Abstract
Introduction
Both dipeptidyl peptidase-4 inhibitors and α-glucosidase inhibitors (α-GI) have been reported to change the incretin and insulin secretion. To examine the effects of acarbose, miglitol, and sitagliptin on glucose metabolism and secretion of gut peptides, we conducted a crossover study in patients with type 2 diabetes mellitus (T2DM).
Methods
Eleven Japanese patients with T2DM underwent four meal tolerance tests with single administration of acarbose, miglitol, sitagliptin, or nothing. Fasting and postprandial plasma levels of glucose, insulin, glucagon, active glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), ghrelin, and des-acyl ghrelin were measured.
Results
Early-phase insulin secretion was reduced following acarbose and miglitol, and the areas under the curve (AUC) of insulin at 180 min following acarbose and miglitol were significantly lower than sitagliptin. AUC of plasma glucose at 180 min after acarbose, miglitol, and sitagliptin tended to be lower than in controls, and plasma glucose levels at 30–60 min following miglitol were significantly lower than in controls. Plasma glucagon, ghrelin, and des-acyl ghrelin levels did not differ among the four conditions. Postprandial plasma active GLP-1 levels and AUC of GLP-1 increased significantly in both the sitagliptin and miglitol groups compared to control. Postprandial plasma total GIP levels increased following sitagliptin but decreased after acarbose and miglitol. Changes in incretin levels tended to be greater with miglitol than acarbose.
Conclusion
These results showed that sitagliptin and α-GIs, miglitol more so than acarbose, improved hyperglycemia in patients with T2DM after single administration, and had different effects on insulin and incretin secretion.
Trial registration: UMIN-CTR number, UMIN000009981.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0113-3) contains supplementary material, which is available to authorized users.
Keywords: Acarbose, Glucagon-like peptide-1, Glucose-dependent insulinotropic polypeptide, Insulin, Japanese patients, Miglitol, Sitagliptin, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) has been increasing worldwide [1] and reduces quality of life due to various complications. To improve blood glucose control, seven types of anti-diabetic oral drugs have been approved for use in Japan. Each type reduces plasma glucose levels by a different mechanism, and recently incretin-related drugs have attracted particular attention. Dipeptidyl peptidase-4 (DPP-4) inhibitors increase active glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) by inhibiting DPP-4 enzymatic activity, and improve hyperglycemia in patients with diabetes in a glucose-dependent manner by increasing insulin secretion [2]. A meta-analysis showed that DPP-4 inhibitors improved glycated hemoglobin (HbA1c) levels by approximately 0.77% after 1 year of treatment [3], and randomized trials to evaluate their cardiovascular safety are in progress [4].
α-Glucosidase inhibitors (α-GIs), including acarbose, voglibose, and miglitol, delay the absorption of carbohydrates from the small intestine by inhibiting their degradation, thus reducing both postprandial glucose and insulin levels [5]. In addition, α-GIs have been reported to increase GLP-1 and decrease GIP levels in healthy subjects [6–8]. These findings can be explained by the fact that α-GIs move the absorption site of carbohydrates from the GIP-producing upper portions of intestine to the GLP-1-producing lower intestine. While acarbose and voglibose did not increase plasma GLP-1 levels after a mixed meal in patients with T2DM [9–11], miglitol significantly increased these levels [10, 12–15]. The reason for this discrepancy is thought to be that only miglitol is rapidly and almost completely absorbed from the small intestine after oral administration, resulting in increased stimulation of GLP-1 secretion in the lower intestine. However, no previous studies have evaluated subjects who each received acarbose, miglitol, and sitagliptin, and the effects of these drugs on glucose metabolism and the levels of gut peptides, including GLP-1, GIP, ghrelin, and des-acyl ghrelin. Therefore, the current study investigated these issues in patients with T2DM using a crossover design.
Methods
Subjects
Eleven Japanese patients aged 20–79 years with T2DM, who had fair to good control of blood glucose levels (HbA1c <8.5%) while on diet and exercise therapies, with or without an oral hypoglycemic agent, were enrolled from our outpatient clinic. Exclusion criteria were as follows: (1) serious infection, pre- or postoperative condition, or severe trauma; (2) pregnancy, possible pregnancy, or breastfeeding; (3) moderate or severe renal dysfunction [estimated glomerular filtration ratio (mL/min/1.73 m2) <50 mL/min, serum creatinine level >1.5 mg/dL in men or >1.3 mg/dL in women]; (4) severe liver dysfunction; and (5) treatment with insulin, α-GI, or DPP-4 inhibitor.
