Abstract
Introduction
New insulin glargine 300 U mL−1 (Gla-300) is a basal insulin that shows more stable and prolonged pharmacokinetic and pharmacodynamic profiles than insulin glargine 100 U mL−1 (Gla-100). This study used continuous glucose monitoring (CGM) to compare 24-h glucose profiles in a Japanese population using Gla-300 versus Gla-100.
Methods
This was an exploratory 8.4-week, single-center, 2-sequence, 2-period, open-label crossover study. Japanese adults with type 1 diabetes mellitus (T1DM) treated with basal–bolus insulin, with glycated hemoglobin (HbA1c) 6.5–10.0% and median fasting self-monitored plasma glucose concentration ≤13 mmol L−1, were randomized to Gla-300 followed by Gla-100 (subgroup 1) or vice versa (subgroup 2), with no washout period. CGM was performed on the last 3 days of the screening period and each treatment period. Primary endpoint was comparison of 24-h glucose variability (area under the curve [AUC]mean_24 h) on the second day of each CGM measurement with Gla-300 versus Gla-100. Baseline and end of treatment period values for HbA1c, fasting plasma glucose (FPG) and daily basal/mealtime insulin doses were recorded. Hypoglycemia and adverse events (AEs) were recorded.
Results
Twenty participants were randomized (10 to subgroup 1 and 10 to subgroup 2). Participants showed comparable glucose variability over 24 h (AUCmean_24 h during treatment with Gla-300 or Gla-100 (treatment ratio 0.96; 90% confidence interval 0.79, 1.16). HbA1c and FPG were generally stable across both treatment periods. There was a trend towards fewer participants experiencing ≥1 hypoglycemia event at any time (24 h) and at night (00:00–05:59 h) with Gla-300 versus Gla-100. Treatment-emergent AEs, reported by 9/20 (45%) and 4/20 (20%) participants during Gla-300 and Gla-100 treatment, respectively, were unrelated to study medication.
Conclusions
In this cohort of Japanese people with T1DM, no between-treatment difference was observed in glucose variability with Gla-300 versus Gla-100, as measured by CGM. There was a trend for less hypoglycemia with Gla-300, particularly at night, versus Gla-100. Both treatments were well tolerated.
Funding
Sanofi, Tokyo, Japan.
Clinical trial registration: NCT01676233, ClinicalTrials.gov.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0115-1) contains supplementary material, which is available to authorized users.
Keywords: Continuous glucose monitoring, Hypoglycemia, Insulin glargine, Type 1 diabetes
Introduction
There is a clinical need for basal insulin products that provide more constant and longer-lasting glycemic control with reduced risk of hypoglycemia to enable more people with diabetes to meet their glycemic targets and improve their quality of life. Recent studies with new insulin glargine 300 U mL−1 (Gla-300) have shown more stable and prolonged pharmacokinetic (PK) and pharmacodynamic (PD) profiles compared with insulin glargine 100 U mL−1 (Gla-100) in both European [1–3] and Japanese populations [3]. In addition, the EDITION clinical trial program has shown lower rates of hypoglycemia with Gla-300 compared with Gla-100 in several different type 1 and type 2 diabetes populations [4–7], including participants from Japan [8, 9].
A recent study using continuous glucose monitoring (CGM) analysis in Western participants with type 1 diabetes mellitus (T1DM) showed that Gla-300 treatment provided more stable glucose levels throughout the day compared with Gla-100 [10]. Intra-subject glucose variability was lower with Gla-300 than with Gla-100, irrespective of morning or evening administration, which confirms some flexibility of injection time is possible with Gla-300 [10]. In turn, rates of nocturnal hypoglycemia were numerically lower with Gla-300 compared with Gla-100, demonstrating a potential clinical benefit of the smoother Gla-300 PK and PD profiles [10]. This reduction in hypoglycemia risk could lead to improved treatment success and quality of life for people with T1DM.
In the present study, CGM was used to compare 24-h glucose profiles at steady state in a Japanese population with T1DM receiving once-daily Gla-300 or Gla-100.
