Abstract
Aim
The purpose of this study was to examine the mediating influence of diabetes health characteristics (diabetes distress, depression symptoms and diabetes symptoms) on the relationship between glucose control and fatigue in adults with type 2 diabetes.
Background
In patients with type 2 diabetes, fatigue is common and can affect diabetes self-management behaviors. Although long thought to result from hyperglycemia, little evidence supports a relationship between fatigue and glucose control.
Design
A cross-sectional, descriptive study design was used.
Method
Data were combined from two studies conducted at a large urban university in the Midwestern United States, resulting in a total sample of 155 urban-dwelling adults with type 2 diabetes. Data were collected over the course of 6 days from 2013 - March 2014. Fatigue and related biological and psychological phenomena were measured to perform path analyses using structural equation modeling methods. The STATA software was used to analyze the data.
Findings
In patients with A1C less than or equal to 7%, fatigue was related to diabetes distress and diabetes symptoms, but not to A1C directly or indirectly. In the group with A1C greater than 7%, fatigue was indirectly related to A1C; this relationship was mediated through diabetes symptoms, depression and diabetes distress.
Conclusion
Our findings suggest that fatigue is indirectly related to glucose control, but only in patients who have elevated A1C levels. In those with adequate glucose control, fatigue is mainly influenced by the presence of diabetes symptoms and distress. In both groups, the number and severity of diabetes symptoms were the strongest predictors of fatigue, regardless of blood glucose control.
Keywords: diabetes, depression, observational research, symptom management, fatigue, nursing
INTRODUCTION
Type 2 diabetes (T2DM) is associated with several long-term complications and symptoms; many are debilitating and compromise the physical and mental health of those affected. The human toll of diabetes symptoms cannot be overestimated. In a recent cohort study of > 13,000 adults with T2DM, there was a relationship between prevalence of symptoms and survival times (Sudore et al. 2012). The most prevalent symptoms were acute and chronic pain, depression and fatigue (Sudore et al. 2012). This study examined the mediating influence of diabetes health characteristics and symptoms on the relationship between glucose control and fatigue in adults with type 2 diabetes.
Background
Fatigue is pervasive among patients with T2DM, but is not well understood. In patients with T2DM, fatigue has been associated with depression and diabetes distress (Fritschi et al. 2012), Body Mass Index (BMI; Hlatky et al. 2010, Fritschi et al. 2012), non-fatigue diabetes symptoms (Fritschi et al. 2012), sleep complaints (Chasens et al. 2014) and (most recently) inflammation (Lasselin et al. 2012). Fatigue is herein defined as a ‘subjective perception of a decreased capacity to perform physical and/or mental tasks due to one or a combination of physiological, psychological, or lifestyle phenomena, including altered glucose control, diabetes symptoms, diabetes emotional distress, depression, physical inactivity and BMI’ (Fritschi & Quinn. 2010).
Fatigue has long been thought to be related to uncontrolled blood glucose levels, but this relationship has not been supported in the literature. While glucose levels have not been directly linked to fatigue, chronic hyperglycemia is strongly related to the development and progression of long-term complications (Stratton et al. 2000, Holman et al. 2008, Nathan et al. 2014) and thus is likely indirectly related to the independent and aggregate symptoms of these long-term complications. Current American Diabetes Association (ADA) recommendations for blood glucose control include maintaining a haemoglobin A1C ≤ 7% (8.6 mmol/L) for most non-pregnant adults with diabetes (American Diabetes Association 2014). This correlates with an average glucose level of 154 mg/dl (8.6 mmol/L). Patients with well-controlled glucose (A1C ≤ 7%) will likely have fewer long-term complications and thus possibly lower diabetes-related symptoms, including fatigue. Prior studies using cross-sectional analyses examined subjects at any level of glucose control and did not explore the possibility of mediating variables. This might account for the lack of relationship between glucose control and fatigue (Fritschi et al. 2012, Lasselin et al. 2012, Zagarins et al. 2012, Goedendorp et al. 2014). For example, Fritschi et al. (2012) reported associations between fatigue, diabetes distress and depressive symptoms (Fritschi et al. 2012), while diabetes distress (Fisher et al. 2010, Hessler et al. 2014), depression (Lustman et al. 2000, Lustman et al. 2005, Bouwman et al. 2010, Gonzalez et al. 2011) and neuropathic symptoms (Van der Does et al. 1996) have been associated with hyperglycaemia, suggesting that other diabetes health outcomes may mediate the relationship between glucose and fatigue.
THE STUDY
Aim
The purpose of this study was to examine the mediating influence of diabetes health characteristics (diabetes distress, depression symptoms, diabetes symptoms) on the relationship between blood glucose control and fatigue in adults with T2DM.
