Abstract
Cancer stem cells (CSCs) represent a small subset of tumor cells which have the ability to self-renew and generate the diverse cells that comprise the tumor bulk. They are responsible for local tumor recurrence and distant metastasis. However, they are resistant to conventional radiotherapy and chemotherapy. Novel immunotherapeutic strategies which specifically target CSCs may improve the efficacy of cancer therapy. To immunologically target CSC phenotypes, innate immune responses to CSCs have been reported using NK cells and γδT cells. To target CSC specifically, in vitro CSC-primed T cells have been successfully generated and shown targeting of CSCs in vivo after adoptive transfer. Recently, CSC-based dendritic cell vaccine has demonstrated significant induction of anti-CSC immunity both in vivo in immunocommpetent hosts and in vitro as evident by CSC reactivity of CSC vaccine-primed antibodies and T cells. In addition, identification of specific antigens or genetic alterations in CSCs may provide more specific targets for immunotherapy. ALDH, CD44, CD133 and HER2 have served as markers to isolate CSCs from a number of tumor types in animal models and human tumors. They might serve as useful targets for CSC immunotherapy. Finally, since CSCs are regulated by interactions with the CSC niche, these interactions may serve as additional targets for CSC immunotherapy. Targeting the tumor microenvironment, such as interrupting the immune cell e.g. myeloid derived suppressor cells, and cytokines e.g. IL-6 and IL-8, as well as the immune checkpoint (PD1/PDL1, et.al) may provide additional novel strategies to enhance the immunological targeting of CSCs.
1. Introduction
Cancer stem cells (CSCs) are defined as malignant cancer cells that retain the ability to self-renew and differentiate generating non-tumorigenic cancer cells that form a tumor mass [1]. CSCs are believed to play important roles in tumor initiation, relapse, metastasis and resistance to traditional therapies [2]. These properties highlight the importance of developing therapeutic strategies to target the CSC population. Major conceptual and technical advances in immunology over the past 25 years have led to a new understanding of cellular and molecular interactions between the immune system and tumor cells. In parallel, recent advances in tumor immunotherapy have provided powerful new therapeutic approaches that have produced durable clinical responses with limited toxicities in a small subset of patients [3]. Although it is currently not known what accounts for these durable remissions receiving immunotherapy, the possibility that this may be related to the ability of these therapies to target CSCs warrants further exploration. If this is demonstrated, then immunologic strategies specifically designed to target CSCs may increase the proportion of patients experiencing these durable remissions. Since CSCs drive tumor progression and metastasis, long term benefit of cancer therapies involving conventional approaches such as surgery, chemotherapy and/or radiation therapy may depend on their ability to effectively target CSCs.
2. CSCs are resistant to conventional therapeutic agents
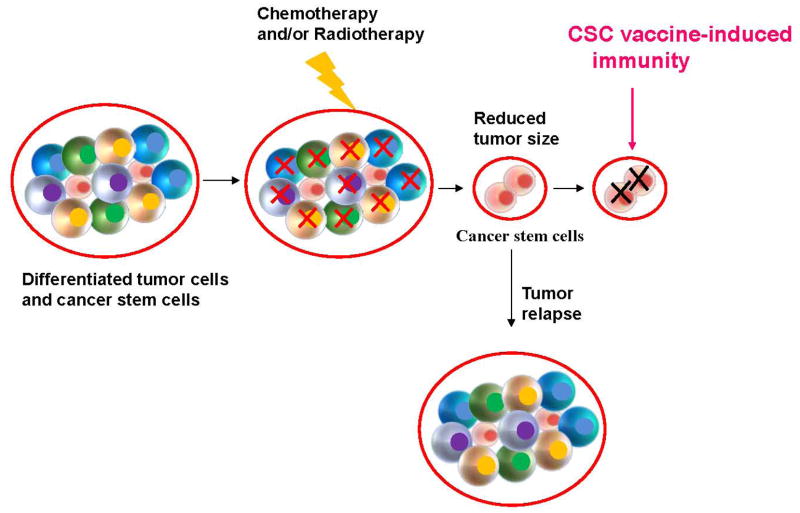
Despite advances in radiation therapy and chemotherapy, the prognosis of patients with advanced malignant tumors remains poor. Ineffective targeting of CSCs has been suggested as one reason for current treatment failure [4]. CSCs have been documented to be resistant to various chemotherapeutic agents and radiotherapy [5–7]. The resistance of CSCs to chemotherapy may involve increased expression of drug efflux pumps, more efficient DNA repair [5, 8], and interactions of CSCs with their microenvironment [9, 10]. In light of CSC resistance to conventional therapeutic agents, development of alternative/novel therapeutic strategies that can specifically and effectively target CSCs are needed to enhance the efficacy of other therapeutic agents (Fig. 1).
Fig. 1.
The inability to target cancer stem cells represents a significant factor contributing to current treatment failure
3. Immunological targeting of cancer stem cell phenotypes
There are a number of theoretical reasons which provide a rationale for developing immune approaches to target CSCs. It is clear that CSCs and their more differentiated progeny display distinct gene expression profiles and therefore express different antigens. Immunologic approaches directed against whole tumors are largely biased toward more differentiated tumor cells which form the bulk of the tumor and which express “differentiation” antigens. This suggests that effective immune targeting of CSC may require the specific targeting of this cell population. In addition, within a tumor, CSCs may themselves exhibit heterogeneity resulting from both genetic and epigenetic regulation associated with tumor progression and metastasis. For instance we [11] have shown that breast CSCs maintain that plasticity to transition between mesenchymal (EMT) and epithelia (MET) states in a process regulated by the tumor microenvironment. The ability of immunotherapies to target multiple antigens make these approaches wells suited to the targeting of these heterogenous CSC populations.
