Abstract
Objectives
Our study examines the relationship between perceived discrimination and levels of C-reactive Protein and blood pressure in low-income youth ages 10–15 years old.
Methods
Data were collected from 10–15 year old focal children and their mothers. Face-to-face interviews were implemented to collect data on stressors including experiences of everyday discrimination from youth. High sensitivity CRP in dried blood spot samples and diastolic and systolic blood pressure were also collected at the time of the interview.
Results
Perceived discrimination among youth was significantly associated with higher levels of CRP, systolic and diastolic blood pressure. CRP, systolic, and diastolic blood pressure remained significant after controlling for age-adjusted BMI, waist circumference, and other factors.
Conclusion
Discrimination is a salient risk factor for inflammation and cardiovascular health. Early life course inflammation and cardiovascular reactivity are important candidate pathways through which the repeated exposure to discrimination for minority group members contributes to racial and economic health inequities in adulthood.
Keywords: C-reactive protein, discrimination, blood pressure, adolescent, health disparities
Introduction
Discrimination is a key factor contributing to minority health disparities (Williams and Mohammed 2013). Perceptions of discrimination among African Americans are associated with increased coronary artery calcification (Lewis et al. 2006), blood pressure (Sawyer et al. 2012; Clark 2000), carotid intima-media thickness (Troxel et al. 2003), lower birth weight offspring (Mustillo et al. 2004), and oxidative stress (Szanton et al. 2012). While these physiological pathways to poor health have been documented for adults, substantially less is known about the degree to which perceived discrimination is associated with inflammatory and cardiovascular symptoms earlier in life. The central purpose of this study, therefore, is to assess whether perceived discrimination during early adolescence places low-income African American youth at cardiovascular risk through systemic inflammation and elevated blood pressure.
Experiencing discrimination early in life may place youth at risk for earlier onset and more severe cardiovascular disease during adulthood, reinforcing durable racial health inequities in the United States (Fuller-Rowell, Williams, Love et al. 2013). To better understand whether the stress of discrimination is associated with adverse health early in the life course, we examined the relationship between discrimination and markers of cardiovascular and immune related inflammation in a sample of low-income, African American youth. We hypothesized that the stress of perceived discrimination is positively associated with systemic inflammation as measured by C-reactive Protein (CRP). Furthermore, because stress triggers increased sympathetic nervous system (SNS) reactivity, we next hypothesized that experiencing or perceiving discrimination is significantly positively related to systolic and diastolic blood pressure among African American youth.
Background
Few studies have examined the physiologic consequences of discrimination and unfair treatment for African American children and adolescents. There is evidence, however, that perceived discrimination among minority adolescents is related to elevated smoking (Guthrie et al. 2002), anger (Wong et al. 2003), alcohol use and abuse (Cheadle and Whitbeck 2011), depressive symptoms, general psychological distress (Sellers et al. 2006), and poorer self-rated health (Priest et al. 2011). Despite the attention given to a broad range of adolescent outcomes, the relationship between discrimination and either systemic inflammation or cardiovascular reactivity in young adolescents remains largely unexplored (Sanders-Phillips et al. 2009).
Although specific links between discrimination, inflammation, and cardiovascular reactivity in early life have not yet been examined, there is growing evidence indicating that these factors play a key role in adult health. For example, there are indications that systemic inflammation in childhood and adolescence play central roles in the progression of atherosclerosis (Groner et al. 2006). Two such potential pathways are through childhood CRP (Reinehr et al. 2006) and blood pressure (Juonala et al. 2005). Assessing the degree to which these CVD risk correlates during early adolescence are associated with the stress of discrimination is essential for understanding the early life course determinants driving inequities in minority health through adulthood.
CRP, an acute phase marker of systemic inflammation, is a protein synthesized in the liver as a downstream response to a rise in other inflammatory factors such as Interlukin-6 elevation (Slopen et al. 2013). CRP is linked to atherosclerosis and other cardiovascular risks in adulthood (Kaptoge et al. 2010). Although the causal order is not fully established (Danesh and Pepys 2009), elevated CRP during childhood predicts adult CRP (Juonala et al. 2006), high blood pressure, and abdominal obesity (Ford et al. 2005; Slopen et al. 2012; Visser 2001). Similarly, elevated childhood blood pressure is positively associated with adult atherosclerosis (Kavey et al. 2003) while elevated systolic blood pressure predicts arterial stiffness (Li et al. 2004). Taken together, these finding suggest that inflammation and cardiovascular response are likely indicators of early life risk for the onset and progression of cardiovascular disease.