Design
This was a secondary data analysis using a cross-sectional, descriptive design.
Participants
A total sample of 155 urban-dwelling adults was recruited from two studies; both of which used convenience sampling methods. Inclusion criteria for both studies included: age ≥ 45 years to include adults at midlife through later adulthood to explore any age-related associations; being diagnosed with T2DM for ≥ one year to avoid psychological and physical issues related to a new diagnosis of diabetes. Subjects were excluded if they had fibromyalgia, any kidney disease, were undergoing cancer therapy or had not been out of remission for at least one year.
By using the maximum likelihood estimation in the path analysis, the sample size met the required sample size-to-parameter ratio of 5:1 (Kline, 2011). Number of used parameters for the path analysis was 13 in the study. (i.e., 65 subjects were required for each group). Required sample size for structural equation modelling methods has not been consistently established (Sivo, Fan, Witta, & Willse, 2006).
Data collection
As part of a secondary analysis, data were combined from two studies that examined fatigue and related biological and psychological phenomena in adults with T2DM (Fritschi et al. 2012, Fritschi 2014, Fritschi et al. 2014) (Table 1). Briefly, the aim of the first observational study was to explore the relationship between fatigue and physiological, psychological and lifestyle phenomena in women with T2DM. The study included 76 women (aged 52.2 SD 6.6 years) who completed questionnaires for health and demographic information, diabetes symptoms (including fatigue) and diabetes-related distress. Data were collected during a single visit from the years from 2006-2007. (Fritschi et al. 2012). The aim of the second study was to examine the influence of selected baseline characteristics (health/physical status, general fatigue, body composition, depression, diabetes-related distress, diabetes symptoms (including fatigue) and demographic factors) on physical activity in adults (both male and female) with T2DM (Fritschi et al. 2014). The study included 79 adults (aged 58.8 SD 9.2 years). Real-time fatigue, glucose and physical activity data were collected over the course of 6 days between September 2011 - March 2014.
Table 1.
Sample characteristics for this study, Study 1 and Study 2 (N = 155)
| Total sample (N = 155) % or Mean (SD) |
Study 1 (n = 76) [2] % or Mean (SD) |
Study 2 (n = 79) [19] % or Mean (SD) |
|
|---|---|---|---|
| Female** | 71.6 | 100.0 | 44.3 |
| Age (years)** | 55.7 (8.7) | 52.2 (6.6) | 58.8 (9.2) |
| Ethnicity | 12.9 | 14.5 | 11.4 |
| % Hispanic | |||
| Race | 46.5 | 38.2 | 54.4 |
| % Black | |||
| A1C (%) | 7.4 (1.9) | 7.3 (1.8) | 7.5 (2.0) |
| A1C (mmol/mol) | 58 (21) | 57 (19) | 58 (21) |
| BMI (kg/m2) | 33.0 (7.0) | 33.6 (8.0) | 32.5 (6.0) |
| DM duration (years)** | 7.3 (6.7) | 6.0 (5.7) | 8.6 (7.3) |
| Depression (yes) | 24.5 | 21.1 | 27.8 |
| Diabetes distress* | 2.33 (1.04) | 2.51 (1.04) | 2.15 (1.01) |
|
| |||
| DSC: | |||
| Fatigue | 2.24 (1.00) | 2.30 (1.07) | 2.19 (0.93) |
| Diabetes symptoms | 1.67 (0.57) | 1.67 (0.58) | 1.67 (0.56) |
p < 0.05,
p < 0.01.
Both studies were conducted at a large urban university in the Midwestern United States. Fatigue and diabetes-related characteristics were measured using stable methods. Capillary blood samples were taken for measurement of A1C using two different point-of-care methods. In each study, subjects were recruited through Internet-based posting sites, flyer dissemination and word of mouth.
Ethical considerations
Institutional Review Board approval was obtained for all study methods and participants gave written, informed consent prior to beginning data collection.
Data analyses
Statistical analyses were performed using SPSS version 22.0 (IBM, Chicago, IL); STATA version 13.0 (StataCorp, College Station, TX) was used to run generalized structural equation modelling for path analyses with a binary dependent variable. We conducted descriptive analyses (chi-square tests and unpaired t tests) to compare differences in sample characteristics between the participants with A1C levels ≤ 7% (controlled glucose) and those whose A1C levels were > 7% (uncontrolled glucose). Pearson correlation coefficients were used to identify relationships among fatigue, A1C values, diabetes symptoms, diabetes distress and other sample characteristics (age, gender, diabetes duration, BMI, self-reported number of diabetes complications and self-reported depression). Z-tests (Preacher 2002) were used to confirm group differences in correlation coefficients.
