Abstract
OBJECTIVE
Plasma soluble CD40L (sCD40L) is increased during human immunodeficiency virus-1 (HIV) infection, but it is unknown whether it circulates in monomeric or multimeric forms, and whether the circulating forms have differential effects on myeloid dendritic cell (DC) function and adaptive regulation.
DESIGN
sCD40L forms were measured in plasma samples from HIV-infected donors. The effects of sCD40L forms on DC function were measured in vitro.
METHODS
To delineate which forms of sCD40L are present in plasma from HIV-infected donors, immunoblots were performed following enrichment of plasma for medium and low abundance proteins. DCs from seronegative donors were exposed to multiple forms of sCD40L prior to Toll-like receptor (TLR) stimulation and DC function and adaptive regulation was assessed in vitro.
RESULTS
Monomeric and multimeric forms of sCD40L were identified in plasma from ART-treated HIV-infected donors. Though monomeric and multimeric forms of sCD40L had differential effects on DC activation when given alone, both strongly suppressed secretion of the Th1 skewing cytokine, IL-12, upon subsequent TLR stimulation. Furthermore, DCs exposed to both monomeric and multimeric sCD40L induced regulatory T cell formation and T cell anergy.
CONCLUSIONS
Elevated sCD40L during HIV infection impairs DC function, contributing to innate and adaptive immune dysfunction. Antiretroviral adjunctive therapies that decrease sCD40L may provide immune modulatory benefits.
Introduction
Myeloid dendritic cells (DCs) are antigen presenting cells (APC) that orchestrate immune responses, serving as critical links between innate and adaptive immunity. DCs are powerful stimulators of CD4+ and CD8+ T cells and are responsible for priming antigen-specific T-cell responses[1]. DCs sense pathogens through pattern recognition receptors, leading to activation of signaling pathways that result in the expression of costimulatory molecules and secretion of cytokines that regulate the adaptive immune responses[2]. During Human Immunodeficiency Virus-1 (HIV) infection there is evidence of DC dysregulation and exhaustion, potentially contributing to generalized immune activation and inadequate adaptive immune responsiveness, which are hallmarks of HIV pathogenesis[3]. The etiology of DC dysregulation during chronic HIV infection is incompletely understood, but we have recently shown that soluble plasma factors, independent of HIV itself, are contributors[4, 5]. Improved understanding of the soluble plasma factors that lead to DC dysregulation could provide therapeutic targets to potentially decrease immune activation, inflammation, and improve adaptive immune responses during HIV infection.
CD40 ligand (CD40L), also known as CD154, is a type II membrane glycoprotein of the tumor necrosis (TNF) family that is expressed as a trimer on activated T cells, B cells, monocytes, macrophages, endothelial cells, and platelets[6]. Cell-associated CD40L binds to its receptor CD40 on the surface of APCs to induce activation and to enhance the expression of B7 molecules to promote T cell expansion [6]. Cell-associated CD40L binding also induces proliferation and immunoglobulin class switching of B cells[7]. Upon activation in vitro, cells shed a soluble form of CD40L in the form of a truncated 18 kDa monomer [8-10]. According to cellular distribution studies, platelets contribute to the majority of circulating soluble CD40 ligand (sCD40L) in plasma[11].
Plasma sCD40L levels are increased in various inflammatory states, such as cancer[12] and HIV infection[13-15], including HIV-associated neuroinflammation[16, 17]. A major cause of increased sCD40L levels during chronic HIV infection is generalized platelet activation. We and others have shown that platelets have an activated phenotype during HIV infection, with increased expression of CD62 P-selectin, and hyperreactive responses to ex-vivo stimulation with platelet activating agents[18-20]. It is currently unknown whether sCD40L circulates as multimeric or monomeric forms in plasma during HIV infection. This distinction is important because these forms have differential immunologic effects. Multimeric forms of sCD40L are immunostimulatory to APCs [6, 21] whereas monomeric forms of sCD40L play an overall immunosuppressive role [12-14]. Monomeric forms of sCD40L stimulate monocytes to express a pro-inflammatory phenotype and render both monocytes and plasmacytoid dendritic cells refractory to stimulation with Toll-like receptor (TLR) ligand stimulation [12, 14, 22]. Monomeric sCD40L exposure also favors the development of myeloid-derived suppressor cells (MDSCs) and regulatory T cells, both of which are elevated during HIV infection[12, 13].
In this study we focused on the characterization of sCD40L forms that circulate in the plasma of HIV-infected individuals. Though it is well established that levels of sCD40L are elevated during HIV infection[13, 15, 23] , it is not understood which forms of sCD40L are circulating, nor their differential effects on DC function. We found that both monomeric and multimeric forms of sCD40L are circulating in plasma. We tested whether there were differences in the ability of monomeric forms as compared to multimeric forms to affect DC phenotype and function as it relates to T cell responsiveness and differentiation. We found that monomeric forms are much less DC-activating than multimeric forms, but both forms ultimately result in DC dysfunction and related T cell suppression upon subsequent TLR stimulation. We further tested plasma samples from our previously published study and detail preliminary findings for the effectiveness of low-dose aspirin in decreasing sCD40L during ART-treated chronic HIV infection, highlighting the potential for further testing of anti-platelet agents as immune modulatory therapy in chronic HIV infection.