Although links between these risk factors and early discrimination exposure have yet to be fully specified, the stress of chronic exposure to adversity may affect the hypothalamic-pituitary-adrenocortical (HPA) axis, reducing efficient cardiovascular response to stress during childhood and adolescence (Pollitt et al. 2007; Evans and Kim 2007). Such stress exposure activates the SNS, leading to elevated blood pressure and hypertension, increasing the likelihood of CVD onset when chronic (Sowers, Epstein, and Frohlich 2001). In addition to the elevation of blood pressure, stress exposure stimulates the SNS, which contributes to elevation of some inflammation markers such as CRP (e.g. McKewen 1998). There is increasing evidence that an array of social stressors, including those in conjunction with discrimination, are proximate determinants of the disproportionate racial differences in cardiovascular and metabolic health outcomes among racial minorities (Williams and Mohammed 2013).
African Americans are disproportionately represented in the low income population and present higher rates of cardiovascular conditions such as hypertension while also suffering from higher cardiovascular disease mortality relative to other racial/ethnic groups (Nwankwo et al. 2013; Colhoun et al. 1998). Moreover, CRP is on average higher among African American than white adults (Khera et al. 2005). After controlling for other cardiovascular risk factors including BMI, African American women have CRP levels that are on average higher than African American males and Caucasian males and females (Khera et al. 2005). During youth, African American low SES adolescents show more arterial stiffness and intima media thickness relative to Caucasian and higher SES youth (Thurston and Matthews 2009).
Such disparities in African American health point to the need for research examining how early in the life course elevations in inflammatory markers emerge in this population leading to later cardiovascular risk. Adolescence is a particularly important period to examine changing health, as it is a time of social expansion when youth reshape and expand their social networks (e.g., Cheadle and Goosby 2012). Moreover, youth appear to be strongly reactive to social conditions as a result of neurobiological development (Steinberg 2008). Consequently adolescence may be a particularly sensitive time to the pain and frustration of experiencing discrimination (Sebastian et al. 2010). Discriminatory experiences become more common for African American youth during adolescence as they begin to spend more time in public places outside of the home (e.g. stores, schools, restaurants) increasing their exposure to discrimination (Fisher et al. 2000; Goosby and Walsemann 2012).
The goal of our study, therefore, is to examine the relationship between perceptions of discrimination among youth, ages 10–15 years old, and cardiovascular risk measured with high sensitivity CRP and blood pressure. We expect to find that discrimination is positively associated with CRP levels and both diastolic and systolic blood pressure.
Methods
Data for this study come from the Omaha Urban Research on Health Study (OURHealth Study). Low-income African American and Caucasian mothers with a focal child between the ages of 10 and 15 were recruited through health fairs. Data was collected between February and July 2013. The purpose of the study was to examine relationships between stressors, such as economic hardship, discrimination, and the manifestations of stress related illness among low-income mothers and their offspring. Mothers were included based on meeting the following criteria: (a) income at 125% or greater of the federal poverty line adjusting for household size, (b) mother’s racial classification as either Black or Caucasian, (c) being born in the U.S., and (d) having a biological child between the ages of 10 and 15 years of age. The final sample included 58 mother/child dyads. For the purpose of this study, we restrict our focus to the relationships between reports of discrimination and health among offspring in the sample.1
Upon completion of screening, separate face-to-interviews were conducted with mothers and their children. Survey data were collected on features of economic hardship, experiences of major and everyday discrimination, health histories of both mother and child, and psychosocial measures of stress. Prior to or upon completion of face-to-face interviews, up to eight drops of capillary whole blood were collected on filter paper (McDade, Williams, and Snodgrass 2007) for subsequent laboratory analysis for CRP. In addition, blood pressure and anthropometric measures of height, weight, and waist circumference were collected from participants during their session following interviews.