Methods
This was a single-center, randomized, open-label, 2-sequence, 2-period, 2-treatment, multiple-dose crossover study. Japanese people of at least 20 years of age with T1DM who were being treated with basal–bolus insulin and had glycated hemoglobin (HbA1c) within the range 6.5–10.0%, and a median fasting self-monitored plasma glucose (SMPG) concentration of ≤13 mmol L−1 (240 mg dL−1) in the 3 days prior to randomization were eligible for inclusion. People were excluded from the study if they had received premix insulin or basal insulin other than Gla-100, neutral protamine Hagedorn insulin, neutral protamine insulin lispro, or insulin detemir, or mealtime insulin other than insulin lispro, aspart, or glulisine during the 4 weeks immediately before screening. Use of an insulin pump or new glucose-lowering medications during the 12 weeks before screening was not permitted. All procedures followed were in accordance with Good Clinical Practice [11], the ethical standards of the responsible committee on human experimentation (institutional and national) and the Declaration of Helsinki [12]. Written informed consent was obtained from all participants before they were included in the study.
Participants received either Gla-300 (using a modified TactiPen®; Haselmeier GmbH, Zürich, Switzerland) in treatment period 1 followed by Gla-100 (using a SoloSTAR® pen; Sanofi, Paris, France) in treatment period 2 (subgroup 1) or vice versa (subgroup 2; Fig. 1), using a 1:1 block randomization protocol to assign subgroups. Study insulin preparations were self-administered subcutaneously once daily at bedtime (preferably >3 h after evening mealtime insulin). The starting dose for both treatment periods was based on the basal insulin dose in the screening period. Owing to differences in the scaling of the two injection devices, starting doses of Gla-300 were divisible by 1.5 U and did not exceed the previous daily dose, while Gla-100 starting doses were equal to the previous daily dose. Participants previously receiving their basal insulin dose in the morning switched to one dose in the evening on Day −7 (start of screening period). Basal insulin dose was titrated to achieve fasting SMPG in the range 4.4–7.2 mmol L−1 (80–130 mg dL−1) during the two treatment periods. The mealtime insulin dose was to continue without adjustment from the participant’s pre-study regimen as much as possible, with adjustment allowed at the discretion of the investigator or participant if postprandial hyperglycemia (2-h postprandial plasma glucose >8.9 mmol L−1 [>160 mg dL−1]) or an abnormality relevant to hypoglycemia caused by mealtime insulin was observed and it was difficult to avoid the occurrence of abnormalities by adjusting the basal insulin dose. No additional antihyperglycemic medications were allowed beyond the study basal insulin, the continued mealtime insulin, and the previous basal insulin utilized in the screening and follow-up periods. The study was open-label and CGM measurements were performed over 3 days (in hospital for the first 2 days and then at home for the third day), on Days −3 to 1 (screening period), Days 26–29 (treatment period 1) and Days 54–57 (treatment period 2) using a CGM meter (CGMS® System Gold™; Medtronic, Northridge, California, USA). During the in-hospital CGM periods, participants received standardized meals and mealtime insulin doses at 08:00 h, 12:00 h and 18:00 h, as well as the basal insulin dose at 23:00 h, to ensure consistency of patient management.
Fig. 1.
