Summary
Bacteria of the genus Brucella are intracellular vacuolar pathogens of mammals that cause the worldwide zoonosis brucellosis, and reside within phagocytes of infected hosts to promote their survival, persistence and proliferation. These traits are essential to the bacterium’s ability to cause disease and have been the subject of much investigation to gain an understanding of Brucella pathogenic mechanisms. Although the endoplasmic reticulum-derived nature of the Brucella replicative niche has been long known, major strides have recently been made in deciphering the molecular mechanisms of its biogenesis, including the identification of bacterial determinants and host cellular pathways involved in this process. Here I will review and discuss the most recent advances in our knowledge of Brucella intracellular pathogenesis, with an emphasis on bacterial exploitation of the host endoplasmic reticulum-associated functions, and how autophagy-related processes contribute to the bacterium’s intracellular cycle.
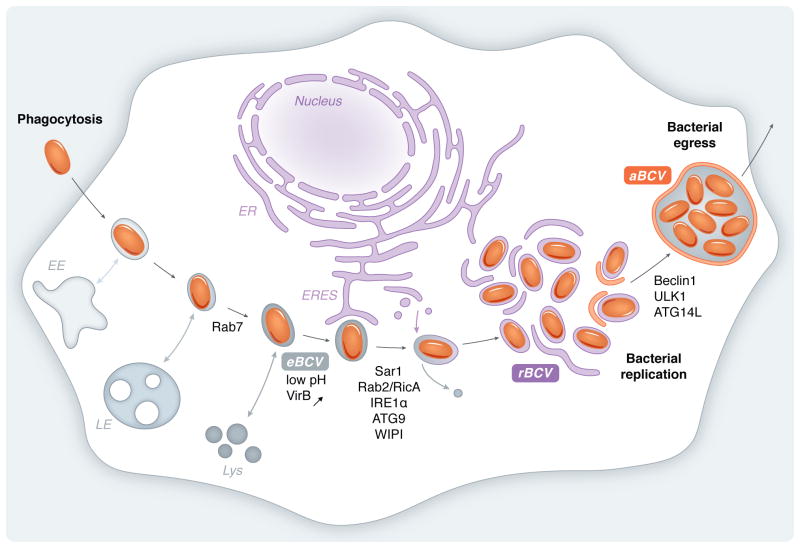
Bacteria of the Brucella genus are the causative agent of brucellosis, a zoonotic disease of worldwide distribution that affects both animals and humans and inflicts economical and public health burden in endemic areas (Pappas et al., 2005). While brucellosis causes abortion and sterility in animals, the human disease is characterized by recurrent fever and debilitating musculoskeletal, cardiac and neurological complications at the chronic stage of the infection. Brucella primarily infects professional phagocytes such as macrophages or dendritic cells (Archambaud et al., 2010) at the onset of infection. These act both as a survival/replication niche and as vectors for systemic dissemination to other organs. Bacteria subsequently infect cells of myeloid lineage, including macrophages in the spleen and liver, and persist within granulomatous lesions, or infect and proliferate within placental trophoblasts in pregnant animals (Atluri et al., 2011). These pathological aspects of brucellosis emphasize the importance of the bacterium’s intracellular cycle to the disease’s development and progression, and rationalize the need to understand the underlying mechanisms of its intracellular cycle. As bacteria that have undergone a long evolution with mammalian hosts, Brucella spp. have selected a sophisticated intracellular cycle that ensures their survival, immune evasion, proliferation and persistence within the host, portraying an exquisite model of pathogen subversion of host cell organelles and functions. Extensive studies of the Brucella intracellular cycle have revealed that this pathogen controls the conversion of its intracellular compartment, the Brucella-containing vacuole (BCV), from an endosomal (eBCV) to a replicative (rBCV) vacuole derived from the endoplasmic reticulum (ER) (Pizarro-Cerdá, Moreno, et al., 1998; Pizarro-Cerdá, Méresse, et al., 1998; Comerci et al., 2001; Delrue et al., 2001; Celli et al., 2003; Salcedo et al., 2008; Starr et al., 2008; Salcedo, Chevrier, et al., 2013), then the subsequent biogenesis of an autophagy-related compartment (aBCV) (Starr et al., 2012) (Fig. 1). Here I will review our current knowledge of how Brucella modulates these sequential changes in its vacuole, with an emphasis on subversion of the host endoplasmic reticulum (ER) and its associated functions.
Figure 1. Model of Brucella intracellular trafficking in mammalian cells.