Measures
C-reactive protein was measured using a high sensitivity immunoassay previously validated for use with dried blood spot (DBS) samples (McDade, Burhop, and Dohnal 2004). Samples were dried overnight following collection and then sent to the Laboratory for Human Biology Research at Northwestern University where they were stored at stored at −30C degrees, prior to analysis. For comparability with serum, estimates were converted to the equivalent serum/plasma value using the Deming regression conversion formula: serum (mg/l) =1.84 × DBS (mg/l). One case with a CRP level over 10mg/L after conversion was excluded from the analyses due to indications of acute, active infection (Pearson et al. 2003). The final CRP measure was log transformed to rescale the distribution to account for non-normality. Systolic and diastolic blood pressure measurements were obtained from participants using the Omron HEM-9070XL digital sphygmomanometer (Omron Healthcare Co, Bannockburn, Illinois). The three blood pressure measurements were taken using the dominant arm over a 15-minute period during data collection while the participants were seated. The three readings for both systolic and diastolic blood pressure were averaged and used in subsequent analyses.
The focal independent variable in these analyses is child’s perceived discrimination measured using a modified version of the Everyday Discrimination scale adapted for adolescents (Forman et al. 1997). The Everyday Discrimination scale appraises chronic, commonplace, and subtler forms of discriminatory experiences without priming respondents to think about race (Williams et al. 1997; Deitch et al. 2003). Although, some literature suggests that discrimination should be ascribed to a particular attribute to be considered discrimination (Major et al. 2002), the Everyday Discrimination scale is strongly associated with institutional and interpersonal racial discrimination (Hughes 2003; Krieger et al. 2005) and is accepted as a valid measure that accounts for discriminatory experiences among people of color (Seaton et al. 2008).
In this study, the questionnaire included thirteen questions regarding the child’s experiences with daily discrimination. The question asked “In your day-to-day life how often have any of the following things happened to you?” followed by thirteen items including: you are treated with less courtesy than others, you are treated with less respect than others, you receive poorer service than other people at restaurants or stores, and et cetera. The response options for each question ranged from never, a few times a year, a few times a month, at least once a week, to almost every day. The items were coded with values ranging from 0 for never to 4 for almost every day and summed to create a continuous scale. After factor analysis, three items were dropped (being served poorer at a restaurant or store, people act afraid of you, and being followed around in stores) due to low loadings. The remaining items loaded on a single factor composed of child’s daily discrimination, which included ten items (alpha = .84).
Control variables include age-adjusted BMI, waist circumference in inches, age (in years), gender (1=female), and mother’s highest education level (1=high school or less, 2=some college, and 3=college or higher). Age adjusted and sex-adjusted BMI was created using the Zanthro package in Stata, which uses standardized methods to calculate age-specific BMI z-scores for children and adolescents using U.S.-specific reference growth charts (Vidmar et al. 2004).
Statistical Analysis
All analyses were conducted using Stata version 13 (StataCorp 2013). Descriptive analyses are reported as means and standard deviations for all variables included in the analyses and reported for each analytic sample in Table 1. In the following bivariate models all measures were standardized for ease of interpretation (standard deviations). Ordinary Least-Squares (OLS) Regression analyses were used to fit the final models of each dependent variable (CRP, diastolic, and systolic blood pressure) on perceived discrimination and all control variables. Final analyses were restricted to African American youth resulting in 9 youth being dropped from the analytic sample (approximately 16%).2
Table 1.
Sample Descriptive Statistics
| Total Sample | CRPa | Systolic BPb | Diastolic BPc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Mean | [SD] | Min | Max | Mean | [SD] | Mean | [SD] | Mean | [SD] | |
| Dependent Variables | |||||||||||
| CRP | 42 | 1.33 | [1.83] | 0.04 | 8.52 | 1.26 | [1.84] | ||||
| Systolic BP | 45 | 114.90 | [13.65] | 83.00 | 149.00 | 114.84 | [13.43] | ||||
| Diastolic BP | 45 | 69.47 | [11.19] | 52.70 | 102.00 | 69.24 | [11.21] | ||||
| Independent Variable | |||||||||||
| Daily Discrimination | 46 | 12.33 | [8.61] | 0.00 | 33.00 | 12.18 | [8.77] | 11.72 | [8.28] | 11.72 | [8.28] |
| Controls | |||||||||||
| Age-Adjusted BMI | 47 | 24.45 | [5.94] | 15.00 | 42.70 | 24.14 | [6.00] | 23.95 | [5.77] | 23.95 | [5.77] |
| Waist Circumference | 47 | 81.16 | [14.79] | 61.00 | 124.00 | 81.06 | [15.41] | 80.41 | [13.75] | 80.41 | [13.75] |
| Age | 47 | 12.32 | [1.64] | 10.00 | 15.00 | 12.40 | [1.60] | 12.33 | [1.57] | 12.33 | [1.57] |
| Female | 47 | 0.79 | 0.00 | 1.00 | 0.78 | 0.77 | 0.77 | ||||
| Mothers Education | 46 | 2.11 | [0.71] | 1.00 | 3.00 | 2.08 | [0.66] | 2.09 | [0.68] | 2.09 | [0.68] |
Note: All measures [except Female] are standardized for analyses. Table represents unstandardized values.