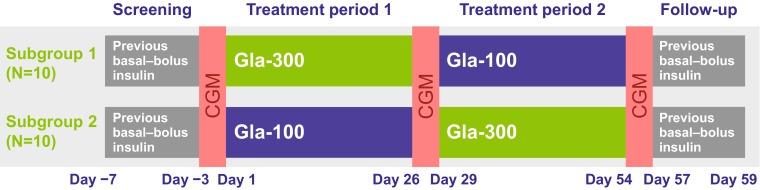
Study design. CGM continuous glucose monitoring, Gla-300 insulin glargine 300 U mL−1, Gla-100 insulin glargine 100 U mL−1
Sample size was estimated using a maximum imprecision approach based on a within-subject standard deviation (SDwithin) of 0.445 obtained for the bedtime time-point blood glucose readings of an 8-point SMPG profile in a parallel study with Gla-100. Thus, with a total of 20 patients, assuming a SDwithin of 0.450, the ratio of Gla-300 versus Gla-100 means for fasting plasma glucose (FPG) at bedtime was estimated with a maximum imprecision of 30% (i.e., the 95% confidence interval (CI) will be 0.70 and 1/0.70 times the observed ratio), with 90% assurance. The primary efficacy endpoint was the comparison of Gla-300 and Gla-100 with regard to glycemic variability over 24 h (area under the curve [AUC]mean_24 h) expressed as the mean area below and above the individual average plasma glucose concentration (BGAVG_24 h) on Day 2 of CGM recording for each treatment period, analyzed using the Riemann sum method. In addition, mean nocturnal glycemic variability (AUCmean_noc; 00:00–06:00 h on Day 2) and mean daytime glycemic variability (AUCmean_daytime; 06:00–00:00 h on Day 2) for both treatments were included as supportive secondary efficacy endpoints and were also analyzed using the Riemann sum method. CGM endpoints were analyzed using the modified intent-to-treat (mITT) population (all participants without any important deviations related to study drug administration, and for whom blood glucose data parameters by CGM were available) with results pooled by treatment. A linear mixed-effect model with sequence, period and treatment as fixed terms and an unstructured 2 by 2 matrix of treatment-specific variances and covariance per participant within sequence blocks was used to obtain the log-transformed AUCmean_24 h ratio and 90% CIs between the two treatments, for the glucose data from the second day of CGM only, using SAS proc MIXED® (Cary, NC, USA). Other efficacy measurements included HbA1c (national glycohemoglobin standardized program values used throughout), laboratory-measured FPG, and daily insulin doses at the start and end of each treatment period (study Days 2 and 28 for treatment period 1; study Days 29 and 56 for treatment period 2), by treatment, and were analyzed using descriptive statistics, using the intent-to-treat population (ITT; all participants without any important deviations related to study drug administration, and for whom efficacy parameters were available).
Hypoglycemic events were recorded according to the American Diabetes Association definitions [13] at any time of day (24 h) and during the night (00:00–05:59 h). All other adverse events (AEs) that were spontaneously reported by the participant or observed by the investigator were recorded. Both were analyzed (SAS ver 9.2, Cary, NC, USA) in the safety population (all participants who received at least one dose of study medication) using descriptive statistics and results were pooled by treatment.
Results
Baseline Characteristics
All 20 randomized participants completed the study and all were included in the mITT, ITT, and safety populations. Demographics and disease characteristics at screening are summarized in Table 1, and were balanced between the two subgroups with the exception of HbA1c and laboratory-measured FPG, which were both slightly higher in subgroup 1 (Gla-300 then Gla-100) than subgroup 2 (Gla-100 then Gla-300). The basal to total daily insulin ratio at the start of treatment period 1 was 0.33 for subgroup 1 and 0.32 for subgroup 2, indicating a preference for mealtime insulin-based blood glucose control (Table 2).
Table 1.
Demographic and disease characteristics at screening for participants included in the study
| Randomized population | Subgroup 1 (Gla-300/Gla-100; N = 10) | Subgroup 2 (Gla-100/Gla-300; N = 10) | All (N = 20) |
|---|---|---|---|
| Age, years | |||
| mean (SD) | 52.1 (17.3) | 52.1 (15.3) | 52.1 (15.9) |
| Sex, male | |||
| n (%) | 4 (40.0) | 4 (40.0) | 8 (40.0) |
| Body weight, kg | |||
| mean (SD) | 61.5 (13.2) | 57.0 (8.0) | 59.3 (10.9) |
| BMI, kg m−2 | |||
| mean (SD) | 24.1 (4.4) | 22.6 (1.9) | 23.4 (3.4) |
| HbA1c, mean (SD) | |||
| % | 8.49 (0.87) | 7.93 (0.70) | 8.21 (0.82) |
| mmol mol−1 | 69.3 (9.5) | 63.2 (7.7) | 66.2 (9.0) |
| FPG, mean (SD) | |||
| mmol L−1 | 8.3 (3.8) | 7.3 (4.1) | 7.8 (3.9) |
| mg dL−1 | 150.2 (68.3) | 130.6 (74.6) | 140.2 (70.4) |
| Prior use of glargine, % | 100.0 | 100.0 | 100.0 |
BMI body mass index, FPG fasting plasma glucose, Gla-300 insulin glargine 300 U mL−1, Gla-100 insulin glargine 100 U mL−1, HbA 1c glycated hemoglobin, SD standard deviation
Table 2.