This study was approved by the ethics committee of the Faculty of Medicine of the University of Miyazaki and was conducted in accordance with the Helsinki Declaration of 1964, as revised in 2013. Written informed consent was obtained from all participants before enrolment. The study was registered in UMIN-CTR number, UMIN000009981.
Study Protocol
Sitagliptin, miglitol, or acarbose was administered according to four different intake schedules: (1) control, no drug; (2) sitagliptin 50 mg administered 30 min before the start of the meal; (3) miglitol 50 mg administered just before the meal; and (4) acarbose 100 mg administered just before the meal. Subjects were randomized to one of the four interventions using a crossover design, and were asked to take each medication after a washout period lasting more than 1 week. After an overnight fast, a meal tolerance test (510 kcal, carbohydrate 56%, protein 17%, fat 27%) was conducted four times. The plasma concentrations of glucose, insulin, glucagon, active GLP-1, total GIP, ghrelin, and des-acyl ghrelin were measured before and 30, 60, 120, and 180 min after the subject consumed the test meal.
Laboratory Measurements
Plasma glucose was measured by the hexokinase method. Serum insulin and plasma glucagon were measured by chemiluminescent enzyme immunoassay kit (Lumipulse Presto insulin, Fujirebio Inc., Tokyo, Japan) and double antibody radioimmunoassay method (Glucagon RIA kit, Cosmic corporation, Tokyo, Japan), respectively. To measure plasma concentrations of active GLP-1 and total GIP, blood was collected directly into chilled tubes containing a DPP-4 inhibitor, protease inhibitor, and esterase inhibitor (P700, Becton, Dickinson and Company, Franklin Lakes, NJ), and then immediately centrifuged at 1468g for 15 min at 4 °C. The plasma samples were subjected to solid-phase extraction as described elsewhere [16]. Oasis HLB Extraction Cartridges (Waters, Milford, MA) were equilibrated with 1 mL of methanol, and then with 1 mL of distilled water. Plasma samples were applied to the wells of the equilibrated plates. Samples were then washed twice with 1 mL of 10% methanol in distilled water, and then peptides were eluted with 0.5 mL of 0.5% NH3/75% ethanol in distilled water. Eluates were freeze-dried and reconstituted in solutions supplemented with commercial immunoassay kits. Active GLP-1 [7–36] NH2 and total GIP were measured using the Glucagon Like Peptide-1 (Active) ELISA kit (Millipore, St. Charles, MO) and the Human GIP Assay kit (Immuno-Biological Laboratories, Gunma, Japan), respectively. To measure plasma ghrelin and des-acyl ghrelin, blood was withdrawn directly into chilled P700 tubes and centrifuged at 1468g for 15 min at 4 °C. The plasma was treated with 10% volume of 1 N hydrochloric acid. The acylated and des-acylated forms of ghrelin were measured using fluorescence enzyme immunoassays (Tosoh Corporation, Tokyo, Japan) [17].
Calculations
The areas under the curve (AUCs) from just before the meal to 180 min after the start of the meal were calculated using the trapezoid method. Test meal-stimulated insulin secretion was evaluated based on the insulinogenic index, oral disposition index (calculated as Δinsulin0–30/Δglucose0–30 × 1/fasting insulin), AUCinsulin/AUCglucose ratio, and homeostasis model assessment of beta cell function (HOMA-β).
Statistical Analysis
Data are shown as mean ± standard deviation. The analyses for each value at 0, 30, 60, 90, 120, and 180 min and the AUCs were performed using one-way layout analysis of variance (ANOVA) with post hoc analysis (Turkey’s test). Statistical analyses of data were performed with Prism 5.0 (GraphPad Software, La Jolla, CA). A P value less than 0.05 was considered to represent a statistically significant difference.