3.1 Innate immune response to CSCs
Natural killer (NK) cells are major effector cells for innate immunity, making them suitable candidates for immunotherapy of both hematologic and solid tumors [12, 13]. However, the role of NK cells in anti-CSC immune surveillance remains controversial. Wu et al., investigated the immunogenicity of CD133+ brain tumor stem cells (BTSCs). Their data revealed that the majority of CD133+ cells do not express detectable MHC I or natural killer (NK) cell activating ligands, which may render them resistant to adaptive and innate immune surveillance [14]. Wang et al., also reported that MICA and MICB (MHC class I-related chain A and B), two ligands for the stimulatory NK cell receptor NKG2D, are downregulated due to aberrant expression of oncogenic miR-20a in human breast CSCs, which resulted in immune escape of these CSCs from NK cell killing [15]. In contrast, Castriconi et al., reported that glioma stem cells (GSCs) express various ligands of NK cell activation receptors that trigger optimal NK cell cytotoxicity. They found that GSCs are highly susceptible to lysis mediated by both allogeneic and autologous IL-2 (or IL-15)-activated NK cells [16]. Tseng et al. also showed that primary oral squamous carcinoma stem cells (OSCSCs) are significantly more susceptible to NK cell-mediated cytotoxicity than their differentiated counterparts [17]. Tallerico et al., analyzed the NK cell recognition of colorectal adenocarcinoma CSCs. They demonstrated that allogeneic NK cells can recognize and kill these CSCs but the non-CSC counterpart is less susceptible to NK cells. Compared with the non-CSCs, these colorectal CSCs express higher levels of ligands for the natural cytotoxicity receptors which mediates NK cell killing and lower levels of MHC class I [18].
Unconventional γδ T cells represent another group of innate immune effector cells, which constitute 1–5% of circulating lymphocytes and are of the Vγ9Vδ2 phenotype. Immunotherapy with γδ T cells is of substantial interest based on their potent non-HLA-restricted cytotoxicity against different tumor entities and their capacity to recognize and present antigens to αβ T cells [19, 20]. γδ T cells primarily target isopentenyl pyrophosphate (IPP), an intermediate of the mevalonate pathway for isoprenoid biosynthesis in eukaryotic cells [21, 22]. Nishio et al., showed that Vγ9Vδ2 T cells mediate cytolysis of sphere-forming neuroblastoma cells sensitized with zoledronate [23]. Todaro et al. also reported that Vγ9Vδ2 T cells are induced to proliferate, secrete TNF-α and IFN-γ, and produce the cytotoxic and apoptotic molecules TRAIL and granzymes after exposure to zoledronate-sensitized human colon CSCs [24]. In a clinical study, activated Vγ9Vδ2 T cells in combination with zoledronate show increased CD69 expression, indicating an activated phenotype. These Vγ9Vδ2 T cells displayed up-regulated expression of peripheral tissue-homing chemokine receptors, CCR5 and CXCR3. In contrast, there was a decrease in expression of the lymphoid-homing receptors, CCR7 and CXCR5 [23]. These Vγ9Vδ2 T cells were cytotoxic in vitro, and adoptively transferred Vγ9Vδ2 T cells trafficked predominantly to the lung, liver and spleen as well as to the metastatic tumor sites outside these organs [25]. These results indicate that in vitro expansion of autologous γδT cells in combination with other anti-tumor agents may benefit cancer treatment via CSC destruction. Further studies are needed to confirm direct targeting of CSCs by γδ T cells. There is a paucity of clinical studies involving the use of non-specific killer cells in the adoptive immunotherapy of solid tumors that have examined effects on CSCs.
3.2 In vitro CSC-primed T cells specifically target CSCs in vivo
CD8+ T cells undergo proliferation and differentiate into cytotoxic T lymphocytes (CTLs) in the presence of appropriate stimulation [26]. Activated CTLs can migrate to peripheral tissues where they exert two main effector functions: direct contact-mediated cytotoxicity and secretion of effector cytokines, such as IFN-γ and TNF-α. Another essential function of activated CD8+ T cells is the acquisition of memory. Memory CD8+ T cells can be maintained for long periods of time without antigenic stimulation and potentiate a more potent and faster immune response of the host upon cancer relapse or development of metastasis [27]. CD4+ T helper cells also play a critical role in the development of effective anti-tumor immunity by increasing clonal expansion of CTL at the tumor site, promoting the generation and maintenance of memory CTLs, preventing activation-induced cell death (AICD) and functioning as APCs for CTLs [28].