Methods
Study population
The HIV-infected subjects enrolled in this cross-sectional study who donated plasma samples for immunoblot analysis of circulating sCD40L were recruited through New York University AIDS Clinical Trial Unit and the Center for AIDS Research as previously described where healthy donors served as controls [4]. The HIV-infected subjects who participated in the aspirin study were recruited and characterized as previously described [18]. Informed consent was obtained from all study participants in accordance with the Declaration of Helsinki. The studies were conducted in accordance with policies of the New York University Langone Medical Center Institutional Review Board, Bellevue Hospital Center, and the central office of the New York City Health and Hospital Corporation.
Immunoblot analysis
1 mL of plasma each from 8 HIV-infected donors virologically suppressed on ART and 8 healthy control donors, randomly selected from our previous study[4] were processed using the ProteoMiner™ protein enrichment large-capacity kit (Bio-Rad), according to the manufacturer specification, to yield 100 uL of plasma enriched for medium- and low- abundance proteins. Enriched plasma samples and recombinant sCD40L monomeric protein and multimeric protein were separated by SDS-PAGE, transferred to PVDF membranes, and probed with mouse anti-CD40L (R&D), followed by horse anti-mouse horseradish peroxidase (HRP) –conjugated secondary antibody (Cell Signaling). The blots were visualized using HRP substrate (Pierce) by chemiluminescent detection.
For IDO detection, a total of 2.0×105 cells were lysed in MPER lysis buffer (Pierce). Lysates were separated by SDS-PAGE, transferred to PVDF membranes, probed with rabbit anti-IDO antibody (Cell Signaling), followed by goat anti-rabbit horseradish peroxidase (HRP) – conjugated secondary antibody (Cell Signaling) and mouse anti-B Actin (Santa Cruz), followed by horse anti-mouse horseradish peroxidase (HRP) –conjugated secondary antibody (Cell Signaling). The blots were visualized using HRP substrate (Pierce) by chemiluminescent detection.
Dendritic cell preparation and stimulation
Peripheral blood mononuclear cells (PBMCs) were purified from buffy coats from uninfected donors purchased from the New York Blood Center (Queens, NY) and plated at 50×106 cells/10 ml/dish in RPMI with 5% PHS. Cells were allowed to adhere for 1–2 hours at 37°C. Nonadherent cells were removed by washing with RPMI. The adherent monocyte-enriched fraction was supplemented with 100 UI/ml rhGM-CSF and 300 UI/ml rhIL-4 (R&D Systems) on days 0 and 2. On day 5 of culture, DC were harvested, washed, and resuspended in RPMI supplemented with 5% PHS, purity >95% (LIN negative, CD11c positive). DC (106/mL) were incubated for 24 hours with either sCD40L monomer (2ug/mL; Enzo Life Sciences, NY, USA, ALX-522-015-6010) or sCD40L multimer, which is prepared using manufacturer's instructions by mixing monomer with Enzo's cross-linking enhancer (ALX-804-034-C050) at a ratio of 10:1, or enhancer alone. To evaluate for nonspecific effects of anti-CD40 ligation, appropriate controls included incubation of DCs in the presence of enhancer, which appeared to be devoid of any functional effect. In certain experiments, DCs were stimulated with Poly-ICLC (Hiltonol, Oncovir, Inc) at 5μg/ml on day 6 for 18-20 hours. To assess DC phenotype following sCD40L stimulation, DCs were stained with conjugated fluorescent antibodies to CD11c (BD Pharmingen) and CD86 (BD Pharmingen). Secretion of IL-12p70, TNFα, and IL-6 was measured in DC supernatants using the Human Inflammatory Cytokine Cytometric Bead Array (BD Pharmingen).
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
RNA from sCD40L-exposed DC (both monomer and multimer) and control-DC (N=3 per group) was isolated using RNAeasy Mini Kit (Quiagen) and converted to cDNA using RT2 Streand Kit (SABiosciences). Expression of 84 genes central to TLR mediated signal transduction were analyzed using the Human TLR signaling Pathway RT2 Profiler PCR Array (SABiosciences) per manufacturer's protocol (for complete list of genes, see http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-018A.html). Reactions were conducted using a BioRad icycler IQ5 RT-PCR detection system. All data and statistics were analyzed via software provided by the manufacturer (http://www.sabiosciences.com/pcrarraydataanalysis.php).