Results
Baseline Characteristics of Subjects
Eleven subjects (eight men and three women) were enrolled in this study. Their characteristics are shown in Table 1. The numbers of patients treated with diet and exercise only, metformin 750 mg/day, gliclazide 20 mg/day, and pioglitazone 15 mg/day were 6, 3, 1, and 1, respectively.
Table 1.
Clinical characteristics of participants
| Characteristic | Mean ± SDa | Range |
|---|---|---|
| Men/women, n | 8/3 | |
| Age (years) | 56.1 ± 16.2 | 23–73 |
| Duration (years) | 10.9 ± 6.3 | 3–24 |
| Body weight (kg) | 71 ± 11.9 | 50.8–90.4 |
| Body mass index (kg/m2) | 26.2 ± 4.7 | 20.5–34.4 |
| HbA1c (%) | 7.1 ± 0.7 | 5.9–8.1 |
| Use of SU, n (%) | 1 (9.1) | |
| Use of MET, n (%) | 3 (27.3) | |
| Use of TZD, n (%) | 1 (9.1) | |
| Diet alone, n (%) | 6 (54.5) |
HbA1c glycated hemoglobin, MET metformin, SD standard deviation, SU sulfonylurea, TZD thiazolidinedione
aData are expressed as mean ± SD unless otherwise stated
Glucose Metabolism
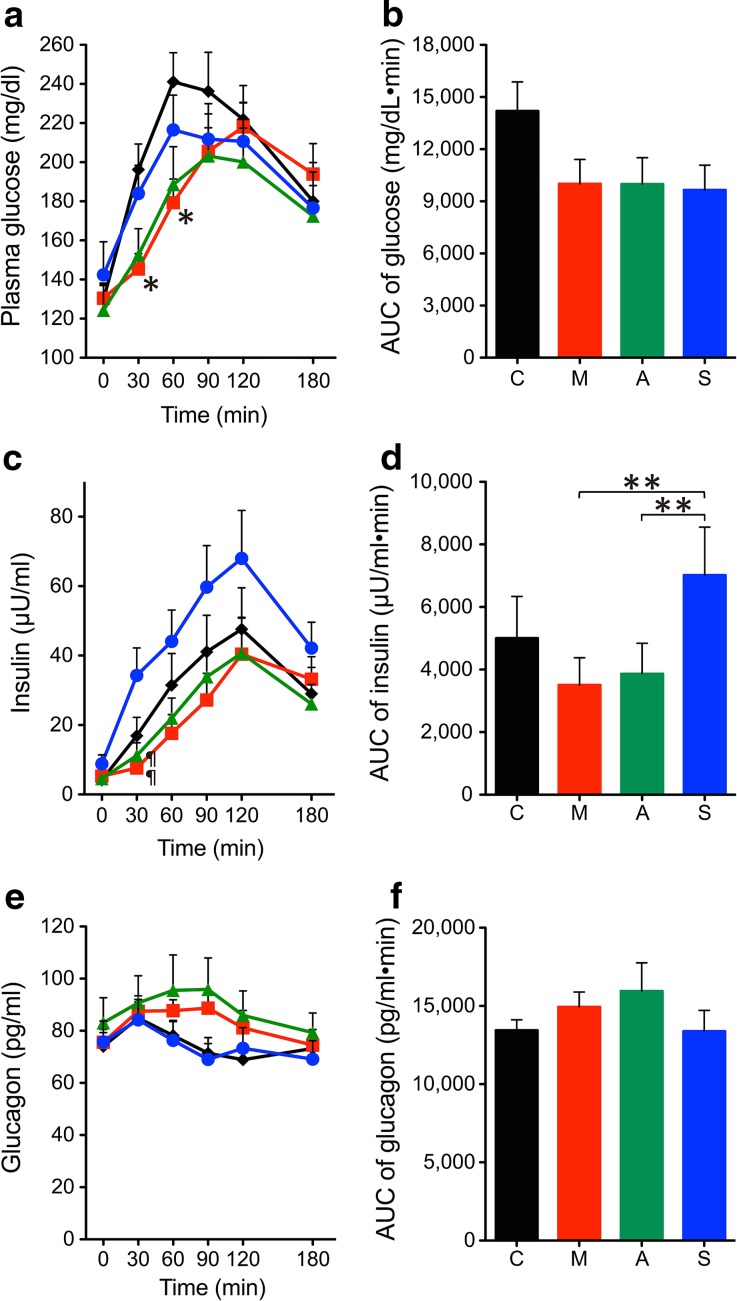
The time profiles and AUCs of plasma glucose, insulin, and glucagon levels are shown in Fig. 1. The plasma glucose levels at 30 and 60 min after the start of the test meal were significantly different among the four conditions analyzed by ANOVA (P = 0.017 and P = 0.043 at 30 and 60 min, respectively), and those in the miglitol group were significantly lower than those in the control group (Fig. 1a). The AUCs of plasma glucose levels were also significantly different among the four conditions (P = 0.032 by ANOVA), but there was no significant difference after post hoc analysis (Fig. 1b). The serum insulin levels at 30 min after the start of the test meal were significantly different among the four conditions (P = 0.004 by ANOVA), and those in the sitagliptin group were significantly higher than those in both the acarbose and miglitol groups (Fig. 1c). The AUCs of serum insulin levels were significantly different among the four conditions (P = 0.007 by ANOVA), and those in the sitagliptin group were significantly higher than those in both the miglitol and acarbose groups (Fig. 1d). Plasma glucagon levels and their AUCs did not differ among the four conditions (Fig. 1e–f). Insulinogenic index, oral disposition index, AUCinsulin/AUCglucose ratio, and HOMA-β also did not differ among the four conditions (data not shown).
Fig. 1.