Analytic sample sizes:
40;
43;
43
Results
The descriptive statistics including unstandardized means and standard deviations of the sample are shown in Table 1 for both the full analytic sample and the samples with complete cases for each dependent variable. Characteristics of the full and analytic samples did not differ significantly. Average diastolic and systolic blood pressure readings in the sample were in the normal range of values of approximately 115 mmHg (SD=13.6) and 70 mmHg (SD=11.2) for diastolic blood pressure (normal systolic <120; normal diastolic <80; DHHS 2005). Youth were on average in the normal BMI range (M=24.5, SD= 5.9; normal BMI <24.9). Approximately 79% of youth in the sample were female and the average age of the sample was 12 years. Mothers had, on average, ‘some college education’.
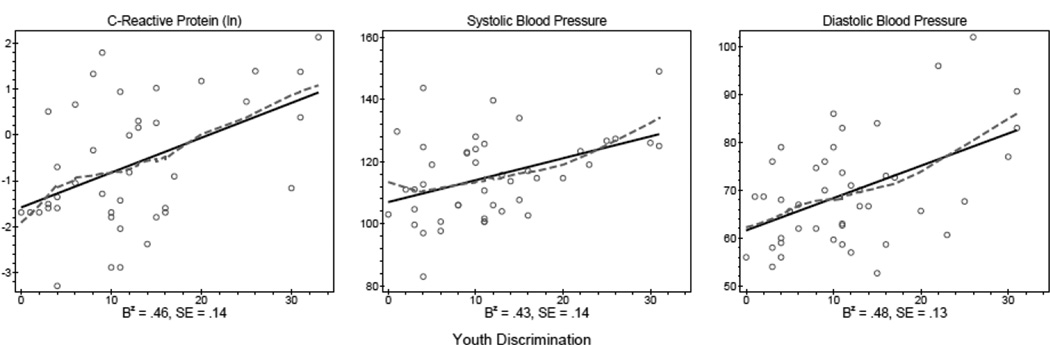
Bivariate standardized results in Table 2 and Figure 1 show evidence of significant relationships between discrimination and inflammation and blood pressure. For every standard deviation increase in discrimination reports, there is a .46 standard deviation increase in log transformed CRP. Similarly, a standard deviation increase in discrimination is associated with a .43 standard deviation increase in systolic blood pressure and a .48 standard deviation increase in diastolic blood pressure. Figure 1 pictorially presents the patterns of the reported bivariate relationships.
Table 2.
Bivariate Regression Analyses of Youth Health Markers on Discrimination
| CRP | Systolic BP | Diastolic BP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | [SE] | β | [SE] | β | [SE] | ||||
| Daily Discrimination | 0.46 | ** | [.14] | 0.43 | ** | [.14] | 0.48 | *** | [.13] |
| Observations | 41 | 44 | 44 | ||||||
Note: Standardized OLS coefficients b and standard errors [SE] are shown.
p ≤ .05,
p ≤ .01,
p ≤.001
Figure 1.
Bivariate Distribution of CVD Risk Markers Regressed on Youth Discrimination
Note: Standardized coefficients reported.
The multivariate models in Table 3 report results controlling for covariates that may also be associated with the dependent variables of interest. For example, youth BMI and waist circumference are associated with CRP and blood pressure in children and adolescents (Visser et al. 2001). The final models also control for adolescent sex, age, and mother’s educational attainment. After accounting for these control variables, the relationships between discrimination and CRP, and between discrimination and systolic and diastolic blood pressure remain statistically significant. For CRP, the inclusion of additional covariates attenuates the discrimination coefficient by approximately 27% (b=.34, SE=.15; p< .05), but the relationship remains statistically significant. Similarly, the effect size for systolic blood pressure is reduced by approximately 21% in the fully estimated model (b=.34, SE=.15; p< .05). Finally, the relationship between discrimination and diastolic blood pressure remains statistically significant with a negligible change in coefficient magnitude relative to the bivariate models (b=.47, SE=.15; p< .01).