CSC-specific CD8+ T cells were generated from human acute myeloid leukemia (AML) stem cells in 1999 by Bonnet et al, and were observed to mediate tumor regression after injection into NOD/SCID mice [29]. Brown and colleagues isolated CD133+ BTSCs, and demonstrated that these BTSCs are susceptible to perforin- dependent CTL-mediated cytolysis [30]. To assess whether the protein processing machinery is sufficiently intact for the BTSCs population to process and present antigen for CD8+ CTL recognition, the authors engineered glioma tumor sphere (TSs) to endogenously express the cytomegalovirus (CMV) pp65 antigen by reconstructed pp65-lentiviral transduction. They found that CMV-specific CTLs mediate the CMV-transducted glioma TSs cytotoxicity. When CMV pp65-expressing TSs and pp65-specific CTLs were co-injected into NOD/SCID mice, the pp65 antigen-positive tumor cells were ablated, while pp65− tumor cells were resistant to the pp65-specific CTL and efficiently engrafted. This result indicated that direct recognition of antigen by CTLs is required to eliminate tumor initiation [30]. In another study, Visus and colleagues generated CSC-specific CD8+ T cells by using antigenic peptide from ALDH1A1. The transfer of ALDH1A1-specific CD8+ T cells eliminated ALDH1A1bright cancer stem cells from squamous cell carcinoma of the head and neck (SCCHN), inhibited tumor growth and metastases, and prolonged survival of xenograft-bearing immunodeficient mice [31, 32]. Together, these studies suggest that CSC-specific T cells can be generated in vitro for subsequent adoptive transfer into tumor-bearing hosts to target CSCs and eradicate tumors in vivo. In addition, these studies have demonstrated that CSCs are sensitive to T cell mediated killing. One problem with targeting CSCs with CSC-specific T cells in an adoptive immunotherapy approach is the immune escape of tumors with antigen loss. In the next Section, we describe a method of generating CSC-specific T and B cell responses in vivo utilizing a vaccine approach and how to mitigate against antigen loss variants.
Interestingly, CSCs seem to be able to evolve strategies to escape T cell attack. Recently Volonté et al., reported that CSCs derived from colorectal cancer (CRC) show weak immunogenicity compared with non-CSC counterparts. This feature may correlate with the expression of high levels of IL-4 by the CSCs because neutralization of CSC-associated IL-4 can rescue the proliferative activity of T lymphocytes [33]. Based on the results in this study, new immunotherapy protocols to target CSCs might involve blocking inhibitory activity of immunomodulatory molecules as well as activating T cell by CSC specific or associated antigens.
3.3 CSC-based vaccines target CSCs in immunocompetent hosts
The use of professional antigen presenting cells, such as dendritic cells (DCs), to initiate tumor-specific T cell responses represents a promising strategy for cancer vaccination approaches. Glioblastoma-derived cancer stem cells express MHC I [34]. After co-culturing human immature, autologous DCs with irradiated brain tumor stem cells, the cancer stem cell-primed mature DCs expressed co-stimulatory molecules CD80, CD86 and CD40, and stimulated significant Th1 (IFN-γ) response in vitro [34]. These studies demonstrate that CSCs may be antigenic and can be used to develop cell-specific vaccines. The efficacy of a tumor-specific vaccine in vivo is dependent upon both cellular and humoral host immunity. However, to date most CSC studies have been performed with human tumor-derived CSCs in NOD/SCID mice [29]. Due to the lack of cellular and humoral immunity, these immunocompromised mice are not suitable for assessing the efficacy of CSC vaccines. The lack of an intact host immune system prevents evaluation of multiple interactions that occur such as epitope spreading, antigen cross-presentation, and immune evasion mechanisms such as T regulatory cells or myeloid-derived suppressor cells; to name a few.
Based on this consideration, our group assessed the effects of CSC-DC vaccine in various syngeneic immunocompetent mouse tumor models, and demonstrated that CSC-DC vaccination significantly prevents lung metastasis of melanoma cells and inhibits tumor growth of squamous carcinoma compared to immunization with bulk tumor cells [35]. In this study, the tumorigenicity of murine aldehyde dehydrogenase (ALDH)+/high CSCs were demonstrated in two histologically different tumors (D5 melanoma and SCC7 squamous cell carcinoma) from two genetically distinct immunocompetent hosts (B6 and C3H mice) [35] We utilized cancer stem cells selected by virtue of their expression of the CSC marker, ALDH as an antigen source to prime DCs, and evaluated the protective effects of cancer stem cell-primed DC vaccines in mouse tumor challenge models [35]. This study demonstrated that CSC-primed DC vaccination was significantly more effective at preventing lung metastasis in the D5 model and subcutaneous tumor growth in the SCC7 model compared with control mice given DCs pulsed with unsorted heterogeneous tumor cells or ALDH−/low non-CSCs. Systemic anti-CSC immunity was associated with CSC-specific IgG and CSC-specific CTLs present in the peripheral blood of CSC-DC vaccinated hosts. These data indicate that enriched CSCs are immunogenic and more effective as an antigen source than unselected tumor cells or non-CSCs in inducing antitumor immunity against CSC epitopes [35]. Consistent with these observations, Phuc and colleagues reported that breast CSC-DC vaccine could migrate to the spleen, activate CD8+ and CD45+ T cells and induce CTL antitumor responses [36]. Currently, we hypothesize that CSCs may have multiple epitopes that are distinct from non-CSCs and which can be targeted by T or B cells. Work is currently underway to identify these antigens. The use of tumor lysates of CSCs as a source of antigen would potentially allow targeting multiple antigens simultaneously; and would be less susceptible to antigen loss as a means of tumor escape.
A number of studies have suggested that tumor vaccines have their greatest efficacy in the setting of micrometastatic disease. Due to the low percentage of CSCs within established tumor masses, CSC-targeted DC vaccines may have minimal effect on tumor size. We postulate that CSC-DC may have maximum utility when deployed in an adjuvant setting after surgical removal of the bulk tumor mass to target microscopic residual CSCs or as combinatorial therapy with radiation and/or chemotherapy in the therapy of established macroscopic tumors. Using an established murine tumor model, we found that treatment of microscopic tumor by CSC-DC was more efficacious that DCs pulsed with non-CSCs (Lin, et al., In press, OncoImmunology, 2015). Furthermore, in the established macroscopic tumor setting, the combination of radiation therapy (RT) plus CSC-DC vaccine was more effective than RT alone or CSC-DC alone in reducing tumor growth and improving survival (Lin, et al., In press, OncoImmunology, 2015). In assessing the percentage of CSC in the treated primary tumors, RT alone resulted in an increased percent of CSC whereas CSC-DC resulted in significant decrease in the percent of CSC (Lin, et al., In press, OncoImmunology, 2015). Mice treated with the combination of RT and CSC-DC vaccine had significantly fewer spontaneous lung metastases compared with other control groups indicating the relationship between the ability to target CSC and a reduction in spontaneous metastatic disease (Lin, et al., In press, OncoImmunology, 2015).