Naive CD4+ T cell co-culture
T cell proliferation and IFNγ secretion assays were performed following co-culture of CFSE labeled allogeneic naïve CD4+ T cells with sCD40L-exposed DCs +/- stimulated with Poly-ICLC as described above. Naïve CD4+ T cells were purified by magnetic cell sorting (EasySep™ Human Naïve CD4+ T cell Enrichment Kit, Stem Cell Technologies, Vancouver, Ca) from uninfected donor PBMCs and stained with 10 μM carboxyfluorescein succinimidyl ester dye (CellTrace™ CFSE Proliferation Kit, Life Technologies, NY, USA) for 10 minutes prior to incubation with DCs at a ratio of 1:20 (DC/Tcell). After 5-6 days, CFSE dilution was analyzed by FACS and culture supernatants were assayed for IFNγ using the Human Th1/Th2/Th17 Cytokine Cytometric Bead Array (CBA) (BD Pharmingen). Regulatory T cell phenotype was analyzed after co-culturing allogeneic naïve CD4+ T cells with sCD40L-exposed DCs at a ratio of 1:10 for 7 days. Cells were stained with CD4 (BD biosciences), FoxP3 (eBioscience), and CD127 (Biolegend) according to the manufacturer's instructions.
T Cell Anergy Assay
DCs were incubated for 48 hours with sCD40L forms, LPS 100 ng/mL(Sigma), LPS 100 ng/mL + IFNγ 200 U/mL (R&D systems), or media control. DCs were washed well and mixed with allogeneic naïve CD4+ T cells at a 1:10 ratio. After 6-7 days, the T cells were stained with CFSE and were mixed at various ratios with allogeneic DCs (from the same donor as the original 48 hours DC incubation) matured overnight with R848 10uM (3M) (1:10, 1:30 and 1:300; DC:Tcell). After 3 days, CFSE dilution was analyzed by FACS.
Inhibitors
1-Methyl-DL-tryptophan (1-MT) was purchased from Sigma-Aldrich and used at a concentration of 200 μM.
PD-1 and CTLA-4 staining
DCs were incubated for 48 hours with sCD40L forms, LPS 100 ng/mL(Sigma), or media control, washed and then co-cultured with allogeneic naïve CD4 T cells for 7 days and stained for CTLA-4 PE Brilliant Violet 605 and PD-1 Brilliant Violet 605 (Biolegend) and analyzed by FACS.
Plasma soluble CD40L quantification
Concentrations of sCD40L were determined in plasma by commercially available quantitative enzyme-linked immunosorbent assay (Bender MedSystems BMS293) according to manufacturer's instructions.
Statistics
Statistical analysis was performed on at least 3 independent experiments. Analyses of variance was performed for an overall comparison among independent groups if ≥3 groups were present, and student's t tests were then used for specific pairwise comparisons. In all cases, P values < 0.05 using two-sided tests were considered significant. Results are presented as mean ± SEM of multiple donors where indicated.
Results
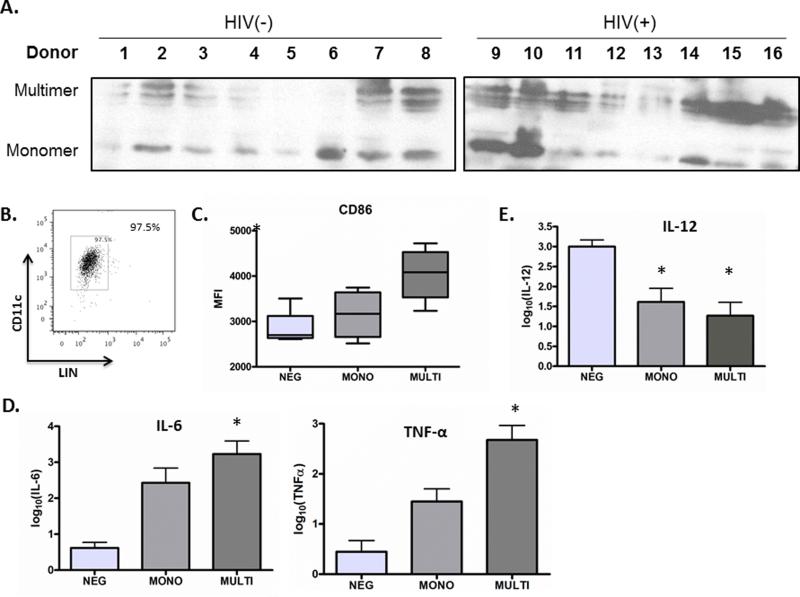
T cells or platelets which are activated in vitro release sCD40L monomers [8-9]. However, it has been reported that sCD40L monomers, dimers, and trimers may circulate in the plasma in vivo[24]. Although it has been observed that total sCD40L is increased in the plasma of HIV-infected subjects as compared to healthy controls [13-15], the circulating form(s) of sCD40L have not been assessed during HIV infection. The immune activating effects of multimeric sCD40L have been extensively studied [6-8], but recent studies suggest an immunosuppressive effect of monomeric sCD40L [12, 25]. To address the question of whether the monomeric or multimeric form is the predominant form of sCD40L circulating in plasma of HIV-infected subjects as compared to healthy control subjects, we enriched plasma from 8 HIV infected donors and 8 healthy control donors for medium- and low- abundance proteins, followed by immunoblot of plasma preparations for sCD40L. We observed that multiple forms of sCD40L, including monomers (18kD) and multimers of various sizes (26-40kD) circulate in the plasma of both HIV-infected subjects and healthy controls with relative ratios that vary by donor (Figure 1A). To better understand the implication of our finding in HIV infection, where overall levels of plasma sCD40L are heightened [13-15], we next sought to determine whether there were differential immunological effects of sCD40L forms on healthy control DCs in vitro, using sCD40L concentrations tested in previous reports[12, 25]. We observed that maturation of DCs (purity>95% CD11c+LINneg, Figure 1B) as assessed by CD86 expression was much higher after stimulation with multimeric sCD40L as compared to monomeric sCD40L (Figure 1C). Along these lines, multimeric sCD40L stimulated DCs to secrete more IL-6 and TNF-α than monomeric sCD40L (Figure 1D). Notably, neither forms of sCD40L resulted in secretion of appreciable amounts of IL-12p70 by DCs (not shown).