Effects of miglitol, acarbose, and sitagliptin on glucose metabolism in the meal tolerance test. Changes are illustrated for a plasma glucose, b glucose AUC0–180, c insulin, d insulin AUC0–180, e glucagon, and f glucagon AUC0–180. Black, red, green, and blue indicate control (C), miglitol (M), acarbose (A), and sitagliptin (S), respectively. *P < 0.05 vs. control, ¶ P < 0.05 vs. sitagliptin. **P < 0.05 between the groups. AUC areas under the curve
Gastrointestinal Peptides
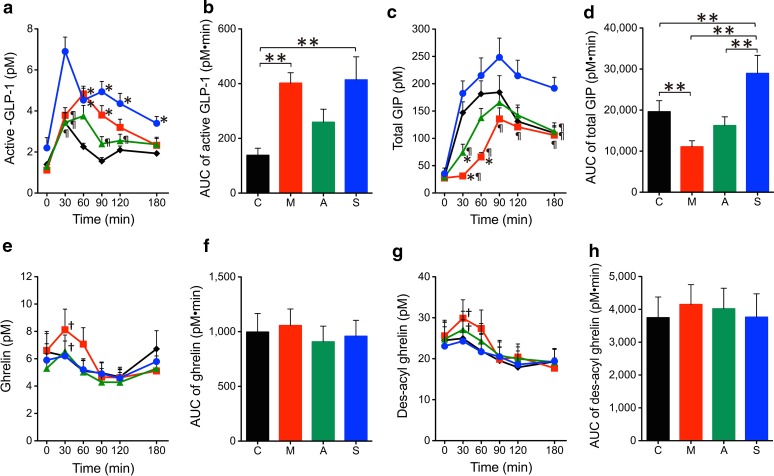
The time profiles and AUCs of plasma active GLP-1, total GIP, ghrelin, and des-acyl ghrelin levels are shown in Fig. 2. The plasma active GLP-1 levels at 30–180 min after the start of the test meal were significantly different among the four conditions analyzed by ANOVA (P < 0.0001, P = 0.0007, P < 0.0001, P = 0.0007, and P = 0.001 at 30, 60, 90, 120, and 180 min, respectively), and those in the sitagliptin group were significantly higher than those in the control group. In addition, plasma active GLP-1 levels at 90 and 120 min in the sitagliptin group were significantly higher than those in the acarbose group. The plasma active GLP-1 levels at 60 and 90 min in the miglitol group were significantly higher than those in the control group (Fig. 2a). The AUCs of plasma active GLP-1 levels were significantly different among the four conditions (P = 0.004 by ANOVA), and those in the miglitol and sitagliptin group were significantly higher than those in the control group (Fig. 2b). The plasma total GIP levels at 30 to 180 min after the start of the test meal were significantly different among the four conditions analyzed by ANOVA (P < 0.0001, P = 0.0002, P = 0.037, P = 0.023, and P = 0.001 at 30, 60, 90, 120, and 180 min, respectively), and those in the sitagliptin group were significantly higher than those in the miglitol group (Fig. 2c). In addition, plasma total GIP levels at 30 and 60 min in the miglitol group were significantly lower than those in the control group. The plasma total GIP levels at 30 min in the acarbose group were significantly lower than those in the control group (Fig. 2c). The AUCs of plasma total GIP levels were significantly different among the four conditions (P < 0.0001 by ANOVA), those in the sitagliptin group were significantly higher than in the other groups, and those in the miglitol group were significantly lower than those in the control group (Fig. 2d). Plasma ghrelin and des-acyl ghrelin levels and their AUCs did not differ among the four conditions (Fig. 2e–h). Both plasma ghrelin and des-acyl ghrelin levels at 30 min after the test meal significantly increased compared to basal levels in the miglitol and acarbose groups but not in the sitagliptin and control groups (Fig. 2e, g).