4. Immunological targeting of CSC antigens
Cancer immunotherapy is based on the ability of the immune system to recognize cancer cells and to affect their growth and expansion. This suggests that proteins express on CSCs may provide targets for CSC immunotherapies. CSCs express various markers, including ALDH, CD44, CD133, and HER2, at levels substantially different from the bulk tumor cell population. These markers have proven useful for identifying and isolating CSCs. These CSC markers may provide specific targets for CSC immunotherapies.
4.1 ALDH
ALDH is responsible for the oxidation of aldehydes to carboxylic acids to prevent cells from oxidative insult and facilitate their survival. Increased ALDH activity has been found in CSCs of various tumor types, such as bladder, breast, colon, gastric, head and neck, lung, pancreatic, prostate, as well as hematopoietic and neural stem/progenitor cells [37–46]. In addition, ALDH- mediated detoxification of toxic aldehyde intermediates produced in cancer cells treated with certain chemotherapy agents has been proposed to confer drug-resistant properties to ALDHhigh tumor cells [45]. Dylla et al., found that using short hairpin RNA against ALDH1 sensitized human colorectal CSCs to cyclophosphamide (CPA) [47]. Raha et al. defined a requirement for ALDH in the maintenance of a drug-tolerant subpopulation of cancer cells that share some properties with cancer stem cells. ALDH protects these CSCs from the potentially toxic effects of elevated levels of reactive oxygen species (ROS) in the cells [48].
Immunological targeting of ALDH activity in vitro and in vivo has been reported by several groups. Visus et al., generated ALDH-specific CD8+ T cells that recognized and eliminated the ALDHhigh tumor cells in human carcinomas [32]. In this work, ALDH-specific CD8+ T cells were induced/ expanded by in vitro stimulation of human CD8+ T cells with ALDH peptide-pulsed autologous dendritic cells. The percentages of ALDHhigh cells were decreased by 60%–89% resulting from ALDH-specific CD8+ T cell-mediated cytotoxicity in vitro. In preclinical models utilizing human tumor xenografts in immunodeficient mice, ALDH-specific CD8+ T cells inhibited xenograft growth and metastases, and prolonged survival after adoptive transfer. These results clearly demonstrated that ALDH can serve as potential target for T cell-based immunotherapy to eliminate CSCs [32].
In addition to ALDH being a CSC marker, there is increasing evidence that it plays an important functional role in these cells. For example, Wang et al. demonstrated that disulfiram (DSF) an irreversible inhibitor of ALDH activity blocked the formation of radiation-induced breast CSCs [49]. In their work, irradiation-induced stemness correlates with increased spontaneous lung metastasis in syngeneic mouse mammary tumor models. However, irradiation-induced stemness was blocked by targeting ALDH activity with DSF. Treatment of mice with radiation and DSF significantly inhibited mammary primary tumor growth and spontaneous lung metastasis, which was associated with decreased cancer stem cells [49] The demonstration of an important role of ALDH in CSC function provides an additional rationale for utilizing it as a target for immunotherapy since it might reduce the likelihood of immune escape through down regulated expression.
4.2 CD44
Cell surface CD44, a highly glycosylated type-1 transmembrane p-glycoprotein (~90 kDa), is among the most widely utilized CSC markers [50]. CD44 is present in multiple species generated by alternative splicing. Differently spliced variants include the V6 isoform in colon cancer CSCs [50] and the standard isoform in breast CSCs [51]. CD44 is involved in multiple signaling functions, e.g. cell proliferation, apoptosis, survival, migration and differentiation [52]. Moreover, a recent study reveals that the CD44 protein plays an important role in a number of CSC functions including self-renewal, niche preparation, epithelial-mesenchymal transition and resistance to apoptosis [53].
Considering that CD44 has its functional roles and is a marker on CSCs, targeting CD44 with immunological approaches represents a promising strategy to eliminate CSCs. In 1993, Seiter et al., firstly reported that monoclonal antibody 1.1ASML against a splice variant of CD44 (CD44v) retarded growth of lymph node and lung metastases from pancreatic adenocarcinoma in rats [54]. Since then, anti-CD44 antibodies have been shown to promote terminal differentiation of AML blasts [55], inhibit growth of murine mammary carcinoma cells and human colon carcinoma cells and induce apoptosis [56], and decrease human melanoma metastasis and increase animal survival in SCID mice [57]. Given these results, recently several anti-CD44 antibodies have been developed and used in anti-CSC approach [58–60]. Jin et al. used the anti-CD44 monoclonal antibody H90 to selectively eradicate AML CSCs in NOD/SCID mice [58, 59]. They found that H90 blocked leukemic stem cells trafficking to their supportive microenvironment and altered stem cell fate [58]. Young et al. also described that H460-16-2, a humanized anti-CD44 monoclonal antibody, is able to reduce the growth of BxPC3 pancreatic cancer xenografts by 80%. In addition, it has been demonstrated that in AML xenografts H460-16-2 binds to CD34+CD38− CSCs increasing mouse survival. Clinical trials utilizing this antibody are planned [60].