Glycemic control parameters and insulin doses during the study
| mITT population Parameter |
Subgroup 1 (N = 10) | Subgroup 2 (N = 10) | ||
|---|---|---|---|---|
| Treatment period 1 Gla-300 |
Treatment period 2 Gla-100 |
Treatment period 1 Gla-100 |
Treatment period 2 Gla-300 |
|
| HbA1c, % (mmol mol−1) | ||||
| Start of treatment perioda | 8.49 (69.3) | 8.21 (66.2) | 7.93 (63.2) | 7.66 (60.2) |
| End of treatment periodb | 8.21 (66.2) | 7.96 (63.5) | 7.66 (60.2) | 7.53 (58.8) |
| Laboratory-measured FPG, mmol L−1 (mg dL−1) | ||||
| Start of treatment perioda | 8.34 (150.2) | 8.70 (156.8) | 7.25 (130.6) | 7.22 (130.1) |
| End of treatment periodb | 8.70 (156.8) | 6.61 (119.0) | 7.22 (130.1) | 7.79 (140.3) |
| Mean daily basal insulin dose, U day−1 (U kg−1 day−1) | ||||
| Start of treatment perioda | 14.85 (0.23) | 18.40 (0.29) | 11.70 (0.21) | 12.75 (0.23) |
| End of treatment periodb | 18.13 (0.29) | 18.10 (0.28) | 13.14 (0.23) | 14.53 (0.26) |
| Mean daily mealtime insulin dose, U day−1 (U kg−1 day−1) | ||||
| Start of treatment perioda | 30.60 (0.48) | 29.80 (0.46) | 24.90 (0.43) | 25.40 (0.44) |
| End of treatment periodb | 29.90 (0.46) | 29.59 (0.45) | 24.50 (0.43) | 25.24 (0.44) |
| Mean daily total insulin dose, U day−1 (U kg−1 day−1) | ||||
| Start of treatment perioda | 45.45 (0.71) | 48.20 (0.75) | 36.60 (0.64) | 38.15 (0.67) |
| End of treatment periodb | 48.03 (0.75) | 47.69 (0.74) | 37.64 (0.66) | 39.77 (0.70) |
| Ratio of daily basal to total insulin dosec | ||||
| Start of treatment perioda | 0.33 | 0.38 | 0.32 | 0.33 |
| End of treatment periodb | 0.38 | 0.38 | 0.35 | 0.37 |
FPG fasting plasma glucose, Gla-300 insulin glargine 300 U mL−1, Gla-100 insulin glargine 100 U mL−1, HbA 1c glycated hemoglobin, mITT modified intent-to-treat
Data presented as the mean from all participants; a Start of treatment period represents Day 1 in treatment period 1 and Day 29 in treatment period 2; b End of treatment period represents Day 28 in treatment period 1 and Day 56 in treatment period 2; c calculated from U day−1 values
Efficacy
The 24-h glucose variability (AUCmean_24 h) was slightly lower with Gla-300 versus Gla-100 (treatment ratio 0.96, 90% CI 0.79–1.16) (Table 3). Glucose variability at night (AUCmean_noc) was also numerically lower for Gla-300 than for Gla-100 (treatment ratio 0.94, 90% CI 0.69–1.27). Neither difference was statistically significant.
Table 3.
Comparison of the effect of Gla-300 and Gla-100 treatment on glucose variability, measured as the absolute AUC above and below the individual average plasma glucose value in Day 2 (of 3) of continuous glucose monitoring (CGM)
| mITT population Parameter |
Screening (N = 20) |
Gla-300a
(N = 20) |
Gla-100a
(N = 20) |
Treatment ratio | 90% CI |
|---|---|---|---|---|---|
| AUCmean_24 h (min mg dL−1) | 65,679b | 59,757b | 60,409 | 0.96 | 0.79–1.16 |
| AUCmean_noc (min mg dL−1) | 6983b | 5337 | 5552 | 0.94 | 0.69–1.27 |
| AUCmean_daytime (min mg dL−1) | 49,108b | 45,654b | 44,875 | 1.01 | 0.84–1.21 |
AUC area under the curve, CI confidence interval, mITT modified intent-to-treat, Gla-300 insulin glargine 300 U mL−1, Gla-100 insulin glargine 100 U mL−1
Data presented as the mean from all participants; a Data pooled from subgroups 1 and 2 by treatment; b N = 19, one participant had missing CGM data so was excluded from this analysis
Glycated hemoglobin and laboratory-measured FPG values at the start and end of treatment periods 1 and 2 are shown in Table 2. HbA1c was generally stable with both Gla-300 and Gla-100, with only small decreases observed across each treatment period. A numerical decrease in FPG was observed over treatment period 2 with Gla-100, while remaining generally stable across treatment periods 1 and 2 with Gla-300 and treatment period 1 with Gla-100.