Fig. 2.
Effects of miglitol, acarbose, and sitagliptin on gut peptides in the meal tolerance test. Changes are illustrated for a plasma active GLP-1, b active GLP-1 AUC0–180, c total GIP, d total GIP AUC0–180, e ghrelin, f ghrelin AUC0-180, g des-acyl ghrelin, and h des-acyl ghrelin AUC0–180. Black, red, green, and blue indicate control (C), miglitol (M), acarbose (A), and sitagliptin (S), respectively. *P < 0.05 vs. control. ¶ P < 0.05 vs. sitagliptin. † P < 0.05 vs. basal level in the same group. **P < 0.05 between the groups. AUC areas under the curve, GIP glucose-dependent insulinotropic polypeptide, GLP-1 glucagon-like peptide-1
Discussion
In this study, a crossover design was used to examine the changes in gastrointestinal peptides and glucose metabolism in a single sample of patients with T2DM following single administration of acarbose, miglitol, or sitagliptin. Although the AUCs of plasma glucose levels were similar in the acarbose, miglitol, and sitagliptin groups, the secretion patterns of insulin, GLP-1, and GIP differed among these drugs.
In the present study, α-GIs increased plasma GLP-1 levels and reduced plasma GIP levels, consistent with previous reports [6, 12, 15, 18, 19]. Miglitol, but not acarbose, significantly increased the AUC of GLP-1 levels and reduced the AUC of GIP levels as compared to controls. These results are similar to those of two previous reports [10, 14], but the current study first examined the effects of single administration in a crossover design. Because miglitol is absorbed from the small intestine after oral administration [20], miglitol is present for a shorter period of time in the lower intestine than other α-GIs. Therefore, GLP-1 producing L cells can be stimulated by reached carbohydrate more with miglitol than acarbose and voglibose. Recently, miglitol, but not acarbose, was reported to increase GLP-1 secretion in rodents via α-glucosidase inhibition as well as an additional mechanism [21]. Because miglitol is structurally different from acarbose and voglibose, only miglitol could activate duodenal enterochromaffin cells, possibly via sodium–glucose co-transporter 3, and potentiate GLP-1 secretion through the parasympathetic nervous system [21]. If this mechanism is also common in humans, the effect of miglitol against GLP-1 secretion will be low in patients with severe autonomic neuropathy. As this study included no subjects with clinically evident autonomic neuropathy, future studies clarifying this mechanism in humans should include patients with this condition. Although postprandial plasma GLP-1 concentrations increased after administration of metformin in healthy subjects [22], they did not differ in our three patients who had been taking metformin compared to other patients (data not shown).
Plasma GIP levels are known to rise after meals in healthy individuals, but this increase was attenuated following α-GI administration in both normal subjects and patients with T2DM [6–10, 12, 15, 18, 23]. This phenomenon was seen unlike the result of GLP-1 in all three α-GIs. In this study, the increment of postprandial GIP levels was the smallest in the miglitol group, but the difference between the miglitol and acarbose groups did not reach statistical significance. The increase in postprandial plasma GIP levels was enhanced by the DPP-4 inhibitor sitagliptin, as shown in previous reports [6, 24]. The incretin effect of GIP is reported to few in the diabetic condition compared to normal, and GIP stimulates fat accumulation and glucagon secretion [25, 26]. Therefore, suppression of postprandial GIP secretion by α-GIs, especially miglitol, is useful for the treatment of obese patients with T2DM.