4.3 CD133
Human CD133 (human prominin-1) is expressed on CSCs in a number of solid tumors [61, 62]. Recent studies have shown that the CD133+ subpopulation displays resistance to chemotherapy and radiotherapy, and high CD133 expression is a marker of poor prognosis [62]. Several monoclonal antibodies to CD133 have been generated [63, 64]. Our group recently generated an anti-CD3/anti-CD133 bispecific antibody (BsAb) and bound it to the cytokine-induced killer (CIK) cells as effector cells (BsAb-CIK) to target CD133high CSCs. We found that killing of CD133high pancreatic (SW1990) and hepatic (Hep3B) cancer cells by the BsAb-CIK cells was significantly higher than the killing by the parental CIK or by CIK cells bound with anti-CD3 (CD3-CIK) without CD133 targeting. In nude mice, the BsAb-CIK cells inhibited CD133high tumor growth significantly more than that by CIK or CD3-CIK cells, or BsAb alone. Mechanistically, treatment with the BsAb-CIK cells significantly down regulated the expression of S100P and IL-18bp, but up-regulated STAT1. These findings suggest a novel immunotherapy for patients with cancer containing CD133high CSCs involving selective targeting of this cell population [65].
4.4 HER2
Human epidermal growth factor receptor-2 (HER2) is over-expressed in several human cancers of epithelial origin where it plays an essential role in tumor development [66, 67][65, 66]. It has been shown that over-expression of HER2 in breast cancer is often associated with an aggressive course characterized by increased disease recurrence and a poor prognosis. Specifically, we have shown that the level of ALDH in HER2+ breast cancer is much higher than that in HER2− breast cancers. HER2 regulates the mammary stem/progenitor cell population, driving tumorigenesis, invasion and HER2-associated radioresistance of breast cancer stem cells [6, 68] [6, 67]. We have shown that in HER2+ breast cancers, HER2 regulates CSC self-renewal [68] and that this is mediated by a pathway involving Akt and B Catenin [69] and have suggested that the ability of HER2 targeting agents to eliminate CSC may contribute to their remarkable clinical efficacy In addition, to playing a role in HER2+ breast cancers we recently showed [67, 70] that in luminal breast cancers that are considered HER2 negative, HER2 is selectively expressed in the ALDHhigh CSC population. FACS analysis of ALDHhigh vs. ALDHlow cells showed enrichment for HER2 expression in ALDHhigh cells. In MCF7 and ZR75-1 human breast cancer luminal cell lines, the level of HER2 expression was considerably lower than in the HER2-amplified cell lines. However, in these cells, HER2 expression was increased 2- to 3-fold in ALDHhigh cells (HER2+ALDHhigh) as compared with ALDHlow cells (HER2−ALDHlow). We have characterized the stem cell nature of the HER2+ALDHhigh cell [71]. We have proposed that HER2 expression in luminal CSCs in the absence of HER2 gene amplification may account for the surprising finding that the benefit of adjuvant trastuzumab may extend to patients whose tumors do not display HER2 gene amplification [71, 72].
In addition to the use of HER2 blocking antibodies, other immunologic approaches have been utilized to target HER2 expressing cells. Sen and colleagues reported that activated T cells armed with anti-CD3 x anti-HER2 bispecific antibody (HER2Bi) mediate high levels of specific cytotoxicity directed at both low and high HER2-expressing breast cancer cell lines [73]. Intravenous infusions of HER2Bi-armed T cells inhibited the growth of established HER2+ PC-3 tumors in SCID/Beige mice and prevented tumor development in co-injection WINN assays [74, 75]. Arming T cells with HER2Bi converts every T cell into a non-MHC restricted HER2-specific cytotoxic T lymphocyte [73]. In a phase I clinical trial led by Lum et al. with infusion of anti-CD3 x anti-HER2 bispecific antibody armed T cells involving 23 HER2 negative (0–2+ IHC) metastatic breast cancer patients, the median OS for the HER2 negative women was 25.9 months, considerably longer than expected from historic controls (personal communication with Dr. LG Lum). Apparently, HER2Bi armed T cells, while intended to target HER2, seem to benefit patients that are HER2-negative by classical criteria including lack of HER2 gene amplification as determined by FISH. Our finding of selective HER2 expression in CSCs in these breast cancers classified as “HER2-negative” may provide a biological explanation for these clinical findings. However, the potential of infused HER2Bi armed T cells to specifically induce immune responses against breast CSCs remains untested.
5. Immunotherapeutic targeting of the CSC niche
CSCs reside in a niche within the tumor, which contributes to the self-renewal and differentiation of these cells. Growth factors, cytokines, and diverse stromal cells, such as mesenchymal stem cells and immune cells in the cellular microenvironment are essential for cell nutrition, intercellular communication, signal transduction and cell fate [76]. For example, Lu et al. [77] recently demonstrated that tumor associated monocytes and macrophages (TAM’s) create a niche through juxtracrine signaling of CSCs. These studies suggest that, components in CSC niche may provide additional therapeutic targets for eliminating CSCs.