Figure 1. Soluble CD40L circulates in plasma as monomers and multimers, which exert differential effects on DCs.
(A) sCD40L forms in plasma from 8 HIV-infected donors and 8 healthy donors were demonstrated by immunoblot analysis. (B) DC purity as measured by FACS, DCs were incubated with either sCD40L monomer (MONO), CD40L multimer (MULTI), as compared to control DC (NEG) overnight and (C) CD86 surface expression was evaluated by flow cytometry and (D) IL-6 and TNF-α production were measured in cell supernatant by cytokine bead array analysis. (E) DCs were incubated with either sCD40L monomer (MONO), CD40L multimer (MULTI) as compared to control DC (NEG) overnight and then were subsequently stimulated overnight with Poly-ICLC, and IL-12 production was measured in cell supernatants by cytokine bead array. Shown in (C), (D), and (E) are data for three donors with statistical analysis using two-tailed Student's t tests. *Represents a p-value < 0.05 and was considered significant.
To further explore differences in activation of DCs by sCD40L monomer as compared to multimer, a qpcr array with 84 genes delineating innate immune activation pathways was performed. Across all genes fold changes, there was no significant difference observed between DCs treated with monomer vs control as compared to multimer vs control (Supplemental Figure 1). However, there was upregulation of more proinflammatory genes by multimeric sCD40L vs. control (IL-2, IRAK2, LY86, MAP2K3, TAB1, MYD88, NFKBIL1, REL, p <0.05) as compared to monomeric sCD40L vs. control (LY86 and MYD88, p<0.05) (Table 1).
To determine whether these circulating forms of sCD40L during HIV infection may contribute to DC dysfunction, we evaluated the ability of sCD40L-exposed healthy donor DCs to produce cytokines, in particular IL-12, upon subsequent stimulation with a TLR 3 ligand, Poly-ICLC (Hiltonol, Oncovir, Inc); an established potent inducer of IL-12 secretion [4, 26-28]. IL-12 is an important regulatory cytokine that plays a pivotal role in in the differentiation of naïve CD4+ T cells towards the Th1 pathway to generate anti-viral cytotoxic T cells and IFNγ secretion[29]. Secretion of IL-12 by DCs in the setting of HIV infection is impaired, and likely impacts the formation of effective immune responses during HIV infection[4, 5, 30-32] The mechanism of this dysfunction is only partially delineated, with a strong contribution by non-viral soluble plasma factors [4, 5] Exposure to both sCD40L monomer and sCD40L multimer resulted in diminished secretion of IL-12p70 secretion by DC upon Poly-ICLC stimulation as compared to control (Figure 1E).
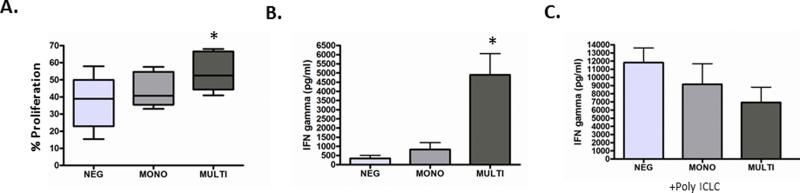
We next studied the effects of healthy donor DCs which were differentially incubated with sCD40L forms on naïve CD4+ T cell skewing and proliferation. As previously reported, DCs activated with multimeric sCD40L induced strong proliferation and Th-1 skewing of CD4 T cells as measured by CFSE dilution and IFNγ secretion (Figure 2A+B)[6]. In contrast, DCs exposed to monomeric sCD40L stimulated T cells to proliferate minimally only in certain donors, with no effect on others. Overall, there was no difference in proliferation by DCs exposed to monomeric sCD40L compared with unstimulated DCs (Figure 2A+B). Furthermore, monomeric sCD40L-exposed DCs did not stimulate secretion of IFNγ by naïve CD4+ T cells (Figure 2A+B). When sCD40L-stimulated DCs were subsequently activated with Poly-ICLC and co-cultured with allogeneic naïve CD4+ T cells, there was a trend for both sCD40L forms to suppress IFNγ secretion (Figure 2C).