Table 3.
Multivariate Regression Analyses of Youth Health Markers on Discrimination
| CRP | Systolic BP | Diastolic BP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | [SE] | β | [SE] | β | [SE] | ||||
| Daily Discrimination | 0.34 | * | [.15] | 0.34 | * | [.15] | 0.47 | ** | [.15] |
| Controls | |||||||||
| Age-Adjusted BMI | 0.28 | [.29] | 0.11 | [.27] | −0.01 | [.27] | |||
| Waist Circumference | 0.24 | [.24] | −0.06 | [.25] | 0.02 | [.26] | |||
| Age | −0.06 | [.18] | 0.17 | [.16] | −0.03 | [.17] | |||
| Female | 0.20 | [.36] | −0.81 | * | [.37] | −0.31 | [.37] | ||
| Mother's Education | −0.10 | [.19] | 0.23 | [.16] | 0.18 | [.17] | |||
| Observations | 40 | 43 | 43 | ||||||
Note: Standardized OLS coefficients b and standard errors [SE] are shown.
p ≤ .05,
p ≤ .01
Discussion
This study provides support for the hypotheses that perceived discrimination among low-income African American youth is associated with increased systemic inflammation and blood pressure. The study is the first of its kind to demonstrate the harmful nature of discrimination for cardiovascular health risks in African American youth as early as 10 years of age. Focusing on younger populations prior to the onset of confounding predominantly adult conditions (e.g., arthritis, atherosclerosis, undetected illness, etc.) appears to hold promise for understanding the pathways through which stressors such as discrimination alter health trajectories to shape long-term health prospects (Lambert et al. 2004; Sanders-Phillips et al. 2009).
Our results support prior research reporting harmful health consequences of discrimination during childhood and adolescence and pointing to the critical need for systematic examination of these factors at early ages (Sanders-Phillips et al. 2009). There is prospective evidence that African Americans who report discrimination at ages 16–18 have higher allostatic load by age 20 (Brody et al. 2014). Our analysis compliments these prior findings by providing new evidence of the physiological consequences of perceived discrimination for African Americans in an even younger sample. These findings thus point to a potential explanatory pathway through which discrimination negatively influences African American health and illness progression: via the stress related activation of the SNS and the upregulation of immune related inflammation.
Over activation of SNS and inflammation are important pathways that may inform African American adults’ disproportionally higher rates of hypertension and cardiovascular disease mortality than other racial groups (Albert et al. 2008), and greater vascular reactivity during resting state (Wyatt et al. 2003). In addition, E-selectin, a set of cell adhesion molecules expressed as part of an inflammatory response to endothelial dysfunction and strongly correlated with elevated CRP, is shown to be positively associated with chronic discrimination in African American men (Friedman et al. 2009; Ross 1999; Pasceri et al. 2000). Moreover, high levels of discrimination are associated with shortened leukocyte telomeres in African American males who report high levels of internalized racial bias in conjunction with discrimination (Chae et al. 2014)—a particularly important factor given the inverse association between telomere length and cardiovascular disease risk (Fitzpatrick et al. 2007). Finally, among both African American men and women chronic discrimination is also linked to elevated levels of the vasoconstrictor endothelin-1 (Cooper et al. 2009). Indeed, in conjunction with our results, these studies further illustrate the need for prospective systematic examinations of the discrimination-health link during childhood and adolescence.
Adolescence is also a critical time to examine the role of discrimination for youth because teens are aware of their social standing during this period. Although there is a growing literature documenting racial discrimination associations with psychological outcomes, less is known about risky or protective psychosocial mechanisms that might offset or exacerbate youth physiological stress outcomes. For example, during adolescence relationships between discrimination and higher levels of depressive symptoms, anger (Wong et al. 2003), and psychological distress (Fisher et al. 2000) may be offset by adaptive coping strategies such as positive racial identity (Sellers 2006). Positive racial identity or the degree to which one attaches high importance and meaning attributed to racial classification (Sellers et al. 1998) is shown to moderate the impact of discrimination exposure on autonomic stress response in young adults (Neblett and Roberts 2013). Future studies should also consider the roles of individual personality characteristics including self-esteem (Utsey et al. 2000), rejection sensitivity (London et al. 2007), and negative affectivity that may either mediate or moderate stress reactivity to the discrimination. Incorporating measures of potential protective mechanisms that may offset the harmful physiological consequences of discrimination exposure for progression of cardiovascular disease risk in African American adolescents would be beneficial to future work in this area. This area of exploration may likely hold clues in helping minority youth deal adaptively with such negative experiences given that racial discrimination remains pervasive and implicit bias against blacks among white Americans is endemic (Schmidt and Nosek 2010).