5. Immune cell/cytokines (Myeloid Derived Suppressor Cells, IL-6 et al)
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population including immature macrophages, dendritic cells, granulocytes and other myeloid cells at earlier stages of differentiation [78]. MDSCs can directly incorporate into tumor endothelium and secrete many proangiogenic factors. They also induce the production of matrix metalloproteinases (MMPs), chemo attractants and create a pre-metastatic environment [78]. Panni et al demonstrated the role of MDSC in promoting the ALDHhigh CSCs in pancreatic cancer in a mouse model [79]. STAT3 signaling in MDSCs can be modulated by IL-6, which has been shown to enhance CSCs and EMT in cancer [80, 81]. Cui et al also reported that MDSCs enhance CSC gene expression, sphere formation, and cancer metastasis in patients with ovarian carcinoma [82]. To the best of our knowledge, there are no published studies on targeting MDSCs for CSC elimination. However, given the roles of MDSCs in tumor invasion and metastasis, immunological targeting of MDSCs represents a rational approach for targeting CSCs.
Recently, Korkaya et al. demonstrated that monocytes and macrophages recruited to breast tumor directly regulate CSC through inflammatory cytokines IL-1, IL-6 and IL-8 in the CSC niche which are involved in driving CSC self-renewal [9]. These cytokines activate STAT3/NF-κB pathways in both tumor and stromal cells, which in turn stimulate further cytokine production, generating positive feedback loops that contribute to CSC self-renewal [10]. Inhibitors of these cytokines and their receptors have been developed and trials to utilize these inhibitors to block CSC self-renewal have been initiated [9, 83]. Immunologically, blockade of the IL-8 receptor CXCR1 using antibody or repertaxin (a small-molecule CXCR1 inhibitor) selectively depletes the cancer stem cell population in human breast cancer cell lines in vitro, followed by the induction of massive apoptosis in the bulk tumor population via FASL/FAS signaling [84]. In addition, IL-6 has been shown to be a direct regulator for CSC self-renewal [85–87]. Anti-IL-6 antibody inhibited JAK1 and STAT3 activation as well as OCT-4 gene expression, thus inhibiting CSCs [88]. These studies suggest that IL-6R blockade may provide attractive therapies in attempt to immunologically target cancer stem cells.
5.1 Immune checkpoints (PD-1/PD-L1)
Immune checkpoints are cell surface molecules that serve as endogenous regulators of the immune response, limiting autoimmunity by mediating co-inhibitory signaling pathways [89]. In cancer, these inhibitory pathways are involved in tumor immune-resistance [90]. To date, two major immunoinhibitory pathways have been recognized, namely the programmed cell death-1 (PD-1)/PD-L1 axis, and the cytotoxic T lymphocyte antigen 4 (CTLA-4)/B7 axis. These negative immune regulatory pathways have been proposed to contribute to a suppressive microenvironment that protects cancer cells from immune destruction [91, 92]. CSCs might reciprocally modulate the immune cells in CSC niche through the secretion of paracrine factors or direct cell-cell contact, based on the concept that physiologic stem cells have immunoprivilege and active immunoregulatory functions [93–97]. Schatton et al., reported evidence that CSCs downregulate T cell activation [98, 99]. They identified a novel type of CSCs, malignant melanoma initiating cells (MMIC), based on their expression of the chemoresistance determinant ABCB5 [98]. Tumorigenic human ABCB5+ MMICs preferentially express PD-1 and B7.2, while having down-regulated expression of PD-L1 compared to ABCD5− cells. Recently PD-1 and PD-L1 antibodies have been shown to have clinical benefit in a variety of cancers including melanoma and lung cancer [100, 101] and most recently in refractory Hodgkins disease [102]. In these studies a subset of patients enjoy prolonged responses often considerably more durable that those produced by cytotoxic or even targeted therapies, It is postulated that expression of PD-L1 on tumors may down-regulate activated T cell responses via the PD-L1/PD-1 axis; and its blockade results in effective T cell responses. Although Schatton et al. reported decreased PD-L1 expression on MMICs [99]; a recent study demonstrated preferential expression of PD-L1 on CSC in head and neck carcinoma [103]. This opens the possibility that subsets of CSCs may down-regulate T cell immunity via the PD-1/PD-L1 axis. Assessment of CSCs in future clinical trials involving immune checkpoint blockade will be necessary to determine whether this is the case. Furthermore, combination of immune checkpoint therapies with CSC targeting immunotherapies such as vaccines may enhance the clinical utility of each approach.
Summary
Multiple immunotherapeutic approaches to target CSCs are in development. As outlined in Fig 2, these include direct targeting of CSCs with immunological methods, e.g. CSC-DC vaccine; blocking the “help” to CSCs from the tumor microenvironment, e.g. anti-IL-6 mAb, and inhibiting CSC-mediated immune suppression, e.g. blockade with anti-PD-1/PD-L1 mAbs. It will be necessary to rigorously test these strategies alone or in combination to determine their therapeutic efficacy. However, immunologic targeting of CSCs represents a promising new direction in cancer therapeutics which we postulate will be more effective as combinatorial therapy with conventional modalities as well as with immunomodulatory agents.
Fig. 2.
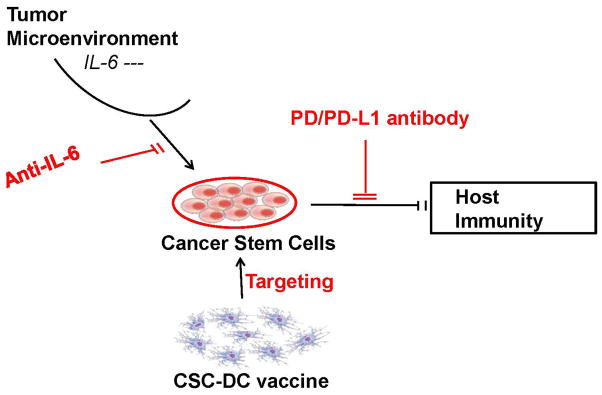
Immunological Targeting of Cancer Stem Cells
Acknowledgments
This work was supported by NIH grant 1R56DE024385-01, the University of Michigan Round 10 MICHR/CTSA Pilot Grant Programs T1-Bench to bedside collaborations award, and was partially supported by the Gillson Longenbaugh Foundation.