Upon phagocytosis, Brucella resides within the Brucella-containing vacuole (BCV), which undergoes interactions with early endosomes (EE), late endosomes (LE) and partially fuse with lysosomes (Lys) to become the eBCV. The eBCV provides cues for induction of the VirB Type IV secretion system (T4SS), which deliver effector proteins that control eBCV interactions with ER exit sites (ERES). Activation of the UPR sensor IRE1α triggers formation of autophagic vesicles in a ATG9 and WIPI-dependent manner, that are thought to fuse with eBCV to promote rBCV biogenesis via accretion of ER-derived membranes and exclusion of endosomal membranes. The small GTPases Sar1 and Rab2 (via its interaction with the Brucella effector RicA) are required for rBCV biogenesis and subsequent bacterial replication in rBCVs. Following replication in the ER, rBCVs are converted into autophagic aBCVs via a process involving the autophagy initiation proteins Beclin1, ULK1 and ATG14L. aBCVs promote completion of the Brucella intracellular cycle by facilitating bacterial egress.
The endosomal BCV: playing with fire
A wealth of knowledge of the Brucella intracellular cycle has been garnered using of a variety of murine and human macrophage or monocyte in vitro models. Additional host cells such as the non-phagocytic HeLa cell line have also been used (Pizarro-Cerdá, Moreno, et al., 1998; Pizarro-Cerdá, Méresse, et al., 1998; Comerci et al., 2001; Delrue et al., 2001; Archambaud et al., 2010) and shown to faithfully recapitulate Brucella intracellular cycle in macrophages (Celli et al., 2003; Celli et al., 2005; Starr et al., 2008; Atluri et al., 2011; Starr et al., 2012). This epithelial cell line is more amenable to manipulation and microscopy, providing a useful tool for cell biological studies of host factors required for Brucella intracellular pathogenesis.
Following phagocytic uptake by macrophages or entry into non-phagocytic cells, Brucella resides within a membrane-bound compartment, the BCV. Acquisition of endocytic markers, such as Rab5, its effector EEA1 and the transferrin receptor TfR (Pizarro-Cerdá, Moreno, et al., 1998; Pizarro-Cerdá, Méresse, et al., 1998; Comerci et al., 2001; Delrue et al., 2001; Celli et al., 2003; Bellaire et al., 2005; Salcedo et al., 2008; Starr et al., 2008; Salcedo, Chevrier, et al., 2013), during early maturation indicates its interaction with early endosomes. These interactions are transient and followed by acquisition of the late endocytic markers LAMP1, CD63 and Rab7 (Pizarro-Cerdá, Méresse, et al., 1998; Celli et al., 2003; Bellaire et al., 2005; Starr et al., 2008) and BCV acidification to pH ~4–4.5 (Porte et al., 1999; Boschiroli, 2002; Starr et al., 2008), suggesting a normal maturation process along the default degradative endocytic pathway. Early studies of BCV trafficking in HeLa cells and primary murine macrophages however failed to detect acquisition of the lysosomal hydrolase Cathepsin D (Pizarro-Cerdá, Méresse, et al., 1998; Celli et al., 2003), which led to the conclusion that Brucella avoids fusion with bactericidal lysosomes as a means of intracellular survival. Yet this model is not consistent with the enrichment of late endosomal/lysosomal markers on the maturing eBCV, or with its rapid acidification, which are considered correlates of fusion with lysosomes. Using live cell imaging techniques, Starr et al. showed significant delivery of a fluid phase marker chased to terminal lysosomes prior to infection, and directly visualized eBCV-lysosome fusion events, indicating that eBCVs indeed undergo fusion with lysosomes, albeit not to the extent of a phagosome containing an inert particle (Starr et al., 2008). This apparent discrepancy in the extent of eBCV fusion with lysosomes likely stems from drastically reduced detection of soluble antigens in fixed and permeabilized samples, due to their leakage, whereas live cell imaging provides optimal sensitivity of detection of fluid phase markers (Drecktrah et al., 2007; Starr et al., 2008).
BCV maturation along the endocytic pathway appears deleterious to bacteria, as some are killed during the eBCV stage (Celli et al., 2003). Nonetheless, inhibition of fusion with late endosomes/lysosomes via overexpression of a dominant negative allele of the small GTPase Rab7 (Rab7T22N) impairs Brucella’s ability to generate its replicative niche (rBCV) and proliferate intracellularly (Starr et al., 2008), indicating that the endosomal stage does not simply reflect a default bactericidal pathway, but serves a specific purpose. Indeed, eBCV acidification constitutes a signal for intracellular induction of Brucella’s major virulence determinant, the VirB Type IV secretion system (T4SS) (Sieira et al., 2000; Boschiroli, 2002; O’Callaghan et al., 2002), an apparatus essential for rBCV biogenesis and replication (Lestrate et al., 2000; Comerci et al., 2001; Delrue et al., 2001; Celli et al., 2003) that delivers effector proteins into the host cell. Hence, the eBCV stage seems to constitute a rite of passage for Brucella towards generating the replication-permissive rBCV.