This study is not without limitations. First, our sample is small and consists largely of female youth. There is, however, little evidence that baseline measures of CRP or blood pressure differ among boys and girls in the age range assessed here after accounting for adiposity and oral contraceptive use (Cook et al. 2000; Lambert, Delvin, Paradis, et al. 2004). However, larger more sex-balanced studies are required to attempt to generalize our results to a larger population. Second, the study is cross-sectional. Prospective, longitudinal data on both discrimination experiences and cardiovascular markers of health are needed. Third, this study specifically targeted woman who were low income and recruited through health fairs. Thus, our sample may include mother/child dyads with worse average health and in need of health care for themselves and/or for their child. Future research employing random selection of more economically diverse samples from known populations needed (Falk et al. 2013). Relatedly, the sample is geographically bound to a large, highly segregated mid-western city. More diverse samples are needed to assess generalizability across places.
Finally, we were unable to distinguish the impact of racial attribution of discrimination with these data. Racial discrimination is thought to be a qualitatively different type of discriminatory experience relative to other types of discrimination, such as gender (Chae et al. 2010). Because adolescence is a tumultuous developmental period where youth become highly cognizant of social ties and relationships, they become more psychologically and physiologically reactive to exclusionary experiences (Cheadle and Goosby 2012). Discriminatory experiences for black youth may in fact be exacerbated and more harmful during this developmental period, but more research is required to understand whether attribution to racial discrimination is as harmful to health as the scalar measurement of non-attributed discrimination.
Despite these limitations, this study moves beyond prior adolescent discrimination research by assessing manifestations of cardiovascular disease risk indicators in early adolescence among disadvantaged youth. These findings support the proposition that discrimination is harmful for cardiovascular disease risk on health early in life, at least for disadvantaged African American female adolescents. Thus, this study provides foundational evidence of the harmful health impacts of discrimination during the early life course and points to a need for ongoing research over longer timelines and with more heterogeneous samples. Assessing the degree to which the physiological consequences of perceived discrimination are exhibited in early life may be essential for understanding racial health inequalities in the U.S., particularly for systemically marginalized groups such as African Americans.
Acknowledgments
Funding: This research was funded by a University of Nebraska Minority Health Disparities Award and is part of a larger study funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K01 HD 064537, Bridget Goosby, PI). The authors are grateful to the mothers and children who participated in this study and the support of Girls Inc. and Ms. Carolyn Green who helped make the health fairs a success. Thanks also to the student volunteers and research assistants including Anna Bellatorre, who assisted with data collection. We also thank Dr. Thom McDade for his helpful insight.
Footnotes
Preliminary analyses showed no significant relationship between maternal and child reports of discrimination and maternal discrimination did not attenuate child discrimination-health relationship and was thus dropped from final analyses. Findings available upon request.
Preliminary analyses indicate that the 9 Caucasian youth in the sample systematically reported fewer incidences of discrimination and are thus a statistically different population from the African American youth. When whites were included in the analyses, the results remained similar to restricted analyses. Results available upon request.