Figure 2. DCs incubated with sCD40L monomeric as compared to multimeric forms have differential effects on naïve CD4+ T cell skewing and proliferation.
DCs were incubated with sCD40L monomer (MONO) as compared to CD40L multimer (MULTI) or control DC (NEG) overnight and were then co-cultured with CFSE-labeled allogeneic naïve CD4 T cells where (A) % CFSE dilution is represented as % proliferation and (B) culture supernatants were assayed for IFNγ using the Human CBA. (C) sCD40L-stimulated DCs were subsequently activated with Poly-ICLC and co-cultured with allogeneic naïve CD4+ T cells. Shown in (A), (B), and (C), are data for three donors with statistical analysis using two-tailed Student's t tests. *Represents a p-value < 0.05 and was considered significant.
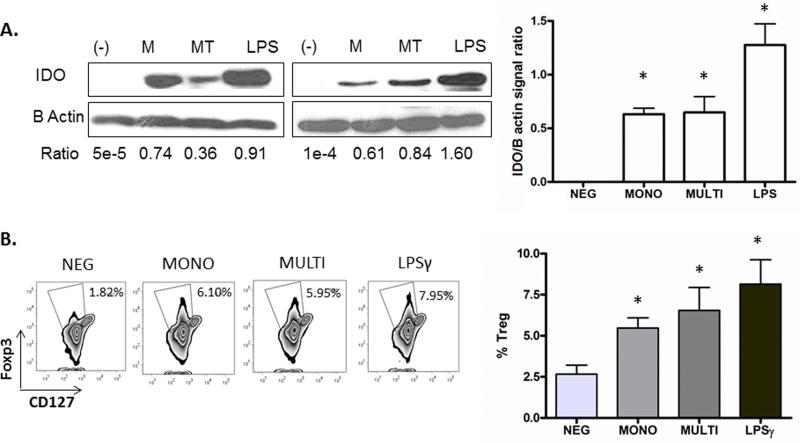
Indoleamine 2,3-dioxygenase (IDO), expressed by APCs such as macrophages and DCs, plays a key role in the differentiation of immunosuppressive regulatory T cells (Treg) by catabolizing Tryptophan (Trp) into Kynurenine (Kyn)[33, 34]. IDO-mediated effects likely tip the balance of Th17/Treg, favoring microbial translocation during chronic HIV infection[35]. Previous reports have presented inconsistent data on the role of sCD40L in IDO expression in DCs[36-39]. Additionally, the monomeric versus multimeric forms have never been directly compared. Because sCD40L circulates in both forms during HIV infection, we sought to investigate whether there are differential effects of sCD40L on IDO expression by DCs. IDO expression as measured via immunoblot was increased by DCs exposed to both monomeric and multimeric forms, as well as LPS, a known inducer of IDO in DCs[38]. To next evaluate whether monomeric as compared to multimeric sCD40L stimulated DCs had differential effects on the ability of DCs to induce Treg formation, allogeneic naïve CD4+ T cells we co-cultured with DCs exposed to either monomeric or multimeric forms of sCD40L. We observed that both monomeric and multimeric sCD40L-stimulated DCs induced increased levels of Tregs, reflected by the increase in FOXP3+ cells, as did DCs stimulated with LPS and LPS/IFNγ, known inducers of Treg formation (Figure 3).
Figure 3. DCs exposed to both monomeric and multimeric sCD40L induce IDO expression and regulatory T cell phenotype.
(A) DCs were incubated with sCD40L monomer (M), CD40L multimer (MT), LPS, or control (-) for 24 hours and IDO expression was measured via immunoblot, shown for 2 donors. The relative abundance of IDO is expressed as the ratio of IDO to B Actin signal intensity and is represented as a histogram on the right as the average of three donors. (B) DCs were incubated with sCD40L monomer (M), CD40L multimer (MT), LPS and interferon-γ, or control (-) for 48 hours, washed and then co-cultured with allogeneic naïve CD4 T cells at a ratio of 1:10. Phenotype of the day 7 T cell population is shown for one representative donor, gated on CD4+ T cells. Percentages of cells in each gate (Foxp3+CD127lo) are indicated, and are represented as a histogram on the right as the average of three donors. *Represents a p-value < 0.05 and was considered significant.