While the eBCV stage represents a transitional step towards rBCV biogenesis, it is also accompanied by cell cycle transitions in the bacterium. Deghelt et al. have elegantly shown that the infectious form of Brucella is arrested in the G1 phase for up to 6 h post infection, but the bacterium resumes its cell cycle and chromosomal replication while still within the eBCV(Deghelt et al., 2014). Bacterial division and replication eventually occurs in rBCVs (Deghelt et al., 2014), indicating that bacteria become primed for proliferation within eBCV and further highlighting their importance in the Brucella intracellular cycle.
Brucella and the ER: biogenesis of the replicative BCV
Brucella residence and replication within the endoplasmic reticulum (ER) of host cells was initially described in seminal ultrastructural studies of infected goat and bovine placentas, indicating bacterial proliferation within ER cisternae of trophoblasts (Anderson et al., 1986; Meador and Deyoe, 1989), together with in vitro studies in Vero cells (Detilleux et al., 1990). These studies also suggested that bacteria were transferred from phagosomes to the ER to undergo replication. The transition of Brucella from an endosomal vacuole to the ER was confirmed by immunolocalization of markers for specific intracellular compartments (Pizarro-Cerdá, Méresse, et al., 1998; Celli et al., 2003). Additional evidence that rBCVs originate from eBCVs, and do not constitute independent populations of bacterial vacuoles with different intracellular fates, was the demonstration that rBCV biogenesis requires host functions associated with endosomal maturation, specifically vacuolar acidification (Porte et al., 1999; Starr et al., 2008) and the late endosomal small GTPase Rab7 (Starr et al., 2008). It is now commonly accepted that Brucella controls the traffic of its intracellular vacuole from the endocytic to the secretory compartment to generate an ER-derived, replication permissive vacuole (rBCV).
An increasing number of bacterial pathogens undergo critical interactions with organelles of the host cell secretory compartment, such as the ER and the Golgi apparatus (recently reviewed in (Celli and Tsolis, 2014)), but only a handful including Brucella spp. and Legionella pneumophila unambiguously establish residence within a vacuole with functional characteristics of the ER (Pizarro-Cerdá, Méresse, et al., 1998; Celli et al., 2003; Robinson and Roy, 2006). While the molecular mechanisms of biogenesis of the ER-derived replicative vacuole of L. pneumophila are well understood (as recently reviewed in (Hubber and Roy, 2010)), details on the mechanisms of rBCV biogenesis are only starting to emerge. Unlike L. pneumophila, which rapidly redirects its original phagosome to the secretory pathway by recruiting and triggering fusion of secretory vesicles via modulation of the host GTPases Rab1 and ARF1 (Nagai, 2002; Dorer et al., 2006; Machner and Isberg, 2006; Murata et al., 2006; Ingmundson et al., 2007), rBCV biogenesis does not require ARF1-dependent vesicular trafficking between the ER and the Golgi apparatus (Celli et al., 2005). Instead, eBCVs localize to ER exit sites (ERES) during their maturation and functional disruption of ERES, via inactivation of the small GTPase Sar1, inhibits rBCV biogenesis indicating that Brucella intercepts the early secretory pathway at the ER interface to promote eBCV to rBCV conversion (Celli et al., 2005). Via a proteomic approach, Fugier et al. also identified Rab2 and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as essential for rBCV biogenesis and bacterial replication (Fugier et al., 2009). Since both proteins also participate in vesicular trafficking between the ER and the Golgi apparatus, these findings lend additional credence to the concept of Brucella subversion of specific components of the early secretory pathway to gain access to the ER.