Daily basal, mealtime and total insulin doses are shown in Table 2. The ratio of the daily basal insulin dose to the total daily insulin dose at each time point is also shown. There was an increase in basal dose with Gla-300 and Gla-100 over treatment period 1 and with Gla-300 over treatment period 2, whereas, per protocol, mealtime insulin doses remained steady over both treatment periods in both groups.
Safety
The total number of confirmed or severe hypoglycemic events and percentage of participants experiencing ≥1 confirmed or severe hypoglycemic event at both ≤3.9 mmol L−1 (≤70 mg dL−1) and <3.0 mmol L−1 (<54 mg dL−1) thresholds were numerically lower during treatment with Gla-300 than with Gla-100 at any time of day (24 h) and particularly during the night (00:00–05:59 h) (Table 4).
Table 4.
Occurrence of hypoglycemic events during the on-treatment period
| Hypoglycemia definition | Hypoglycemia at any time of day (24 h) | Nocturnal hypoglycemia (00:00–05:59 h) | ||
|---|---|---|---|---|
| Gla-300a
(N = 20) |
Gla-100a
(N = 20) |
Gla-300a
(N = 20) |
Gla-100a
(N = 20) |
|
| Any hypoglycemia | ||||
| Participants experiencing ≥1 event during the treatment period, n (%) | 17 (85.0) | 20 (100.0) | 4 (20.0) | 8 (40.0) |
| Total number of events during the treatment period | 126 | 192 | 6 | 20 |
| Confirmed (≤3.9 mmol L−1 [≤70 mg dL−1]) or severe hypoglycemiab | ||||
| Participants experiencing ≥1 event during the treatment period, n (%) | 17 (85.0) | 20 (100.0) | 4 (20.0) | 8 (40.0) |
| Total number of events during the treatment period | 126 | 189 | 6 | 20 |
| Confirmed (<3.0 mmol L−1 [<54 mg dL−1]) or severe hypoglycemiab | ||||
| Participants experiencing ≥1 event during the treatment period, n (%) | 8 (40.0) | 12 (60.0) | 1 (5.0) | 4 (20.0) |
| Total number of events during the treatment period | 28 | 44 | 1 | 6 |
Gla-300 insulin glargine 300 U mL−1, Gla-100 insulin glargine 100 U mL−1
aData pooled from subgroups 1 and 2 by treatment
bConfirmed or severe hypoglycemia includes documented symptomatic, asymptomatic and severe hypoglycemia
Treatment-emergent AEs (TEAEs) were reported by 9/20 (45%) participants who received Gla-300 and 4/20 (20%) who received Gla-100; none were considered to be related to the study medication (Table 5). The most commonly reported TEAEs were gastrointestinal disorders, infections and infestations, nervous system disorders and musculoskeletal and connective tissue disorders. No TEAEs led to study discontinuation and there were no serious AEs.
Table 5.