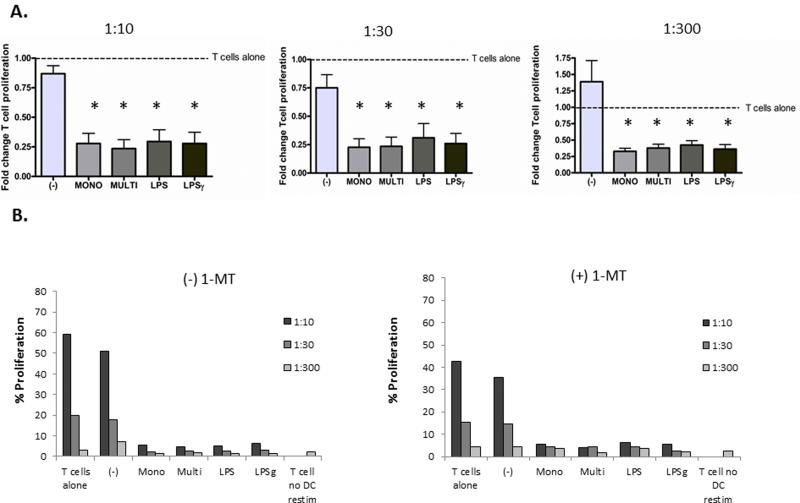
To further investigate the suppressive effects of sCD40L-exposed DCs on T cell function, anergy assays were performed. Co-culture of naïve CD4 T cells with both sCD40L monomer and multimer-exposed allogeneic DCs resulted in T cell anergy as evidenced by decreased proliferation upon subsequent co-culture with TLR-matured allogenic DCs from the original DC donor. Induction of anergy by both forms of sCD40L was to a similar degree as LPS and LPS and IFNγ, potent TLR agonists and DC activators (Figure 4A). We next tested whether IDO and Treg formation played a role in the induction of anergy by DCs differentially activated by sCD40L forms. To test this we added an inhibitor of IDO, 1-Methyl-DL-tryptophan (1-MT), to the DC-T cell co-culture experiments. There was no reversal of anergy by 1-MT, thus demonstrating that IDO and regulatory T cells do not play a major role in the induction of T cell anergy by sCD40L activated DCs (Figure 4B, Supplemental Figure 2).
Figure 4. DCs exposed to both monomeric and multimeric sCD40L induce anergy, which is not reversed by IDO inhibition.
(A) DCs were incubated with sCD40L monomer (MONO), CD40L multimer (MULTI), LPS, LPS and interferon-γ (LPS γ), or control (-) for 48 hours, washed and then co-cultured with allogeneic naïve CD4 T cells at a ratio of 1:10. After 6-7 days, the T cells were stained with CFSE and were mixed at various ratios with allogeneic DCs, from the original DC donor, matured overnight with R848 10uM (3M) (1:10, 1:30 and 1:300; DC: T cell ratio). After 3 days, CFSE dilution was analyzed by FACS. Data is shown for 3 donors with T cell proliferation (CFSE dilution) normalized to untreated T cells (T cells alone) stimulated with R848-DCs. *Represents a p-value < 0.05 and was considered significant. (B) The same anergy experiment was performed in the absence (-) MT or presence (+) MT of 1-MT a specific inhibitor of IDO activity. Data is shown for one donor, representative of 4 donor experiments.
Discussion
DC dysregulation contributes to HIV pathogenesis and may blunt adaptive immune responses to various pathogens and vaccines in infected individuals[3]. The defect in cytokine secretion, in particular IL-12, that has been observed by DCs in the setting of HIV infection is particularly relevant to the induction of Th1-type responses to facilitate the formation of strong cytotoxic T cells that are critical for killing of infected cells, including latently infected cells[40]. Here we demonstrate that multiple circulating forms of sCD40L may contribute to this DC dysfunction during HIV infection.
It is well described that circulating levels of sCD40L are elevated overall during both treated and untreated HIV infection [13-15], at least in part secondary to platelet activation[18]. Here, we demonstrate that circulating sCD40L is comprised of multiple forms including monomeric and multimeric, in both seronegative and ARV-treated seropositive donors. Previous data delineating the circulating forms of sCD40L in vivo are scant[24], and often it has been assumed that the circulating forms are monomeric [12-14]. Prior assessments of circulating forms of sCD40L have been limited by technical challenges in the detection of low abundance small plasma proteins. Here we utilize a protein enrichment kit to concentrate small plasma proteins (ProteoMiner™) which enhanced our ability to detect these forms via immunoblot. While plasma sCD40L monomeric forms appeared as discrete bands, we observed that multimeric forms varied in size. While our immunoblot study was meant to be descriptive, further proteomic analysis could better define whether these multimeric forms are modified[41].