The VirB type IV secretion system (T4SS) is essential for biogenesis of the replicative vacuole and intracellular replication (Lestrate et al., 2000; Comerci et al., 2001; Delrue et al., 2001; Celli et al., 2003). Loss-of-function studies have clearly established that inactivation of the VirB T4SS via deletion or insertional mutagenesis of virB genes sequesters the corresponding bacterial mutants in eBCVs, unable to sustain interactions with ERES and convert their endosomal vacuole into an rBCV (Comerci et al., 2001; Delrue et al., 2001; Celli et al., 2003; Celli et al., 2005). Given that the VirB T4SS is thought to deliver effector proteins across the BCV membrane into the host cell (De Jong et al., 2008; de Barsy et al., 2011; Ines Marchesini et al., 2011; Myeni et al., 2013; Salcedo, Marchesini, et al., 2013), a reasonable hypothesis is that Brucella modulates host functions involved in the early secretory pathway via the action of these effectors to generate the rBCV. In support of this, the Brucella secreted protein RicA binds Rab2 (de Barsy et al., 2011). Deletion of RicA in B. abortus reduces recruitment of GTP-locked Rab2Q65L on BCVs and potentiates the bacterium’s trafficking to the ER and its replication, suggesting that RicA may have a downmodulating function in BCV trafficking. Although RicA preferentially binds GDP-bound Rab2, it does not exhibit any guanine nucleotide exchange factor (GEF) activity (de Barsy et al., 2011), so whether it affects Rab2 activity remains unclear. Additional evidence that Brucella T4SS effectors modulate secretory trafficking to promote rBCV biogenesis comes from the identification of a series of effectors (BspA, BspB, BspC, BspD, BspF, BspK) that either target compartments of the early secretory pathway or impair secretory trafficking when ectopically expressed in HeLa cells (Myeni et al., 2013). Furthermore, Brucella infection impairs secretory trafficking in a BspA-, BspB- and BspF-dependent manner (Myeni et al., 2013). Inhibition of secretory trafficking in Brucella-infected cells is evidenced by retention of a secretory marker (VSV-G-GFP) within the Golgi apparatus and its decreased delivery to the plasma membrane (Myeni et al., 2013). Since inhibition of host secretory trafficking occurs prior to bacterial replication in the ER, it appears to be temporally consistent with eBCV to rBCV conversion events. Deletion of bspB reduces bacterial replication in primary murine macrophages, an effect that is potentiated by additional deletions of bspA and bspF (Myeni et al., 2013), yet whether these mutants display altered BCV trafficking remains to be established. Nonetheless, these findings may reflect bacterial effector-mediated alterations of early secretory trafficking associated with rBCV biogenesis. Yet, it cannot be ruled out that these may also reflect changes in secretory trafficking that promote bacterial replication within rBCVs. Similarly, it could represent changes aimed at altering surface expression of immune molecules, consistent with the retention of MHC Class I molecules in the Golgi apparatus of Brucella-infected monocytes (Barrionuevo et al., 2012), or secretion of pro-inflammatory cytokines. Altogether, further identification and characterization of VirB effector targets and mode of action will likely elucidate the exact contribution of early secretory trafficking in rBCV biogenesis, and also shed light onto the reasons and consequences of Brucella impairment of secretory trafficking.
While Brucella replication is commonly associated with biogenesis of its ER-derived vacuole, alternate replication niches have been documented. Immunoglobulin G (IgG)-opsonized B. abortus replicates in the human monocytic cell line THP1 within LAMP1-positive, non-acidic endosomal vacuoles that do not fuse with lysosomes (Bellaire et al., 2005). Although this suggests that Brucella intracellular trafficking may be altered by opsonization and cause bacterial replication in a modified eBCV, these endosomal vacuoles may alternatively correspond to autophagic aBCVs (see below; (Starr et al., 2012)), given the late time point analyzed, and their further characterization is warranted. Nonetheless, a recent investigation of Brucella spp. infection of human trophoblasts revealed that strains of B. abortus and B. suis replicate in large endosomal inclusions in extra-villous trophoblasts (EVTs) or in the EVT-like cell line JEG-3, although they seem to reach, and replicate within, the ER in trophoblast cell lines of other lineages (BeWo, JAR, HTR8 cells) and in syncytiotrophoblasts (Salcedo, Chevrier, et al., 2013). This suggests that EVTs have the capacity to interfere with normal BCV trafficking and restrict bacteria to an endosomal vacuole, possibly the eBCV, where they nonetheless exhibit replication that does not depend upon the VirB T4SS (Salcedo, Chevrier, et al., 2013). Interestingly, B. melitensis strains reach, and replicate within, the ER in EVTs (Salcedo, Chevrier, et al., 2013), indicating that this species is more resistant to EVT trafficking restriction. EVTs therefore constitute an interesting model to dissect Brucella mechanisms of replication within an endosomal environment, and to tease out differences in the ability of Brucella species to achieve an optimal intracellular cycle.