Plasma ghrelin levels were shown to increase after fasting and decrease in the postprandial state in both healthy subjects and patients with T2DM [27, 28]. However, in this study both plasma ghrelin and des-acyl ghrelin levels at 30 min after the test meal significantly increased compared to basal levels in the miglitol and acarbose groups but not in the sitagliptin and control groups. Kaku et al. [29] reported that miglitol resulted in decreased plasma ghrelin levels at 60–180 min after a test meal in healthy subjects, but they did not measure plasma ghrelin levels at 30 min. Four weeks of treatment with acarbose significantly enhanced postprandial total ghrelin suppression at 120 min in patients with T2DM [30]; however, in healthy subjects a single treatment with acarbose increased postprandial total ghrelin levels at 120 min only compared to drug-free controls [31]. Plasma ghrelin levels were reported to inversely correlate with serum insulin levels, and increased after taking protein-rich meat when serum insulin level increased only just a little [32]. In this study, the increments of serum insulin levels at 30 min in the acarbose and miglitol groups were significantly smaller than those of the sitagliptin and control groups (12.7 ± 4.5, 2.3 ± 1.2, 6.6 ± 3.4, and 25.5 ± 7.0 µU/ml in the control, miglitol, acarbose, and sitagliptin control groups, respectively). These findings may be the reason for the increased plasma ghrelin levels observed at 30 min in miglitol and acarbose groups, but the mechanisms should be examined in more detail in the future. On the other hand, previous studies reported that the single administration of sitagliptin did not change postprandial total ghrelin levels in healthy subjects, and the administration of vildagliptin for 10 days did not change these levels in patients with T2DM [33, 34]. These results are consistent with our own.
Since both α-GIs and sitagliptin increase plasma GLP-1 levels, plasma glucagon concentrations were similar at all time points under the four conditions evaluated in this study. A previous report found that plasma glucagon levels decreased after a meal tolerance test following 4 weeks administration of linagliptin in patients with T2DM [35]. Postprandial plasma glucagon levels following acarbose administration increased in normal subjects and were unchanged in patients with T2DM [9, 18]. Although plasma glucagon levels are thought to be suppressed by increased levels of plasma GLP-1, all plasma glucagon measurement kits have relatively low specificity and sensitivity, including that used in our study [36]. Therefore, the true effects of α-GIs and DPP-4 inhibitors on glucagon secretion must be examined in the future.
The present study had several limitations. The number of subjects was small, the drugs were only administered once to each patient, and only the total GIP level was measured. Therefore, larger-scale and longer-term studies are needed in the future.
Conclusions
Our results show that sitagliptin and α-GIs, miglitol more so than acarbose, improved hyperglycemia in patients with T2DM, and had different effects on insulin and incretin secretion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are very thankful to M. Oshikawa in the Division of Neurology, Respirology, Endocrinology and Metabolism at the Department of Internal Medicine, University of Miyazaki. This study was supported by a Grant-in-Aid for Clinical Research from Miyazaki University Hospital, whose support is deeply appreciated. The article processing charges for this publication were funded by a Grant-in-Aid for Clinical Research from Miyazaki University Hospital. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Hiroaki Ueno, Wakaba Tsuchimochi, Hong-Wei Wang, Eiichiro Yamashita, Chikako Tsubouchi, Kazuhiro Nagamine, Hideyuki Sakoda, and Masamitsu Nakazato declare no conflicts of interest.
Compliance with ethics guidelines
This study was approved by the ethics committee of the Faculty of Medicine of the University of Miyazaki and was conducted in accordance with the Helsinki Declaration of 1964, as revised in 2013. Written informed consent was obtained from all participants before enrolment. The study was registered in UMIN-CTR number, UMIN000009981.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Contributor Information
Hiroaki Ueno, Email: intron@med.miyazaki-u.ac.jp.