Listing of treatment-emergent adverse events observed during the study
| Adverse events Primary system organ class Preferred term, number of participantsa (%) |
Gla-300 (N = 20)b |
Gla-100 (N = 20)b |
|---|---|---|
| Any class | 9 (45.0) | 4 (20.0) |
| Infections and infestations | 2 (10.0) | 2 (10.0) |
| Nasopharyngitis | 2 (10.0) | 2 (10.0) |
| Nervous system disorders | 2 (10.0) | 0 (0.0) |
| Dizziness postural | 1 (5.0) | 0 (0.0) |
| Headache | 1 (5.0) | 0 (0.0) |
| Gastrointestinal disorders | 3 (15.0) | 0 (0.0) |
| Abdominal discomfort | 1 (5.0) | 0 (0.0) |
| Dental caries | 1 (5.0) | 0 (0.0) |
| Stomatitis | 1 (5.0) | 0 (0.0) |
| Vomiting | 1 (5.0) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | 0 (0.0) | 1 (5.0) |
| Pruritus | 0 (0.0) | 1 (5.0) |
| Rash | 0 (0.0) | 1 (5.0) |
| Musculoskeletal and connective tissue disorders | 2 (10.0) | 0 (0.0) |
| Plantar fasciitis | 1 (5.0) | 0 (0.0) |
| Tenosynovitis | 1 (5.0) | 0 (0.0) |
| Reproductive system and breast disorders | 1 (5.0) | 0 (0.0) |
| Menstruation irregular | 1 (5.0) | 0 (0.0) |
| Injury, poisoning and procedural complications | 1 (5.0) | 1 (5.0) |
| Intentional overdose | 0 (0.0) | 1 (5.0) |
| Accidental overdose | 1 (5.0) | 0 (0.0) |
Gla-300 insulin glargine 300 U mL−1, Gla-100 insulin glargine 100 U mL−1
aSome participants experienced more than one adverse event
bData pooled from subgroups 1 and 2 by treatment
Discussion
In this pilot study with fixed daytime mealtime insulin and evening basal insulin injections and an in-house/hospital setting for CGM recording in a cohort of Japanese people with T1DM, 24-h glucose variability was not statistically different between the treatment groups, but there was a trend for lower variability with Gla-300 compared with Gla-100. The changes in HbA1c and FPG seen across the treatment periods were generally small and comparable between Gla-300 and Gla-100, although a numerical decrease in FPG was observed with Gla-100 during treatment period 2.
In addition, the percentage of participants experiencing ≥1 hypoglycemic event of any kind and total number of hypoglycemic events during the treatment period were lower for Gla-300 than for Gla-100, particularly during the night. This may be a result of more stable glucose control during the night with Gla-300 and is aligned with the more constant PK/PD profiles that have been described for Gla-300 compared with Gla-100 [1, 3]. Interestingly, a recent CGM study in a Western population, which used a basal to total insulin ratio of 0.5, described more stable glucose levels and lower variability between subjects, within day and between days, regardless of morning or evening injection time, as well as a reduction in the rate of confirmed (<3.0 mmol L−1 [<54 mg dL−1]) or severe hypoglycemia at any time and during the night with Gla-300 compared with Gla-100 [10]. Overall, Gla-300 and Gla-100, administered in the evening, were well tolerated during the study period and no specific safety concerns were observed.
The small, non-significant trend observed in this pilot study gives a first insight into the potential for lower glucose variability and lower incidence of hypoglycemia with Gla-300 compared with Gla-100 in a Japanese population. However, the study is limited by the small study population, requiring a larger study to confirm and assess the potential significance of the observations reported here. Other limitations include the open-label design that was necessary because of the different doses and injection pens that were used for the Gla-300 and Gla-100 treatments, the lack of a washout period, the short treatment period that may not provide a full picture of the insulin dose titration (treatment continued over a longer period in the main EDITION studies), and the potential impact that hospitalizing participants who are normally outpatients may have on glycemic control.
Conclusion
This exploratory study showed no between-treatment difference in glucose variability with Gla-300 versus Gla-100, over 24 h and at night, in Japanese people with T1DM. There was, however, a numerical reduction in the percentage of participants experiencing ≥1 hypoglycemic event, across the 24-h period and particularly at night. Gla-300 was well tolerated, with no specific safety concerns observed. Larger studies are needed to confirm these observations and assess whether the findings in this study could translate into clinical benefits for people with diabetes, allowing them to better manage their condition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The study was sponsored by Sanofi. The authors thank the study participants, trial staff and investigators for their participation. Medical writing services were provided by Catriona Marshall of Fishawack Communications Ltd, funded by Sanofi, Tokyo, Japan. Article processing charges were funded by Sanofi, Tokyo, Japan. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interest
Masayoshi Koyama: Employee and stock/shareholder of Sanofi.
