL22 levels were associated with reported allergy history or measured serum IgE levels. Null relationships were observed between CCL22 concentration and any reported allergy, total number of allergies reported, or IgE measurements in both cases and controls.
Discussion
Although systemic defects in APC function have been observed in glioma patients15, 22, 23, there are no established biomarkers for assessing this dimension of the immune response for clinical prognostication. Here we focused on serum CCL22 as a simple protein assay applicable to routinely collected blood sera. A strong rationale for considering CCL22 as a biomarker of APC function is that DCs and monocyte derived macrophages are the most potent producers of CCL22 in vitro and in vivo31. The maturation of monocyte precursors into DCs with a functional APC phenotype leads to a many-fold increased expression and secretion of CCL2231. Given our current observation of suppressed CCL22 levels in glioma patients and its association with CD4 lymphopenia, it is reasonable to propose that this reflects depressed DC function and T cell trafficking.
We did not find a significant association between chemotherapy taken before blood draw and CCL22 levels, but found that GBM cases who had radiation before blood draw had higher CCL22 levels than those who had not yet had radiation. We explored the time elapsed since the last dose of radiation or chemotherapy and the day of the blood draw but found no correlation between time since last dose of either radiation or chemotherapy and CCL22 concentrations. Medications such as dexamethasone may also influence CCL22 expression32, 33 and in our data current dexamethasone exposure was associated with significantly lower CCL22 levels among glioma cases.
We found a strong correlation between CCL22 values and CD4 counts in cases. We also found that cases who received chemotherapy or radiation before blood draw had significantly lower CD4 counts than cases who had not yet received these treatments. Prospective studies during TMZ and radiation therapy indicate an inhibitory effect on CD4 and B cells followed by a recovery phase in some but not all exposed patients. Prolonged lymphopenia has been associated with the patient’s ability to generate appropriate cytokine responses including the production of IL7 and IL1534. Perhaps chemokine directed cell trafficking as marked by CCL22 also fails to recover in some patients and could explain our observed CD4 lymphopenia. It is interesting that another CCR4 responsive chemokine, CCL2, was found to be depressed in sera of GBM patients along with CD4 cells, and both appeared to correlate with increases in a CD14+ HLA-DR- subtype of blood monocyte35. Our earlier study showed there were similar percentages of cases and controls positive for antibodies to three herpes viruses (herpes simplex virus, cytomegalovirus, and Epstein-Barr virus)36. In addition, circulating CD14 levels in our past study37 were significantly higher in cases compared with controls. Furthermore, in our current study we did not observe decreased total leukocyte counts in glioma cases versus controls. These observations argue against the hypothesis that lower CCL22 levels in glioma patients might be primarily the result of nonspecific effects of advanced tumors or therapies. Taken together, these observations implicate monocyte and DC derived trafficking molecules with suppression of T cell immunity. Further study is required to identify whether suppression of CCL22 is an intrinsic patient related defect, or a tumor driven effect or some combinations of all these possibilities.
IDH mutation, 1p19q codeletion, and MGMT methylation are tumor markers that have been shown to be associated with improved prognosis in glioma patients50. We controlled for the effect of IDH mutation in most patients and 1p19q codeletion in patients with oligodendroglial tumors, but were not able to examine MGMT methylation. However, MGMT methylation is highly correlated with IDH status and we have IDH mutation results for most cases.
The present study is, to our knowledge, the first to assess the relationship of peripheral CCL22 levels in glioma survival, which can be more easily applied as a routine assay for prognosis than most tumor markers. Our findings are consistent with two cohort studies on metastatic breast cancer showing that low CCL22 levels were associated both with low CD4 counts and reduced overall survival16. This raises the possibility that in the context of radio-chemotherapy for cancer, CCL22 might be considered an indicator of DC related immune suppression. However, the association of low CCL22 and abnormal CD4 counts was not observed in our control group, even though a small number of subjects demonstrated <200 CD4 cells/µL. In these exceptional control patients, a history of HIV, hepatitis or autoimmune disorders were common though their CCL22 values were normal. The specificity of chemokine measurements as indicators of DC function in different clinical populations requires further study.
Abnormalities in T cell immunity are most extreme in high grade (grades III and IV) glioma compared with lower grades. Our observations parallel this grade dependence in the sense that CCL22 concentrations were not significantly depressed in grade II glioma irrespective of histological subtype. There were individual exceptions, however, with some grade II patients demonstrating very low CCL22 and CD4 counts. The survival curve among non-GBM patients demonstrated a survival advantage among patients with higher CCL22 levels although the sample size of non-GBM cases was limited. Tumor grade and histology subtype analyses revealed that patients with grade III astrocytoma had the lowest CCL22 concentrations compared to those with the grade II oligodendroglioma, grade II astrocytoma, and oligoastrocytoma. Further work will be necessary to assess whether specific morphologies drive the survival associations in non-GBM patients and whether CCL22 may be useful in identifying lower grade glioma patients at high risk for early progression.