Footnotes
Author Contributions:
Qin Pan: Manuscript writing, Collection and/or assembly of data, Data analysis and interpretation
Qiao Li: Conception and design, Financial support, Manuscript writing, Data analysis and interpretation, Final approval of manuscript
Shuang Liu: Collection and/or assembly of data
Ning Ning: Collection and/or assembly of data
Xiaolian Zhang: Collection and/or assembly of data
Yingxin Xu: Collection and/or assembly of data
Alfred E. Chang: Conception and design, Financial support, Manuscript writing, Data analysis and interpretation, Final approval of manuscript
Max S. Wicha: Conception and design, Financial support, Administrative support, Manuscript writing, Data analysis and interpretation, Final approval of manuscript
References
- 1.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 2.Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med. 2008;12:374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung DS, Shin HJ, Hong YK. A new hope in immunotherapy for malignant gliomas: adoptive T cell transfer therapy. J Immunol Res. 2014;2014:326545. doi: 10.1155/2014/326545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Becker MW, Wicha M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 6.Duru N, Fan M, Candas D, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18:6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond) 2012;7:597–615. doi: 10.2217/nnm.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer’s inflammatory roots. Clin Cancer Res. 2011;17:6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretta A, Locatelli F, Moretta L. Human NK cells: from HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol Rev. 2008;224:58–69. doi: 10.1111/j.1600-065X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 13.Baker GJ, Chockley P, Yadav VN, et al. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer Res. 2014;74:5079–5090. doi: 10.1158/0008-5472.CAN-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu A, Wiesner S, Xiao J, et al. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Wang Q, Wang Z, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746–5757. doi: 10.1158/0008-5472.CAN-13-2563. [DOI] [PubMed] [Google Scholar]
- 16.Castriconi R, Daga A, Dondero A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 17.Tseng HC, Arasteh A, Paranjpe A, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS One. 2010;5:e11590. doi: 10.1371/journal.pone.0011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tallerico R, Todaro M, Di Franco S, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–2390. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
- 19.Braza MS, Klein B. Anti-tumour immunotherapy with Vgamma9Vdelta2 T lymphocytes: from the bench to the bedside. Br J Haematol. 2013;160:123–132. doi: 10.1111/bjh.12090. [DOI] [PubMed] [Google Scholar]
- 20.Fournie JJ, Sicard H, Poupot M, et al. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10:35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka Y, Kobayashi H, Terasaki T, et al. Synthesis of pyrophosphate-containing compounds that stimulate Vgamma2Vdelta2 T cells: application to cancer immunotherapy. Med Chem. 2007;3:85–99. doi: 10.2174/157340607779317544. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y. Human gamma delta T cells and tumor immunotherapy. J Clin Exp Hematop. 2006;46:11–23. doi: 10.3960/jslrt.46.11. [DOI] [PubMed] [Google Scholar]
- 23.Nishio N, Fujita M, Tanaka Y, et al. Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human gammadelta T cells. J Immunother. 2012;35:598–606. doi: 10.1097/CJI.0b013e31826a745a. [DOI] [PubMed] [Google Scholar]
- 24.Todaro M, D’Asaro M, Caccamo N, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 25.Nicol AJ, Tokuyama H, Mattarollo SR, et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Shah S, Qiao L. Tumor resistance to CD8+ T cell-based therapeutic vaccination. Arch Immunol Ther Exp (Warsz) 2007;55:205–217. doi: 10.1007/s00005-007-0029-3. [DOI] [PubMed] [Google Scholar]
- 27.Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 29.Bonnet D, Warren EH, Greenberg PD, et al. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc Natl Acad Sci U S A. 1999;96:8639–8644. doi: 10.1073/pnas.96.15.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CE, Starr R, Martinez C, et al. Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res. 2009;69:8886–8893. doi: 10.1158/0008-5472.CAN-09-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visus C, Ito D, Amoscato A, et al. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumor antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–10545. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 32.Visus C, Wang Y, Lozano-Leon A, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells. Clin Cancer Res. 2011;17:6174–6184. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volonte A, Di Tomaso T, Spinelli M, et al. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J Immunol. 2014;192:523–532. doi: 10.4049/jimmunol.1301342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Q, Liu G, Yuan X, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ning N, Pan Q, Zheng F, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phuc PVHC, Nguyet NTM, Thuy DT. Effects of breast cancer stem cell extract primed dendritic cell transplantation on breast cancer tumor murine models. Ann Rev Res Biol. 2011;1:13. [Google Scholar]
- 37.Su Y, Qiu Q, Zhang X, et al. Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsuno Y, Ehata S, Yashiro M, et al. Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-beta. J Pathol. 2012;228:391–404. doi: 10.1002/path.4020. [DOI] [PubMed] [Google Scholar]
- 40.Duarte S, Loubat A, Momier D, et al. Isolation of head and neck squamous carcinoma cancer stem-like cells in a syngeneic mouse model and analysis of hypoxia effect. Oncol Rep. 2012;28:1057–1062. doi: 10.3892/or.2012.1904. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 42.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MP, Fleming JB, Wang H, et al. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger PE, Gupta R, Xiong X, et al. High aldehyde dehydrogenase activity: a novel functional marker of murine prostate stem/progenitor cells. Stem Cells. 2009;27:2220–2228. doi: 10.1002/stem.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 46.Balber AE. Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 2011;29:570–575. doi: 10.1002/stem.613. [DOI] [PubMed] [Google Scholar]