Masamitsu Nakazato, Email: nakazato@med.miyazaki-u.ac.jp.
References
- 1.Zimmet PZ, Magliano DJ, Herman WH, et al. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 2.Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2:101–121. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito K, Chiodini P, Maiorino MI, et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open. 2015;5:e005892. doi: 10.1136/bmjopen-2014-005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethel MA, Green JB, Milton J, et al. Regional, age and sex differences in baseline characteristics of patients enrolled in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) Diabetes Obes Metab. 2015;17:395–402. doi: 10.1111/dom.12441. [DOI] [PubMed] [Google Scholar]
- 5.Scheen AJ. Is there a role for alpha-glucosidase inhibitors in the prevention of type 2 diabetes mellitus? Drugs. 2003;63:933–951. doi: 10.2165/00003495-200363100-00002. [DOI] [PubMed] [Google Scholar]
- 6.Aoki K, Masuda K, Miyazaki T, et al. Effects of miglitol, sitagliptin or their combination on plasma glucose, insulin and incretin levels in non-diabetic men. Endocr J. 2010;57:667–672. doi: 10.1507/endocrj.K10E-103. [DOI] [PubMed] [Google Scholar]
- 7.Goke B, Fuder H, Wieckhorst G, et al. Voglibose (AO-128) is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserve. Digestion. 1995;56:493–501. doi: 10.1159/000201282. [DOI] [PubMed] [Google Scholar]
- 8.Ranganath L, Norris F, Morgan L, et al. Delayed gastric emptying occurs following acarbose administration and is a further mechanism for its anti-hyperglycaemic effect. Diabet Med. 1998;15:120–124. doi: 10.1002/(SICI)1096-9136(199802)15:2<120::AID-DIA529>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Hucking K, Kostic Z, Pox C, et al. alpha-Glucosidase inhibition (acarbose) fails to enhance secretion of glucagon-like peptide 1 (7-36 amide) and to delay gastric emptying in Type 2 diabetic patients. Diabet Med. 2005;22:470–476. doi: 10.1111/j.1464-5491.2005.01451.x. [DOI] [PubMed] [Google Scholar]
- 10.Hiki M, Shimada K, Kiyanagi T, et al. Single administration of alpha-glucosidase inhibitors on endothelial function and incretin secretion in diabetic patients with coronary artery disease—Juntendo University trial: effects of miglitol on endothelial vascular reactivity in type 2 diabetic patients with coronary heart disease (J-MACH) Circ J. 2010;74:1471–1478. doi: 10.1253/circj.CJ-10-0013. [DOI] [PubMed] [Google Scholar]
- 11.DeLeon MJ, Chandurkar V, Albert SG, et al. Glucagon-like peptide-1 response to acarbose in elderly type 2 diabetic subjects. Diabetes Res Clin Pract. 2002;56:101–106. doi: 10.1016/S0168-8227(01)00359-X. [DOI] [PubMed] [Google Scholar]
- 12.Narita T, Katsuura Y, Sato T, et al. Miglitol induces prolonged and enhanced glucagon-like peptide-1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese Type 2 diabetic patients. Diabet Med. 2009;26:187–188. doi: 10.1111/j.1464-5491.2008.02651.x. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa M, Ebato C, Mita T, et al. Miglitol suppresses the postprandial increase in interleukin 6 and enhances active glucagon-like peptide 1 secretion in viscerally obese subjects. Metabolism. 2008;57:1299–1306. doi: 10.1016/j.metabol.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Narita T, Yokoyama H, Yamashita R, et al. Comparisons of the effects of 12-week administration of miglitol and voglibose on the responses of plasma incretins after a mixed meal in Japanese type 2 diabetic patients. Diabetes Obes Metab. 2012;14:283–287. doi: 10.1111/j.1463-1326.2011.01526.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Patrick P, Wishart J, et al. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab. 2002;4:329–335. doi: 10.1046/j.1463-1326.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- 16.Yabe D, Watanabe K, Sugawara K, et al. Comparison of incretin immunoassays with or without plasma extraction: incretin secretion in Japanese patients with type 2 diabetes. J Diabetes Investig. 2012;3:70–79. doi: 10.1111/j.2040-1124.2011.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akamizu T, Sakura N, Shigematsu Y, et al. Analysis of plasma ghrelin in patients with medium-chain acyl-CoA dehydrogenase deficiency and glutaric aciduria type II. Eur J Endocrinol. 2012;166:235–240. doi: 10.1530/EJE-11-0785. [DOI] [PubMed] [Google Scholar]