We, and others, have shown that T helper 2 (a subset of CD4 cells) related phenotypes such as atopic allergy are underrepresented in glioma patients38, 39. Atopic conditions have been linked to CCL2240. One study41 showed that increased cord blood CCL22 levels were associated with development of allergic sensitization and asthma; CCL22 increases preceded allergy development during the first 6 years of life. Another group demonstrated that cord blood CCL22 levels were associated with elevated total IgE levels during preschool age42. Our analyses did not reveal any associations of allergy history or serum IgE concentrations with CCL22 among cases or controls. Previous reports linking serum CCL22 with atopic conditions concerned children and it is not known whether circulating levels in adulthood are expected to signal greater atopic risk. It is also possible that the repertoire of cells responsive to CCL22 is broader than just CD4 Th2 cells. Targeted knock down of CCL22 in DCs affected CD8 cytotoxic cells as well as CD4 and CD25+ Foxp3+ regulatory cells43. Finally, a limitation of our studies in cases is that serum samples were obtained after glioma diagnosis and it would be very interesting to know if CCL22 depression precedes diagnosis and may therefore reflect a preexisting alteration in macrophage/DC function.
Our study focused on a peripheral marker of immune suppression and we did not evaluate the potential relationship between serum CCL22 and production of this chemokine by the tumor. CCL22 and CCL2 are implicated in the trafficking of T cells including T regs into the tumor microenvironment44. IFN-γ, IL1β and TNF-α will favor CCL22 overproduction by tumor cells, which promotes the recruitment of CCR4+ blood T reg that favors the development of a tolerogenic environment45. Trafficking and accumulation of T regs at the tumor site may have a negative effect on patient survival46. However, not all studies support this view47 and the issue is complicated by the strong correlation of immune infiltrates with WHO glioma grade. In any case, it is not clear what effect local tumor related production of CCL22 would have on the peripheral blood concentration. Both CCL22 and CCL2, the primary chemokines directing T regs, are markedly depressed in sera of glioma patients, at least following tumor resection. It will be necessary to characterize the T cell infiltrate in glioma tissues and assess the peripheral chemokine profile in the same patients to address whether the blood parameter reflects the T cell immune response of the tumor microenvironment48.
In conclusion, our observations combined with previous work suggest that monocyte and DC produced CCL22 and, possibly, CCL2 may serve as markers in glioma patients that signal patients at risk of prolonged CD4 cell suppression and poorer survival. New strategies to augment antitumor immunity49 can be adversely affected by persistent defects in APC cells and CCL22 may prove a useful marker to assess APC function in patients receiving immunotherapy.
Supplementary Material
Description of the novelty and impact of the work.
Here, we present the novel observation that depressed levels of CCL22 are common in glioma patients. We further have found that these depressed CCL22 levels are associated with low CD4 cell counts and poorer survival times. Thus, CCL22 could be a useful biomarker reflecting the suppressed immunity of glioma patients. It may also serve as a significant prognostic factor of gliomas and a possible marker of cancer associated suppression of immune function.
Acknowledgements
Work at University of California, San Francisco was supported by the National Institutes of Health (grant numbers R01CA52689, P50CA097257, R01CA126831, R01CA139020 and R25CA112355), as well as the National Brain Tumor Foundation, the UCSF Robert Magnin Newman Chair in Neuro-oncology, the Stanley D. Lewis and Virginia S. Lewis Chair in Brain Tumor Research and by donations from families and friends of John Berardi, Helen Glaser, Elvera Olsen, Raymond E. Cooper, and William Martinusen. This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. We acknowledge the expert pathological evaluation of Drs. Kenneth Aldape and Tarik Tihan.