Autophagy, the unfolded protein response and the Brucella intracellular cycle
Although significant strides have been made in identifying host factors that specifically contribute to rBCV biogenesis, how Brucella controls conversion of its endosomal eBCV into the ER-derived rBCV has largely remained elusive, in part because this process does not intuitively abide by known host cell trafficking pathways. Early studies of BCV intracellular trafficking proposed the involvement of the autophagy pathway in rBCV biogenesis (Pizarro-Cerdá, Moreno, et al., 1998; Pizarro-Cerdá, Méresse, et al., 1998). Autophagy invokes the capture of cytosolic components, damaged organelles, protein aggregates and intracellular microbes (whether cytosolic or vacuolar) into double membrane vacuoles called autophagosomes. These then mature into degradative autolysosomes via interactions with late endocytic compartments and fusion with lysosomes, which allows degradation and recycling of autolysosome content. While originally identified as a process of nutrient recycling under starvation conditions, autophagy fulfills many homeostatic functions in eukaryotic cells and also contributes to innate immunity via its antibacterial function (Levine et al., 2011). Based on multimembrane BCV structures, increased bacterial replication upon amino-acid starvation, and accumulation of the lysomotropic probe monodansylcadaverine (MDC) in BCVs, it was proposed that these vacuoles traffic via the autophagy pathway in HeLa cells (Pizarro-Cerdá, Moreno, et al., 1998; Pizarro-Cerdá, Méresse, et al., 1998), but this assumption requires validation using more specific tools and assays for autophagy that have since become available (Klionsky et al., 2012). In an elegant genetic screen in Drosophila S2 cells for ER-associated proteins required for Brucella replication, Qin et al., identified the unfolded protein response (UPR) transmembrane sensor IRE1α as necessary for bacterial replication (Qin et al., 2008). The UPR is an ER stress response activated under physiological conditions that overwhelm the ER protein folding capacity, leading to the accumulation of unfolded proteins. Altered gene expression, mRNA turnover, translation and protein folding capacity are initiated by activation of three UPR sensors IRE1α, ATF6 and PERK, which together resolve ER stress and restore cellular homeostasis (Walter and Ron, 2011). One of these responses is IRE1α-dependent induction of autophagy, which contributes to controlling ER expansion during the UPR (Yorimitsu et al., 2006; Bernales et al., 2006) and is independent of ATF6 or PERK (Ogata et al., 2006). Since Brucella replication was not affected by ATF6 and PERK depletion, Qin et al proposed that IRE1α affects bacterial replication via activation of autophagy to promote eBCV to rBCV conversion, but this was not further examined (Qin et al., 2008). In contrast to this model, depletion or deletion of various host proteins involved in the canonical cascade of autophagosome biogenesis, namely Beclin1, ULK1, LC3B, ATG5, ATG7, ATG16L1 and ATG4B, does not impair rBCV biogenesis in either HeLa cells or primary macrophages, arguing against a role of canonical autophagy in this step of the Brucella intracellular cycle (Starr et al., 2012).
Three recent studies have however shed new light on the potential roles of autophagy and the UPR in rBCV biogenesis and Brucella replication (de Jong et al., 2013; Smith et al., 2013; Taguchi et al., 2015). First, they all showed that Brucella infection of macrophages or HeLa cells induces activation of only IRE1α in the case of B. abortus (de Jong et al., 2013; Taguchi et al., 2015), and IRE1α, ATF6 and PERK in the case of B. melitensis (Smith et al., 2013). While the discrepancy in the UPR pathways activated by different Brucella species needs to be reconciled, these findings further implicate IRE1α in Brucella replication. These studies did not determine whether UPR activation precedes rBCV biogenesis, or results from bacterial replication in the ER. Yet, Taguchi et al. detected IRE1α activation as early as 4 h post infection, which argues for UPR induction at the eBCV stage(Taguchi et al., 2015). Indeed, phosphorylation of IRE1α upon B. abortus infection occurs via Yip1A, a host protein that binds to the COPII coat components, Sec23 and Sec24, and localizes to ERES. Both IRE1α and Yip1A are required for rBCV biogenesis and Brucella replication(Taguchi et al., 2015). Interestingly, Yip1A-dependent activation of IRE1α was associated with an upregulation of COPII components and the GTPase Sar1 (Taguchi et al., 2015), suggesting an enhancement of vesicle budding at ERES, consistent with the requirement for Sar1 in rBCV biogenesis (Celli et al., 2005). Moreover, Yip1A-dependent activation of IRE1α also triggered formation of large vacuoles, whose occurrence also depended upon the autophagy-associated proteins ATG9 and WIPI (Taguchi et al., 2015). Depletion of either ATG9 or WIPI, but not the autophagy-associated protein DFCP1, inhibited rBCV biogenesis and restrained B. abortus in eBCVs(Taguchi et al., 2015). The emerging picture from these findings is that IRE1α activation upon Brucella infection occurs at ERES and leads to the formation of vacuoles of autophagic origin that may promote eBCV to rBCV conversion, a scenario consistent with ERES being a site of autophagosome biogenesis (Carlos Martín Zoppino et al., 2010; Graef et al., 2013; Wang et al., 2014). ATG9 and WIPI requirement for this process provides a strong argument for a role of autophagy in rBCV biogenesis, but it will be important to further define if other autophagy-associated proteins are required, since many key complexes involved in initiation and elongation of canonical autophagosomes have been ruled out (Starr et al., 2012). It is therefore possible that Brucella subverts a subset of autophagy-associated proteins, including ATG9 and WIPI, to promote accretion of ER-derived membranes on the eBCV and its conversion into an rBCV, in a process that may functionally differ from canonical autophagy.