- 47.Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raha D, Wilson TR, Peng J, et al. The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 2014;74:3579–3590. doi: 10.1158/0008-5472.CAN-13-3456. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Li W, Patel SS, et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 2014;5:3743–3755. doi: 10.18632/oncotarget.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todaro M, Gaggianesi M, Catalano V, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–778. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh SC, Neslihan Alpay S, Klostergaard J. CD44: a validated target for improved delivery of cancer therapeutics. Expert Opin Ther Targets. 2012;16:635–650. doi: 10.1517/14728222.2012.687374. [DOI] [PubMed] [Google Scholar]
- 54.Seiter S, Arch R, Reber S, et al. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charrad RS, Li Y, Delpech B, et al. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat Med. 1999;5:669–676. doi: 10.1038/9518. [DOI] [PubMed] [Google Scholar]
- 56.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 57.Guo Y, Ma J, Wang J, et al. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–1565. [PubMed] [Google Scholar]
- 58.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 59.Dick J. 2007237761. University Health Network; France: Patent US. 2007
- 60.Young D. 2007098571. Arius Research Inc; Patent WO. 2007
- 61.Kryczek I, Liu S, Roh M, et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer. 2012;130:29–39. doi: 10.1002/ijc.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S, Song X, Chen Z, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e56380. doi: 10.1371/journal.pone.0056380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swaminathan SK, Olin MR, Forster CL, et al. Identification of a novel monoclonal antibody recognizing CD133. J Immunol Methods. 2010;361:110–115. doi: 10.1016/j.jim.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 65.Huang J, Li C, Wang Y, et al. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clin Immunol. 2013;149:156–168. doi: 10.1016/j.clim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Duffy MJ, Lamerz R, Haglund C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134:2513–2522. doi: 10.1002/ijc.28384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korkaya H, Wicha MS. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013;73:3489–3493. doi: 10.1158/0008-5472.CAN-13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korkaya H, Paulson A, Iovino F, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ithimakin S, Day KC, Malik F, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013;73:1635–1646. doi: 10.1158/0008-5472.CAN-12-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korkaya H, Kim GI, Davis A, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miles DW. Recent advances in systemic therapy. When HER2 is not the target: advances in the treatment of HER2-negative metastatic breast cancer. Breast Cancer Res. 2009;11:208. doi: 10.1186/bcr2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sen M, Wankowski DM, Garlie NK, et al. Use of anti-CD3 x anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu+ tumors. J Hematother Stem Cell Res. 2001;10:247–260. doi: 10.1089/15258160151134944. [DOI] [PubMed] [Google Scholar]
- 74.Davol PA, Smith JA, Kouttab N, et al. Anti-CD3 x anti-HER2 bispecific antibody effectively redirects armed T cells to inhibit tumor development and growth in hormone-refractory prostate cancer-bearing severe combined immunodeficient beige mice. Clin Prostate Cancer. 2004;3:112–121. doi: 10.3816/cgc.2004.n.021. [DOI] [PubMed] [Google Scholar]
- 75.Lum HE, Miller M, Davol PA, et al. Preclinical studies comparing different bispecific antibodies for redirecting T cell cytotoxicity to extracellular antigens on prostate carcinomas. Anticancer Res. 2005;25:43–52. [PubMed] [Google Scholar]
- 76.Borovski T, De Sousa EMF, Vermeulen L, et al. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 77.Lu H, Clauser KR, Tam WL, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye XZ, Yu SC, Bian XW. Contribution of myeloid-derived suppressor cells to tumor-induced immune suppression, angiogenesis, invasion and metastasis. J Genet Genomics. 2010;37:423–430. doi: 10.1016/S1673-8527(09)60061-8. [DOI] [PubMed] [Google Scholar]
- 79.Panni RZ, Sanford DE, Belt BA, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63:513–528. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Yi T, Kortylewski M, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui TX, Kryczek I, Zhao L, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang DR, Ding XF, Luo J, et al. Increased chemosensitivity via targeting testicular nuclear receptor 4 (TR4)-Oct4-interleukin 1 receptor antagonist (IL1Ra) axis in prostate cancer CD133+ stem/progenitor cells to battle prostate cancer. J Biol Chem. 2013;288:16476–16483. doi: 10.1074/jbc.M112.448142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SY, Kang JW, Song X, et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25:961–969. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917–4924. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer. 2014;111:2214–2219. doi: 10.1038/bjc.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004;363:1411–1412. doi: 10.1016/S0140-6736(04)16134-5. [DOI] [PubMed] [Google Scholar]
- 95.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 96.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 97.Maccalli C, Volonte A, Cimminiello C, et al. Immunology of cancer stem cells in solid tumours. A review. Eur J Cancer. 2014;50:649–655. doi: 10.1016/j.ejca.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 98.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schatton T, Schutte U, Frank NY, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma P, Wagner K, Wolchok JD, et al. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N Engl J Med. 2014 Dec 6; doi: 10.1056/NEJMoa1411087. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee Y, Sunwoo J. PD-L1 is preferentially expressed on CD44+ tumor-initiating cells in head and neck squamous cell carcinoma. J Immunother Cancer. 2014;2(Suppl 3):270. [Google Scholar]