Abbreviations
- DC
dendritic cell
- FACS
fluorescence-activated cell sorting
- GBM
glioblastoma multiforme
- CCL22
C-C motif chemokine 22
- MDC
macrophage derived chemokine
- APC
antigen presenting cell
- IDH
isocitrate dehydrogenase
- TMZ
temozolomide
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis FG, Kupelian V, Freels S, McCarthy B, Surawicz T. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro-oncology. 2001;3:152–158. doi: 10.1093/neuonc/3.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a "state of the science" review. Neuro-oncology. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins RB, Wrensch MR, Johnson D, Fridley BL, Decker PA, Xiao Y, Kollmeyer TM, Rynearson AL, Fink S, Rice T, McCoy LS, Halder C, et al. Distinct germ line polymorphisms underlie glioma morphologic heterogeneity. Cancer Genet. 2011;204:13–18. doi: 10.1016/j.cancergencyto.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins RB, Xiao Y, Sicotte H, Decker PA, Kollmeyer TM, Hansen HM, Kosel ML, Zheng S, Walsh KM, Rice T, Bracci P, McCoy LS, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. 2012;44:1122–1125. doi: 10.1038/ng.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice T, Zheng S, Decker PA, Walsh KM, Bracci P, Xiao Y, McCoy LS, Smirnov I, Patoka JS, Hansen HM, Hsuang G, Wiemels JL, et al. Inherited variant on chromosome 11q23 increases susceptibility to IDH-mutated but not IDH-normal gliomas regardless of grade or histology. Neuro-oncology. 2013;15:535–541. doi: 10.1093/neuonc/nos324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen BC, Smith AA, Zheng S, Koestler DC, Houseman EA, Marsit CJ, Wiemels JL, Nelson HH, Karagas MR, Wrensch MR, Kelsey KT, Wiencke JK. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103:143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parney IF. Basic concepts in glioma immunology. Adv Exp Med Biol. 2012;746:42–52. doi: 10.1007/978-1-4614-3146-6_4. [DOI] [PubMed] [Google Scholar]
- 11.Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol. 2012;746:53–76. doi: 10.1007/978-1-4614-3146-6_5. [DOI] [PubMed] [Google Scholar]
- 12.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 2010;21:31–42. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, Consortium NC. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson MPLY, LaPlant B, Liwski CJ, Maas ML, League SC, et al. Immun monitoring using the predictive power of immune profiles. Journal for ImmunoTherapy of Cancer. 2013;1:11. doi: 10.1186/2051-1426-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gousias K, von Ruecker A, Voulgari P, Simon M. Phenotypical analysis, relation to malignancy and prognostic relevance of ICOS+T regulatory and dendritic cells in patients with gliomas. J Neuroimmunol. 2013;264:84–90. doi: 10.1016/j.jneuroim.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Tredan O, Manuel M, Clapisson G, Bachelot T, Chabaud S, Bardin-dit-Courageot C, Rigal C, Biota C, Bajard A, Pasqual N, Blay JY, Caux C, et al. Patients with metastatic breast cancer leading to CD4+ T cell lymphopaenia have poor outcome. Eur J Cancer. 2013;49:1673–1682. doi: 10.1016/j.ejca.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, Guastalla JP, Bachelot T, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peron J, Cropet C, Tredan O, Bachelot T, Ray-Coquard I, Clapisson G, Chabaud S, Philip I, Borg C, Cassier P, Labidi Galy I, Sebban C, et al. CD4 lymphopenia to identify end-of-life metastatic cancer patients. Eur J Cancer. 2013;49:1080–1089. doi: 10.1016/j.ejca.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Moore C, Eslin D, Levy A, Roberson J, Giusti V, Sutphin R. Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatr Blood Cancer. 2010;55:1096–1102. doi: 10.1002/pbc.22673. [DOI] [PubMed] [Google Scholar]
- 20.Ceze N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305–1313. doi: 10.1007/s00280-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 21.Borg C, Ray-Coquard I, Philip I, Clapisson G, Bendriss-Vermare N, Menetrier-Caux C, Sebban C, Biron P, Blay JY. CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer. 2004;101:2675–2680. doi: 10.1002/cncr.20688. [DOI] [PubMed] [Google Scholar]
- 22.Ogden AT, Horgan D, Waziri A, Anderson D, Louca J, McKhann GM, Sisti MB, Parsa AT, Bruce JN. Defective receptor expression and dendritic cell differentiation of monocytes in glioblastomas. Neurosurgery. 2006;59:902–909. doi: 10.1227/01.NEU.0000233907.03070.7B. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 23.Tyrinova TV, Leplina OY, Mishinov SV, Tikhonova MA, Shevela EY, Stupak VV, Pendyurin IV, Shilov AG, Alyamkina EA, Rubtsova NV, Bogachev SS, Ostanin AA, et al. Cytotoxic activity of ex-vivo generated IFNalpha-induced monocyte-derived dendritic cells in brain glioma patients. Cell Immunol. 2013;284:146–153. doi: 10.1016/j.cellimm.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-oncology. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC) J Leukoc Biol. 2000;68:400–404. [PubMed] [Google Scholar]