Whether Brucella infection triggers a complete or partial UPR remains to be established. Studies using B. abortus have only observed activation of IRE1α and linked it to host cell sensing of VirB T4SS-mediated delivery of the effector, VceC (de Jong et al., 2013). By contrast, Smith et al. have observed activation of all three arms of the UPR in B. melitensis-infected macrophages, via delivery of the Brucella TIR-domain containing protein TcpB (Smith et al., 2013), and also proposed that UPR activation benefits bacterial replication, since counteracting it with the chemical chaperone tauroursodeoxycholic acid (TUDCA), which facilitates protein folding, affects B. melitensis intracellular growth (Smith et al., 2013). While this requires confirmation by more targeted means of inhibiting the UPR, it also remains to be determined whether this requirement of the UPR for bacterial replication only reflects activation of IRE1α, possibly as a sole means to promote rBCV biogenesis, or additional signaling that may benefit bacterial growth within the rBCV. It is worth noting that additional effectors (BspC, BspG, BspH, BspK) also trigger ER stress upon ectopic expression in HeLa cells (Myeni et al., 2013), suggesting that the mechanisms of UPR induction by Brucella are more complex than currently envisioned.
The recent findings that autophagy-associated proteins are required for rBCV biogenesis have highlighted the possibility that Brucella subverts specific components of host cell membrane trafficking pathways to undergo its intracellular cycle. In agreement, Starr et al. observed that, by 48h post infection, following extensive replication in the ER bacteria become enclosed in multi-membrane structures consistent with autophagosomes (Starr et al., 2012). Named aBCVs for autophagic BCVs, these vacuoles form both in macrophages and epithelial cells, contain one to many bacteria, and functionally differ from rBCVs as they do not display ER markers, but instead acquire late endosomal features consistent with a maturing autophagosome (Starr et al., 2012). rBCV to aBCV conversion requires a subset of autophagy-associated proteins, since depletion of the autophagy initiation proteins Beclin1, ULK1 and ATG14L, but not of the autophagy elongation proteins ATG5, ATG7, LC3B, ATG16L1 or ATG4B, abolishes aBCV formation (Starr et al., 2012). These findings further exemplify the ability of Brucella to exploit specific membrane trafficking complexes involved in autophagy to promote its intracellular cycle. Importantly, aBCVs correlate with bacterial release and cell-to-cell spread, since blocking aBCV biogenesis reduced formation of infection foci in HeLa cells (Starr et al., 2012), arguing that aBCVs contribute to completion of the Brucella cycle following its proliferation in the ER. How aBCVs form without requiring canonical autophagy elongation complexes remains to be established but is not inconsistent with reports of non-canonical, ATG5- and LC3-independent autophagy processes (Collins et al., 2009; Nishida et al., 2009). Altogether, these recent studies highlight complex interactions of BCVs with autophagy-associated membrane trafficking processes. Future studies need to address the molecular details of these critical trafficking steps, including the identification of bacterial factors that modulate the functional evolution of BCVs.
Concluding remarks
Major advances in our understanding of the Brucella intracellular cycle have recently been made regarding the evolutive nature of the BCV and host factors that are required for its functional transition from an endosomal to an ER-derived organelle to an autophagic vacuole. These findings highlight the complexity of the bacterium’s cycle within mammalian cells and how studying this bacterium constitutes, beyond the need to understand its pathogenesis, a rich model of bacterial subversion of cell biological processes. Future challenges in this field are to identify the bacterial effectors that modulate the host processes involved in BCV trafficking, and characterize their mode of action to comprehend Brucella molecular mechanisms of its pathogenesis.
Acknowledgments
I wish to thank Leigh Knodler for critical reading of this manuscript and apologize to the authors whose studies I could not cite due to space limitiations. This work was supported by the NIH/NIAID grant AI112649, the Stanley Alder Research Fund and the Paul G. Allen School for Global Animal Health.