Literature Cited
- Albert MA, Ravenell J, Glynn RJ, Khera A, Halevy N, de Lemos JA. Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas Heart Study. American Heart Journal. 2008;156(6):1103–1109. doi: 10.1016/j.ahj.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Brody GH, Lei M-K, Chae DH, Yu T, Kogan SM, Beach SRH. Perceived discrimination among african american adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Development. 2014;85(3):989–1002. doi: 10.1111/cdev.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Lincoln KD, Adler NE, Syme SL. Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Soc Sci Med. 2010;71(6):1182–1188. doi: 10.1016/j.socscimed.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, Epel ES. Discrimination, racial bias, and telomere length in african-american men. American Journal of Preventive Medicine. 2014;46:103–111. doi: 10.1016/j.amepre.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle JE, Goosby BJ. The small-school friendship dynamics of adolescent depressive symptoms. Society and Mental Health. 2012;2:99–119. doi: 10.1177/2156869312445211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle JE, Whitbeck LB. Alcohol use trajectories and problem drinking over the course of adolescence: a study of north american indigenous youth and their caretakers. Journal of Health and Social Behavior. 2011;52(2):228–245. doi: 10.1177/0022146510393973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. Perceptions of interethnic group racism predict increased vascular reactivity to a labratory challenge in college women. Ann Behav Med. 2000;22(3):214–222. doi: 10.1007/BF02895116. [DOI] [PubMed] [Google Scholar]
- Colhoun H, Hemingway H, Poulter N. Socio-economic status and blood pressure: An overview analysis. Journal of Human Hypertension. 1998;12:91–110. doi: 10.1038/sj.jhh.1000558. [DOI] [PubMed] [Google Scholar]
- Cook DG, Mendall MA, Whincup PH. C-reative protein concentration in children: Relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- Cooper DC, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among blacks and whites. Am J Hypertens. 2009;22(7):698–704. doi: 10.1038/ajh.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Pepys MB. C-reactive protein and coronary disease: is there a causal link? Circulation. 2009;120(21):2036–2039. doi: 10.1161/CIRCULATIONAHA.109.907212. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Barsky A, Butz RM, Chan S, Brief AP, Bradley JC. Subtle yet significant: The existence and impact of everyday racial discrimination in the workplace. Human Relations. 2003;56:1299–1324. [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R. What is representative brain? Neuroscience meets population science. Proceedings of the National Academy of Sciences. 2013;110:17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CB, Wallace SA, Fenton RE. Discrimination distress during adolescence. Journal of Youth and Adolescence. 2000;29(6):679–695. [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of c-reative protein among U.S. youth. Diabetes Care. 2005;28(4):878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- Forman T, Williams DR, Jackson JS. Race, place and discrimination. Social Problems. 1997;9:231–261. [Google Scholar]
- Friedman EM, Williams DR, Singer BH, Ryff CD. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: the MIDUS study. Brain, Behavior, and Immunity. 2009;23(5):684–692. doi: 10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell T, Williams DR, Love GD, McKinley PS, Sloan RP, Ryff C. Race difference in age-trends of autonomic nervous system functioning. Journal of Aging and Health. 2013;25:839–862. doi: 10.1177/0898264313491427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Walsemann KM. School racial composition and race/ethnic differences in early adulthood health. Health & Place. 2012;18:296–304. doi: 10.1016/j.healthplace.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner JA, Joshi M, Bauer JA. Pediatric precursors of adult cardiovascular disease: noninvasive assessment of early vascular changes in children and adolescents. Pediatrics. 2006;118(4):1683–1691. doi: 10.1542/peds.2005-2992. [DOI] [PubMed] [Google Scholar]
- Guthrie BJ, Young AM, Williams DR, Boyd CJ, Kintner EK. African american girls' smoking habits and day-to-day experiences with racial discrimination. Nursing Research. 2002;51(3):183–190. doi: 10.1097/00006199-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Hughes D. Correlates of african american and latino parents’ messages to children about ethnicity and race: A comparative study of racial socialization. American Journal of Community Psychology. 2003;31:15–33. doi: 10.1023/a:1023066418688. [DOI] [PubMed] [Google Scholar]
- Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JSA, Raitakari OT. Risk factors identified in childhood and decreased cartoid artery elasticity in adulthood. The cardiovascular risk in young finns study. Circulation. 2005;112:1489–1496. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- Juonala M, Viikari JSA, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood c-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the cardiovascular risk in young finns study. Arteriosclerosis, Thrombosis, and Vascular biology. 2006;26(8):1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C. Reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–139. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavey R-E, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American heart association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107(11):1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in c-reactive protein levels. Journal of the American College of Cardiology. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine. 2005;61:1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lambert M, Delvin EE, Paradis G, O'Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clinical Chemistry. 2004;50(10):1762–1768. doi: 10.1373/clinchem.2004.036418. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN heart study. Psychosomatic Medicine. 2006;68(3):362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: The bogalusa heart study. Hypertension. 2004;43:541–546. doi: 10.1161/01.HYP.0000115922.98155.23. [DOI] [PubMed] [Google Scholar]