- 27.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 29.Szeinbach SL, Barnes JH, Sullivan TJ, Williams PB. Precision and accuracy of commercial laboratories' ability to classify positive and/or negative allergen-specific IgE results. Ann Allergy Asthma Immunol. 2001;86:373–381. doi: 10.1016/S1081-1206(10)62481-7. [DOI] [PubMed] [Google Scholar]
- 30.Wiemels JL, Wiencke JK, Li Z, Ramos C, Nelson HH, Karagas MR. Risk of squamous cell carcinoma of the skin in relation to IgE: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2011;20:2377–2383. doi: 10.1158/1055-9965.EPI-11-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vulcano M, Albanesi C, Stoppacciaro A, Bagnati R, D'Amico G, Struyf S, Transidico P, Bonecchi R, Del Prete A, Allavena P, Ruco LP, Chiabrando C, et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol. 2001;31:812–822. doi: 10.1002/1521-4141(200103)31:3<812::aid-immu812>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di Carlo V. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- 33.Moser M, De Smedt T, Sornasse T, Tielemans F, Chentoufi AA, Muraille E, Van Mechelen M, Urbain J, Leo O. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–2824. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 34.Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, Luznik L, Drake CG. Sustained CD4 T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3:e27357. doi: 10.4161/onci.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-oncology. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrensch M, Weinberg A, Wiencke J, Miike R, Sison J, Wiemels J, Barger G, DeLorenze G, Aldape K, Kelsey K. History of chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls. Am J Epidemiol. 2005;161:929–938. doi: 10.1093/aje/kwi119. [DOI] [PubMed] [Google Scholar]
- 37.Zhou M, Wiemels JL, Bracci PM, Wrensch MR, McCoy LS, Rice T, Sison JD, Patoka JS, Wiencke JK. Circulating levels of the innate and humoral immune regulators CD14 and CD23 are associated with adult glioma. Cancer Res. 2010;70:7534–7542. doi: 10.1158/0008-5472.CAN-10-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiemels JL, Wilson D, Patil C, Patoka J, McCoy L, Rice T, Schwartzbaum J, Heimberger A, Sampson JH, Chang S, Prados M, Wiencke JK, et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009;125:680–687. doi: 10.1002/ijc.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544–1550. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto S, Nakamura K, Oyama N, Kaneko F, Tsunemi Y, Saeki H, Tamaki K. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J Dermatol Sci. 2006;44:93–99. doi: 10.1016/j.jdermsci.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Abelius MS, Ernerudh J, Berg G, Matthiesen L, Nilsson LJ, Jenmalm MC. High cord blood levels of the T-helper 2-associated chemokines CCL17 and CCL22 precede allergy development during the first 6 years of life. Pediatr Res. 70:495–500. doi: 10.1203/PDR.0b013e31822f2411. [DOI] [PubMed] [Google Scholar]
- 42.Folsgaard NV, Chawes BL, Bonnelykke K, Jenmalm MC, Bisgaard H. Cord blood Th2-related chemokine CCL22 levels associate with elevated total-IgE during preschool age. Clin Exp Allergy. 2012;42:1596–1603. doi: 10.1111/j.1365-2222.2012.04048.x. [DOI] [PubMed] [Google Scholar]
- 43.Kang S, Xie J, Ma S, Liao W, Zhang J, Luo R. Targeted knock down of CCL22 and CCL17 by siRNA during DC differentiation and maturation affects the recruitment of T subsets. Immunobiology. 2010;215:153–162. doi: 10.1016/j.imbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, Adema GJ. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225:195–199. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Heimberger AB, Kong LY, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Wei J, Qiao W, Schmittling RJ, Archer GE, Sampson JH, Hiraoka N, Priebe W, et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- 48.Zisakis A, Piperi C, Themistocleous MS, Korkolopoulou P, Boviatsis EI, Sakas DE, Patsouris E, Lea RW, Kalofoutis A. Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine. 2007;39:99–105. doi: 10.1016/j.cyto.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Ahn BJ, Pollack IF, Okada H. Immune-checkpoint blockade and active immunotherapy for glioma. Cancers (Basel) 2013;5:1379–1412. doi: 10.3390/cancers5041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zalatimo O, Zoccoli CM, Patel A, Weston CL, Glantz M. Impact of genetic targets on primary brain tumor therapy: what's ready for prime time? Adv Exp Med Biol. 779:267–289. doi: 10.1007/978-1-4614-6176-0_12. 013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.