Footnotes
The author declares no conflict of interest.
References
- Anderson TD, Cheville NF, Meador VP. Pathogenesis of Placentitis in the Goat Inoculated with Brucella abortus. II. Ultrastructural Studies. Veterinary Pathology. 1986;23:227–239. doi: 10.1177/030098588602300302. [DOI] [PubMed] [Google Scholar]
- Archambaud C, Salcedo SP, Lelouard H, Devilard E, de Bovis B, Van Rooijen N, et al. Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur J Immunol. 2010;40:3458–3471. doi: 10.1002/eji.201040497. [DOI] [PubMed] [Google Scholar]
- Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the Human Pathogenic BrucellaSpecies with Their Hosts. Annu Rev Microbiol. 2011;65:523–541. doi: 10.1146/annurev-micro-090110-102905. [DOI] [PubMed] [Google Scholar]
- Barrionuevo P, Delpino MV, Pozner RG, Velásquez LN, Cassataro J, Giambartolomei GH. Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8 +T cell responses. Cell Microbiol. 2012;15:487–502. doi: 10.1111/cmi.12058. [DOI] [PubMed] [Google Scholar]
- Bellaire BH, Roop RM, Cardelli JA. Opsonized Virulent Brucella abortus Replicates within Nonacidic, Endoplasmic Reticulum-Negative, LAMP-1-Positive Phagosomes in Human Monocytes. Infect Immun. 2005;73:3702–3713. doi: 10.1128/IAI.73.6.3702-3713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschiroli ML. The Brucella suis virB operon is induced intracellularly in macrophages. Proceedings of the National Academy of Sciences. 2002;99:1544–1549. doi: 10.1073/pnas.032514299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos Martín Zoppino F, Damián Militello R, Slavin I, Álvarez C, Colombo MI. Autophagosome Formation Depends on the Small GTPase Rab1 and Functional ER Exit Sites. Traffic. 2010;11:1246–1261. doi: 10.1111/j.1600-0854.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- Celli J, Tsolis RM. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes? Nat Rev Micro. 2014;13:71–82. doi: 10.1038/nrmicro3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, Salcedo SP, Gorvel JP. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc Natl Acad Sci USA. 2005;102:1673–1678. doi: 10.1073/pnas.0406873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, De Mazière A, van Dijk S, Carlsson F. Atg5-independent sequestration of ubiquitinated mycobacteria. PLoS Pathog. 2009;5:e1000430. doi: 10.1371/journal.ppat.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerci DJ, Lorenzo MM, Sieira R, Gorvel JP, Ugalde RA. Essential role of the VirB machinery in the maturation of the Brucella abortus containing vacuole. Cell Microbiol. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- de Barsy M, Jamet A, Filopon D, Nicolas C, Laloux G, Rual JF, et al. Identification of a Brucella spp. secreted effector specifically interacting with human small GTPase Rab2. Cell Microbiol. 2011;13:1044–1058. doi: 10.1111/j.1462-5822.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- de Jong MF, Starr T, Winter MG, den Hartigh AB, Child R, Knodler LA, et al. Sensing of bacterial type IV secretion via the unfolded protein response. MBio. 2013;4:e00418–12. doi: 10.1128/mBio.00418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong MF, Sun YH, Den Hartigh AB, van Dijl JM, Tsolis RM. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol Microbiol. 2008;70:1378–1396. doi: 10.1111/j.1365-2958.2008.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deghelt M, Mullier C, Sternon JF, Francis N, Laloux G, Dotreppe D, et al. G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus. Nat Commun. 2014;5:4366. doi: 10.1038/ncomms5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrue RM, Martinez-Lorenzo M, Lestrate P, Danese I, Bielarz V, Mertens P, et al. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 2001;3:487–497. doi: 10.1046/j.1462-5822.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- Detilleux PG, Deyoe BL, Cheville NF. Entry and Intracellular Localization of Brucella spp. in Vero Cells: Fluorescence and Electron Microscopy. Veterinary Pathology. 1990;27:317–328. doi: 10.1177/030098589002700503. [DOI] [PubMed] [Google Scholar]
- Dorer MS, Kirton D, Bader JS, Isberg RR. RNA Interference Analysis of Legionella in Drosophila Cells: Exploitation of Early Secretory Apparatus Dynamics. PLoS Pathog. 2006;2:e34. doi: 10.1371/journal.ppat.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Knodler LA, Howe D, Mortimer OS. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic. 2007;8:212–225. doi: 10.1111/j.1600-0854.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugier E, Salcedo SP, de Chastellier C, Pophillat M, Muller A, Arce-Gorvel V, et al. The Glyceraldehyde-3-Phosphate Dehydrogenase and the Small GTPase Rab 2 Are Crucial for Brucella Replication. PLoS Pathog. 2009;5:e1000487. doi: 10.1371/journal.ppat.1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef M, Friedman JR, Graham C, Babu M, Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubber A, Roy CR. Modulation of Host Cell Function by Legionella pneumophilaType IV Effectors. Annu Rev Cell Dev Biol. 2010;26:261–283. doi: 10.1146/annurev-cellbio-100109-104034. [DOI] [PubMed] [Google Scholar]