Based on the results of bivariate correlations for each group, we extracted those diabetes health factors that would most likely exert mediating effects between A1C and fatigue. Pearson bivariate correlations were performed among interval level variables. Spearman’s rho bivariate correlations were conducted for a dichotomously measured variable (i.e. self-reported depression = ‘yes’ or ‘no’).
Path analyses using structural equation modelling methods were used to estimate significance tests of direct and indirect effects of A1C on fatigue mediated through depression, diabetes distress and diabetes symptoms. All analyses were controlled for gender. Model fit was assessed by examining Chi-square (χ2) statistic (p value > 0.05 is considered an acceptable fit), root mean square error of approximation (RMSEA; < 0.08 is acceptable fit), comparative fit index (CFI; values close to 1 indicates goof fit), standardized root mean squared residual (SRMR; < 0.08 is good fit) and coefficient of determination (CD, overall R2; values close to 1 indicate good fit) (Byrne 2009, Acock 2013).
Validity and reliability
Fatigue and diabetes symptoms were measured using the Diabetes Symptom Checklist-Revised (DSC-R) (Grootenhuis et al. 1994). The DSC-R includes 34 items that measure both the occurrence and perceived burden of physical and psychological symptoms related to T2DM and its complications. The questionnaire measures 8 symptom dimensions: hyperglycaemic, hypoglycaemic, cardiovascular, polyneuropathic sensory, polyneuropathic pain, psychological fatigue, psychological/cognitive and ophthalmologic. Participants indicated whether they experienced any of the symptoms during the past month and used a Likert-type scale to rate the extent to which they were bothered by the symptoms from 1 = ‘not at all’ - 5 = ‘extremely’. In a trial of ~4,000 patients with T2DM, internal consistency reliability of all DSC-R subscales ranged 0.69-0.87 (Arbuckle et al. 2009). For the purposes of this study, we used the psychological fatigue subscale as our measure of fatigue and separated it from the rest of the scale. In a prior study of fatigue in women with T2DM, internal consistency reliability of the combined DSC-R subscales excluding the psychological fatigue subscale was acceptable (Cronbach’s alpha = 0.93) (Fritschi et al. 2012). In the current study, Internal consistency reliability was again supported for the combined DSC-R subscales excluding the fatigue subscale (Cronbach’s alpha = 0.92) and the separate psychological fatigue subscale (Cronbach’s alpha = 0.88).
Diabetes distress was measured using the Diabetes Distress Scale (DDS) (Polonsky et al. 2005). The DDS contains 17 items that measure four domains of diabetes-related emotional distress: Emotional Burden, Physician-related Distress, Regimen-related Distress and Diabetes-related Intrapersonal Distress. Subjects use a Likert-type scale to rate the degree to which each item has been problematic during the past month. The answers range from 1 = ‘no problem’ - 6 = ‘serious problem-. In the current study internal consistency reliability for the DDS was acceptable (Cronbach’s alpha = 0.92).
All of the item standardized coefficient factor loadings to each component was acceptable (>.05) for both the DSC-R and the DDS scales in the current study.
Depression was measured dichotomously by self-report. Participants were asked if they had ever been diagnosed with depression or were currently being treated for depression.
Blood glucose control was measured using two point-of-care methods for A1C, both of which have demonstrated high levels of accuracy in comparison with laboratory methods using high-performance liquid chromatography (HPLC). In 81 female participants, fingerstick blood samples were analyzed for haemoglobin A1C using the DCA 2000® + Analyzer (Siemens Diagnostics, Tarrytown, NY). The DCA 2000® is a point-of-care analyzer that provides A1C results within 6 minutes. A sample of 1 μL of whole blood was collected by capillary tube and inserted into a reagent cartridge, which used immunoassay methods to determine haemoglobin A1C. Results obtained from the DCA 2000® have been found to be comparable to laboratory methods using HPLC (Tamborlane et al. 2005, St John et al. 2006).
A1C was measured in the remaining participants using the A1CNow+™ by Bayer Healthcare. The A1CNow+™ is a portable, point-of-care analyzer that provides A1C results within 5 minutes. A sample of 5 μL of whole blood is collected by capillary tube and inserted into a reagent cartridge, which uses immunoassay (monoclonal antibody) and chemistry methods to determine A1C. Results obtained from the A1CNow+™ have been found to be comparable to HPLC (Bode et al. 2007). The DCA 2000® and the A1CNow+™ have both been certified by the National Glycohaemoglobin Standardization Program, which requires rigorous assessment of accuracy and bias through testing by reference laboratories (Little et al. 2001, Little 2003).
RESULTS
A total of 155 adults with T2DM aged 55.7 (SD 8.7) years were included in this analysis. Most were White or Black. The average duration of diabetes was 7.3 (SD 6.7) years and almost 73% were female. The average A1C of the total sample was 7.4% (SD 1.9) and 87% of participants were overweight or obese (Table 1).