- 18.Seifarth C, Bergmann J, Holst JJ, et al. Prolonged and enhanced secretion of glucagon-like peptide 1 (7-36 amide) after oral sucrose due to alpha-glucosidase inhibition (acarbose) in Type 2 diabetic patients. Diabet Med. 1998;15:485–491. doi: 10.1002/(SICI)1096-9136(199806)15:6<485::AID-DIA610>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Enc FY, Imeryuz N, Akin L, et al. Inhibition of gastric emptying by acarbose is correlated with GLP-1 response and accompanied by CCK release. Am J Physiol Gastrointest Liver Physiol. 2001;281:G752–G763. doi: 10.1152/ajpgi.2001.281.3.G752. [DOI] [PubMed] [Google Scholar]
- 20.Ahr HJ, Boberg M, Brendel E, et al. Pharmacokinetics of miglitol. Absorption, distribution, metabolism, and excretion following administration to rats, dogs, and man. Arzneimittelforschung. 1997;47:734–745. [PubMed] [Google Scholar]
- 21.Lee EY, Kaneko S, Jutabha P, et al. Distinct action of the alpha-glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J Endocrinol. 2015;224:205–214. doi: 10.1530/JOE-14-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin Pharmacol Ther. 2010;88:801–808. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
- 23.Nagai E, Katsuno T, Miyagawa J, et al. Incretin responses to oral glucose load in Japanese non-obese healthy subjects. Diabetes Ther. 2011;2:20–28. doi: 10.1007/s13300-010-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–4619. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- 25.Irwin N, Flatt PR. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia. 2009;52:1724–1731. doi: 10.1007/s00125-009-1422-8. [DOI] [PubMed] [Google Scholar]
- 26.Meier JJ, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801. doi: 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 27.Tschop M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21. [DOI] [PubMed]
- 28.Ueno H, Shiiya T, Mizuta M, et al. Plasma ghrelin concentrations in different clinical stages of diabetic complications and glycemic control in Japanese diabetics. Endocr J. 2007;54:895–902. doi: 10.1507/endocrj.K07-007. [DOI] [PubMed] [Google Scholar]
- 29.Kaku H, Tajiri Y, Yamada K. Anorexigenic effects of miglitol in concert with the alterations of gut hormone secretion and gastric emptying in healthy subjects. Horm Metab Res. 2012;44:312–318. doi: 10.1055/s-0032-1304563. [DOI] [PubMed] [Google Scholar]
- 30.Zheng F, Yin X, Lu W, et al. Improved post-prandial ghrelin response by nateglinide or acarbose therapy contributes to glucose stability in Type 2 diabetic patients. J Endocrinol Invest. 2013;36:489–496. doi: 10.3275/8811. [DOI] [PubMed] [Google Scholar]
- 31.Tai K, Hammond AJ, Wishart JM, et al. Carbohydrate and fat digestion is necessary for maximal suppression of total plasma ghrelin in healthy adults. Appetite. 2010;55:407–412. doi: 10.1016/j.appet.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Erdmann J, Topsch R, Lippl F, et al. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89:3048–3054. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 33.Huang CL, Hsu CH, Huang KC, et al. Preprandial single oral dose of sitagliptin does not affect circulating ghrelin and gastrin levels in normal subjects. Pharmacology. 2010;85:131–135. doi: 10.1159/000280583. [DOI] [PubMed] [Google Scholar]
- 34.Oz O, Kiyici S, Ersoy C, et al. Effect of sitagliptin monotherapy on serum total ghrelin levels in people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:212–216. doi: 10.1016/j.diabres.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Rauch T, Graefe-Mody U, Deacon CF, et al. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Ther. 2012;3:10. doi: 10.1007/s13300-012-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bak MJ, Albrechtsen NW, Pedersen J, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014;170:529–538. doi: 10.1530/EJE-13-0941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.