Consistent with prior studies[42, 43], we reveal a primary immunostimulatory role of multimeric sCD40L on DC. In contrast we found minimal stimulation of DCs by monomeric sCD40L. However, despite these differential primary effects, we found that both forms induce a hyporesponsive or exhausted phenotype in the setting of subsequent TLR stimulation, with significant reduction in IL-12 secretion by DC that leads to a trend towards downstream Th1-skewing defects. This closely resembles the phenotype of DCs upon exposure to plasma from HIV infected individuals that has been previously attributed to non-viral plasma factors [4, 5]. Given the fact that these forms of sCD40L have differential effects on DCs in terms of activation, it is likely that the mechanism of DC hyporesponsiveness to subsequent stimulation also differs. Following strong activation by multimeric sCD40L, it stands to reason that DCs may be refractory to subsequent stimulation due to a certain level of exhaustion as has been observed with sequential TLR stimulation[3, 4, 26]. However, in regards to monomeric sCD40L, where minimal DC activation is observed, it is less clear how further responsiveness to TLR stimulation is inhibited. In accordance with our findings in DCs, decreased cytokine secretion, including IL-12, has been observed by monocytes following exposure to monomeric sCD40L[12]. Future studies investigating the mechanisms underlying these effects are warranted to better define intracellular pathways that regulated by monomeric sCD40L in DCs and other cell types.
Additionally, we describe that exposure of DCs to both monomeric and multimeric sCD40L leads to the induction of IDO expression, T regulatory cell formation, and T cell anergy that further blunts adaptive cytotoxic T cell responses. The findings related to increased IDO expression and Treg formation by sCD40L are consistent with previous studies demonstrating this link[12, 13], however, we found that blocking T regulatory cell formation using the IDO inhibitor 1-MT did not reverse T cell unresponsiveness in these experiments. This suggests that sCD40L exposure of DCs leads to T cell anergy through additional mechanisms. The induction of T cell anergy is a complex and often multifactorial process for which many pathways have been implicated including the absence of costimulation and the presence of inhibitory signals, including PD-1 and CTLA-4 engagement[44]. In studies using cells from individuals with cancer, sCD40L is found to result in increased upregulation of PD-1 on T cells compared with cells from control donors[12]. Indeed, we also found that naïve CD4 T cells that were co-cultured with sCD40L-activated DCs (both monomeric and multimeric forms) resulted in upregulation of PD-1 and CTLA-4 on T cells (Supplemental Figure 3).
We had previously published data from a study of one week of low-dose aspirin therapy in HIV-infected individuals receiving suppressive ART that revealed decreases in markers of immune activation and modulation of innate immune function [18]. Now, upon further analysis of plasma samples for total sCD40L from this study, we found that one week of low dose aspirin therapy significantly decreased sCD40L (Supplementary Figure 4). Therefore, by reducing platelet activation and resultant release of sCD40L, aspirin therapy may improve innate immune function and decrease immune activation during chronic infection. The reduction of sCD40L following one week of low dose aspirin was modest(mean 2.66 vs 2.41, p=0.04), but it remains possible that more prolonged or more potent anti-platelet therapy may have additive benefit in lowering these levels, resulting in further augmentation of innate and adaptive immune function. In order to address this question, a randomized, placebo-controlled clinical trial is currently underway to better evaluate the immunomodulatory role(s) of aspirin therapy during HIV infection (NCT02155985).
Supplementary Material
Acknowledgements
E.A.M. and M.P.O. designed the study and performed experiments, analyzed and interpreted data, and drafted and revised the article. R.G. and V.V. collected data and performed selected experiments. N.B. and J.S.B. contributed to experimental design and N.B. revised the article. This work has been supported by National Institutes of Health [K08 AI84578 to E.A.M., K08 AI093153 to M.P.O., R37 AI044628 and U01 A1067854 to N.B.; Center for AIDS Research P30 AI027742 and National Center for the Advancement of Translational Science (NCATS) UL1 TR000038 to M.P.O. and J.S.B], and an American Heart Association Fellow to Faculty Award [0775074N] and a Doris Duke Clinical Scientist Development Award [2010055] to J.S.B.