- London B, Downey G, Bonica C, Paltin I. Social causes and consequences of rejection sensitivity. Journal of Research on Adolescence. 2007;17:481–506. [Google Scholar]
- Major B, Quinton WJ, McCoy S. Antecedents and consequences of attributions to discrimination: Theoretical and empirical advances. Advances in Experimental Psychology. 2002;34:254–330. [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for c-reactive protein in dried blood spots. Clinical chemistry. 2004;50(3):652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: Dried spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- McKewen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–40. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reportede experiences of racial discrimination and black-white differences in preterm and low-birthweight deliveries: The CARDIA study. American Journal of Public Health. 2004;94(12):2125–2131. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neblett E, Roberts SO. Racial identity and autonomic responses to racial discrimination. Psychophysiology. 2013;5:1–11. doi: 10.1111/psyp.12087. [DOI] [PubMed] [Google Scholar]
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National health and nutrition examination survey, 2011–2012. National Center for Health Statistics: United States Department of Health and Human Services. 2013:1–7. [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL. Markers of inflammation and cardiovascular disease: Application to clincal and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European journal of epidemiology. 2007;22(1):55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Priest N, Paradies Y, Stewart P, Luke J. Racism and health among urban aboriginal young people. BMJ Public Health. 2011;11:1–9. doi: 10.1186/1471-2458-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism: Clinical and Experimental. 2006;55(1):113–118. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis- An inflammatory disease. The New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sanders-Phillips K, Settles-Reaves B, Walker D, Brownlow J. Social inequality and racial discrimination: risk factors for health disparities in children of color. Pediatrics. 2009;124:176–186. doi: 10.1542/peds.2009-1100E. [DOI] [PubMed] [Google Scholar]
- Sawyer PJ, Major B, Casad BJ, Townsend SS, Mendes WB. Discrimination and the stress response: psychological and physiological consequences of anticipating prejudice in interethnic interactions. American journal of public health. 2012;102(5):1020–1026. doi: 10.2105/AJPH.2011.300620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Nosek BA. Implicit (and explicit) racial attitudes barely changed during Barack Obama’s presidential campaign and early presidency. Journal of Experimental Social Psychology. 2010;38:308–314. [Google Scholar]
- Seaton E, Seller R, Caldwell C, Jackson J. The prevalence of perceived discrimination among african american and carribean black youth. Developmental Psychology. 2008;44:1288–1297. doi: 10.1037/a0012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore S-J. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sellers RM, Copeland-Linder N, Martin PP, Lewis RLH. Racial identity matters: The relationship between racial discrimination and psychological functioning in African American adolescents. Journal of Research on Adolescence. 2006;16:187–216. [Google Scholar]
- Sellers RM, Smith M, Shelton JN, Rowley SJ, Chavous TM. Multidimensional model of racial identity: A reconceptualization of African American racial identity. Personality and Social Pyschology Review. 1998;2:18–39. doi: 10.1207/s15327957pspr0201_2. [DOI] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular disease risk in youth: A systematic review. Brain, Behavior and Immunity. 2012;26:239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, Koenen KC. Internalizing and externalizing behaviors predict elevated inflammatory markers in childhood. Psychoneuroendocrinology. 2013;38(12):2854–2862. doi: 10.1016/j.psyneuen.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- StateCorp. Statistical Software. College Station, TX: StataCorp LP; 2013. Stata: Release 13. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanton SL, Rifkind JM, Mohanty JG, Miller ER, 3rd, Thorpe RJ, Nagababu E, Epel ES, Zonderman AB, Evans MK. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. International journal of behavioral medicine. 2012;19(4):489–495. doi: 10.1007/s12529-011-9188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68(5):807–813. doi: 10.1016/j.socscimed.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychology. 2003;22(3):300–309. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- Utsey SO, Ponterrotto JG, Reynolds A, Cancelli A. Racial discrimination, coping, life satisfaction, and self-esteem among african americans. Journal of Counseling and Development. 2000;78:72–80. [Google Scholar]
- Vidmar S, Carlin J, Hesketh K. Standardizing anthropometric measures in children and adolescents with new functions for egen. The Stata Journal. 2004;4(1):50–55. [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107(1):1–6. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Racism and health I: Pathways and scientific evidence. American Behavioral Scientist. 2013;57:1152–1173. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CA, Eccles JS, Sameroff A. The influence of ethnic discrimination and ethnic identification on African American adolescents' school and socioemotional adjustment. Journal of Personality. 2003;71:1197–1232. doi: 10.1111/1467-6494.7106012. [DOI] [PubMed] [Google Scholar]
- Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in african americans. The American Journal of the Medical Sciences. 2003;325(6):315–331. doi: 10.1097/00000441-200306000-00003. [DOI] [PubMed] [Google Scholar]