- Ines Marchesini M, Herrmann CK, Salcedo SP, Gorvel JP, Comerci DJ. In search of Brucella abortus type IV secretion substrates: screening and identification of four proteins translocated into host cells through VirB system. Cell Microbiol. 2011;13:1261–1274. doi: 10.1111/j.1462-5822.2011.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestrate P, Delrue RM, Danese I, Didembourg C, Taminiau B, Mertens P, et al. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol Microbiol. 2000;38:543–551. doi: 10.1046/j.1365-2958.2000.02150.x. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. Targeting of Host Rab GTPase Function by the Intravacuolar Pathogen Legionella pneumophila. Developmental Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Meador VP, Deyoe BL. Intracellular localization of Brucella abortus in bovine placenta. Veterinary Pathology. 1989;26:513–515. doi: 10.1177/030098588902600609. [DOI] [PubMed] [Google Scholar]
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nature Cell Biology. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- Myeni S, Child R, Ng TW, Kupko JJ, III, Wehrly TD, Porcella SF, et al. Brucella Modulates Secretory Trafficking via Multiple Type IV Secretion Effector Proteins. PLoS Pathog. 2013;9:e1003556. doi: 10.1371/journal.ppat.1003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H. A Bacterial Guanine Nucleotide Exchange Factor Activates ARF on Legionella Phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, et al. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 2002;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biology. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerdá J, Méresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goñi I, et al. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerdá J, Moreno E, Sanguedolce V, Mege JL, Gorvel JP. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte F, Liautard JP, Köhler S. Early Acidification of Phagosomes ContainingBrucella suis Is Essential for Intracellular Survival in Murine Macrophages. Infect Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin QM, Pei J, Ancona V, Shaw BD, Ficht TA, De Figueiredo P. RNAi Screen of Endoplasmic Reticulum–Associated Host Factors Reveals a Role for IRE1α in Supporting Brucella Replication. PLoS Pathog. 2008;4:e1000110. doi: 10.1371/journal.ppat.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CG, Roy CR. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell Microbiol. 2006;8:793–805. doi: 10.1111/j.1462-5822.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- Salcedo SP, Chevrier N, Lacerda TLS, Ben Amara A, Gerart S, Gorvel VA, et al. Pathogenic Brucellae Replicate in Human Trophoblasts. J Infect Dis. 2013;207:1075–1083. doi: 10.1093/infdis/jit007. [DOI] [PubMed] [Google Scholar]
- Salcedo SP, Marchesini MI, Degos C, Terwagne M, von Bargen K, Lepidi H, et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front Cell Infect Microbiol. 2013;3:28. doi: 10.3389/fcimb.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, et al. Brucella control of dendritic cell maturation is dependent on the TIR-Containing protein btp1. PLoS Pathog. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieira R, Comerci DJ, Sánchez DO, Ugalde RA. A Homologue of an Operon Required for DNA Transfer in Agrobacterium Is Required in Brucella abortus for Virulence and Intracellular Multiplication. Journal of Bacteriology. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Khan M, Magnani DD, Harms JS, Durward M, Radhakrishnan GK, et al. Brucella Induces an Unfolded Protein Response via TcpB That Supports Intracellular Replication in Macrophages. PLoS Pathog. 2013;9:e1003785. doi: 10.1371/journal.ppat.1003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T, Child R, Wehrly TD, Hansen B, Hwang S, López-Otín C, et al. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Taguchi Y, Imaoka K, Kataoka M, Uda A, Nakatsu D. Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Brucella Infection. PLoS Pathog. 2015;10:e1004747. doi: 10.1371/journal.ppat.1004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang J, Tan D, Cai Y, Reinisch KM, Walz T, Ferro-Novick S. A requirement for ER-derived COPII vesicles in phagophore initiation. Autophagy. 2014;10:708–709. doi: 10.4161/auto.28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic Reticulum Stress Triggers Autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]