Atsushi Amano: Employee and stock/shareholder of Sanofi.
Yoshinori Takahashi: Employee and stock/shareholder of Sanofi.
Reinhard Becker: Employee of Sanofi.
Hideaki Jinnouchi: Research support—Novo Nordisk Pharma Ltd., Eli Lilly and Company, Sumitomo Dainippon Pharma, GlaxoSmithKline K.K., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Taisho Pharmaceutical Co., Ltd, Astellas Pharma Inc., Boehringer Ingelheim GmbH. Honoraria for consulting and/or speaking—Boehringer Ingelheim GmbH, Sanofi K.K., Novo Nordisk Pharma Ltd., Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Company, Limited, Kissei Pharmaceutical Co., Ltd., Kowa Pharmaceutical Company Ltd., AstraZeneca K.K., Mochida Pharmaceutical Co., Ltd., Novartis Pharma K.K.
Kunio Hieshima: Other—Sanofi K.K., Novo Nordisk Pharma Ltd.
Seigo Sugiyama: Honoraria for consulting and/or speaking—MSD K.K., Boehringer Ingelheim GmbH, Novo Nordisk Pharma Ltd.
Noboru Kurinami: Honoraria for consulting and/or speaking—Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation.
Tomio Jinnouchi has no conflicts to declare.
Akira Yoshida: Honoraria for consulting and/or speaking—Sanofi K.K., MSD K.K., Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Boehringer Ingelheim GmbH, Abbott Japan Co., Ltd.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all participants before being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units mL−1. Diabetes Care. 2015;38:637–643. doi: 10.2337/dc14-0006. [DOI] [PubMed] [Google Scholar]
- 2.Becker RH, Nowotny I, Teichert L, Bergmann K, Kapitza C. Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab. 2015;17:261–267. doi: 10.1111/dom.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiramoto M, Eto T, Irie S, et al. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17:254–260. doi: 10.1111/dom.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle MC, Bolli GB, Zieman M, et al. New insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using basal and mealtime insulin: glucose and hypoglycemia in a 6-month randomized controlled trial (EDITION I) Diabetes Care. 2014;37:2755–2762. doi: 10.2337/dc14-0991. [DOI] [PubMed] [Google Scholar]
- 5.Yki-Järvinen H, Bergenstal RM, Zieman M, et al. New insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2) Diabetes Care. 2014;37:3235–3243. doi: 10.2337/dc14-0990. [DOI] [PubMed] [Google Scholar]
- 6.Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/mL compared with glargine 100 U/mL in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3) Diabetes Obes Metab. 2015;17:386–394. doi: 10.1111/dom.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Home PD, Bergenstal R, Riddle MC, et al. New insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015. doi:10.2337/dc15-0249 (In press). [DOI] [PubMed]
- 8.Matsuhisa M, Koyama M, Cheng X, Shimizu S, Hirose T. New insulin glargine 300 U/mL: glycemic control and hypoglycemia in Japanese people with T1DM (EDITION JP 1) Diabetologia. 2014;57:S400. [Google Scholar]
- 9.Terauchi Y, Koyama M, Cheng X, Shimizu S, Hirose T. Glycemic control and hypoglycaemia in Japanese people with type 2 diabetes mellitus receiving new insulin glargine 300 U/mL in combination with OADs (EDITION JP 2) Diabetologia. 2014;57:S401. [Google Scholar]
- 10.Bergenstal R, Bailey T, Robard D, et al. Insulin glargine 300 U/ml vs 100 U/ml: glucose profiles of morning vs evening injections in adults with T1DM measured with continuous glucose monitoring (CGM). Diabetes Technol Ther. 2015;17:A16-17 (abstract 39).
- 11.ICH. ICH harmonized tripartite guideline: Guideline for Good Clinical Practice E6 (R1). 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 21 May 2015. [PubMed]
- 12.World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. 2013. http://www.wma.net/en/30publications/10policies/b3/index.html.pdf?print-media-type&footer-right=[page]/[toPage]. Accessed 21 May 2015. [DOI] [PubMed]
- 13.Workgroup on Hypoglycemia ADA Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