References
- 1.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Miller E, Bhardwaj N. Dendritic cell dysregulation during HIV-1 infection. Immunol Rev. 2013;254(1):170–89. doi: 10.1111/imr.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller EA, et al. Plasma factors during chronic HIV-1 infection impair IL-12 secretion by myeloid dendritic cells via a virus-independent pathway. J Acquir Immune Defic Syndr. 2012;61(5):535–44. doi: 10.1097/QAI.0b013e31826afbce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frleta D, et al. HIV-1 infection-induced apoptotic microparticles inhibit human DCs via CD44. J Clin Invest. 2012;122(12):4685–97. doi: 10.1172/JCI64439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Mazzei GJ, et al. Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem. 1995;270(13):7025–8. doi: 10.1074/jbc.270.13.7025. [DOI] [PubMed] [Google Scholar]
- 9.Graf D, et al. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25(6):1749–54. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- 10.Nannizzi-Alaimo L, Alves VL, Phillips DR. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 2003;107(8):1123–8. doi: 10.1161/01.cir.0000053559.46158.ad. [DOI] [PubMed] [Google Scholar]
- 11.Andre P, et al. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120(15):3030–8. doi: 10.1182/blood-2012-05-427799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenabian MA, et al. Soluble CD40-ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T-cell expansion in HIV infection. Clin Exp Immunol. 2014 doi: 10.1111/cei.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donhauser N, et al. Chronic immune activation in HIV-1 infection contributes to reduced interferon alpha production via enhanced CD40:CD40 ligand interaction. PLoS One. 2012;7(3):e33925. doi: 10.1371/journal.pone.0033925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olmo M, et al. Impact of antiretroviral therapy interruption on plasma biomarkers of cardiovascular risk and lipids: 144-week final data from the STOPAR study. HIV Med. 2012;13(8):488–98. doi: 10.1111/j.1468-1293.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- 16.Davidson DC, et al. Excess soluble CD40L contributes to blood brain barrier permeability in vivo: implications for HIV-associated neurocognitive disorders. PLoS One. 2012;7(12):e51793. doi: 10.1371/journal.pone.0051793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson DC, Jackson JW, Maggirwar SB. Targeting platelet-derived soluble CD40 ligand: a new treatment strategy for HIV-associated neuroinflammation? J Neuroinflammation. 2013;10:144. doi: 10.1186/1742-2094-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukaetova-Ladinska EB, et al. Platelet Tau Protein as a Potential Peripheral Biomarker in Alzheimer's disease: An Explorative Study. Curr Alzheimer Res. 2013 doi: 10.2174/1567205015666180404165915. [DOI] [PubMed] [Google Scholar]
- 19.Damien P, et al. Highly Active Antiretroviral Therapy Alters Inflammation Linked to Platelet Cytokines in HIV-1-Infected Patients. Journal of Infectious Diseases. 2013;208(5):868–870. doi: 10.1093/infdis/jit260. [DOI] [PubMed] [Google Scholar]
- 20.Mayne E, et al. Increased Platelet and Microparticle Activation in HIV Infection: Upregulation of P-Selectin and Tissue Factor Expression. J Acquir Immune Defic Syndr. 2012;59(4):340–6. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez CE, et al. Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus vectors MVA and NYVAC expressing HIV antigens. Vaccine. 2009;27(24):3165–74. doi: 10.1016/j.vaccine.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 22.Devaraj S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55(3):774–9. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 23.Donhauser N, et al. Chronic immune activation in HIV-1 infection contributes to reduced interferon alpha production via enhanced CD40:CD40 ligand interaction. PLoS One. 2012;7(3):e33925. doi: 10.1371/journal.pone.0033925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura K, et al. Study of plasma levels of soluble CD40 ligand in systemic lupus erythematosus patients who have undergone plasmapheresis. Ther Apher Dial. 2005;9(1):64–8. doi: 10.1111/j.1774-9987.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 25.Donhauser N, et al. Chronic immune activation in HIV-1 infection contributes to reduced interferon alpha production via enhanced CD40:CD40 ligand interaction. PLoS One. 7(3):e33925. doi: 10.1371/journal.pone.0033925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogunovic D, et al. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res. 2011;71(16):5467–76. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouas R, et al. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int Immunol. 2004;16(5):767–73. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- 28.Zobywalski A, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med. 2007;5:18. doi: 10.1186/1479-5876-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 30.Buisson S, et al. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J Infect Dis. 2009;199(12):1862–71. doi: 10.1086/599122. [DOI] [PubMed] [Google Scholar]
- 31.Smed-Sorensen A, et al. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood. 2004;104(9):2810–7. doi: 10.1182/blood-2003-07-2314. [DOI] [PubMed] [Google Scholar]
- 32.Yonkers NL, et al. Systemic immune activation in HIV infection is associated with decreased MDC responsiveness to TLR ligand and inability to activate naive CD4 T-cells. PLoS One. 2011;6(9):e23884. doi: 10.1371/journal.pone.0023884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118(10):3431–9. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terness P, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase- expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2(32):32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grohmann U, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166(1):277–83. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 37.Grohmann U, et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003;171(5):2581–7. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- 38.Hwang SL, et al. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005;15(3):167–75. doi: 10.1038/sj.cr.7290282. [DOI] [PubMed] [Google Scholar]
- 39.Tas SW, et al. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110(5):1540–9. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 40.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1(11):845–67. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 42.Stone GW, et al. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006;80(4):1762–72. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miconnet I, Pantaleo G. A soluble hexameric form of CD40 ligand activates human dendritic cells and augments memory T cell response. Vaccine. 2008;26(32):4006–14. doi: 10.1016/j.vaccine.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 44.Valdor R, Macian F. Induction and stability of the anergic phenotype in T cells. Semin Immunol. 2013;25(4):313–20. doi: 10.1016/j.smim.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.