There were correlations among fatigue, A1C, depression, diabetes symptoms and diabetes distress in the total sample. In bivariate correlational analyses among the total sample, fatigue was correlated with non-fatigue diabetes symptoms (r = 0.680, p = 0.001) and diabetes distress (r = 0.359, p = 0.001). Like fatigue, A1C was also positively associated with diabetes symptoms (r = 0.379, p < 0.001) and diabetes distress (r = 0.305, p < 0.001). However, A1C was not associated with fatigue. Diabetes symptoms were also correlated with self-reported depression (r = 0.219, p = 0.006) and diabetes distress (r = 0.393, p <0.001), although there was no relationship between depression and distress (Table 2).
Table 2.
Correlations among fatigue, A1C, distress in the total sample depression, diabetes symptoms and diabetes
| Fatigue | A1C | Depression (yes) |
DM symptoms |
|
|---|---|---|---|---|
| A1C | 0.107 | |||
| Depression (yes) | 0.217** | −0.090 | ||
| DM Symptoms | 0.680** | 0.278** | 0.219** | |
| DM Distress | 0.379** | 0.305** | 0.113 | 0.393** |
p < .01
Findings by Glucose Control Group
The total sample was divided by A1C values: the controlled glucose group had A1C values ≤ 7%; the uncontrolled glucose group had A1C values > 7%. The A1C values of the uncontrolled glucose group were significantly higher at 8.9% vs. 6.2%, respectively (p < 0.0001) and this group reported that they felt higher levels of diabetes symptoms (1.78 vs. 1.57, respectively; p = 0.028) and diabetes distress (2.63 vs. 2.08, respectively; p = 0.001) than the controlled glucose group. No significant demographic differences were found between the two groups (Table 3).
Table 3.
Differences in characteristics between the controlled glucose and uncontrolled glucose groups (N = 155)
| Total sample (N = 155) % or Mean (SD) |
Controlled glucose (n = 85) % or Mean (SD) |
Uncontrolled glucose (n = 70) % or Mean (SD) |
Chi- square, t |
p | |
|---|---|---|---|---|---|
| Female | 71.6 | 67.1 | 77.1 | 1.456 | .166 |
| Age (years) | 55.7 (8.7) | 56.3 (9.0) | 55.0 (8.3) | −0.935 | .351 |
| Ethnicity | |||||
| Non-Hispanic | 87.1 | 91.8 | 81.4 | 2.787 | .056 |
| Hispanic | 12.9 | 8.2 | 18.6 | ||
| Race | |||||
| White | 38.1 | 45.9 | 28.6 | 10.412 | .083 |
| Black | 46.5 | 43.5 | 50.0 | ||
| Asian | 6.5 | 5.9 | 7.1 | ||
| Native American | 1.9 | 1.2 | 2.9 | ||
| Others | 7.1 | 3.6 | 11.4 | ||
| A1C (%) | 7.4 (1.9) | 6.2 (0.5) | 8.9 (1.8) | 12.544 | <.0001** |
| A1C (mmol/mol) | 58 (21) | 44 (6) | 74 (19) | 12.544 | <.0001** |
| eAG (mg/dl) | 166.20 (53.30) | 130.53 (15.49) | 209.51 (50.77) | 12.544 | <.0001** |
| BMI (kg/m2) | 33.0 (7.0) | 32.8 (6.5) | 33.3 (7.7) | 0.404 | .687 |
| Overweight or obese | 87.1 | 88.2 | 85.7 | 0.051 | .064 |
| DM duration (years) | 7.3 (6.7) | 6.6 (6.5) | 8.0 (6.7) | 1.375 | .171 |
| Depression (yes) | 24.5 | 30.6 | 17.1 | 3.059 | .053 |
| Diabetes distress | 2.33 (1.04) | 2.08 (0.94) | 2.63 (1.08) | 3.358 | .001** |
|
| |||||
|
Diabetes Symptom
Checklist: |
|||||
| Fatigue | 2.24 (1.00) | 2.20 (0.96) | 2.30 (1.05) | 0.720 | .565 |
| Diabetes symptoms | 1.67 (0.57) | 1.57 (0.54) | 1.78 (0.59) | 2.225 | .028* |
p<0.05,
p<0.01.
Similar bivariate correlation coefficients were computed by groups based on A1C. In both the uncontrolled and controlled glucose groups, fatigue was significantly correlated with diabetes symptoms (r = 0.732, p < 0.001; r = 0.635, p < 0.001, respectively), modestly with diabetes distress (r = 0.267, p = 0.026; r = 0.500, p < 0.001, respectively) and self-reported depression (r = 0.239, p = 0.046; r = 0.221, p = 0.43, respectively). Fatigue was not associated with A1C in either group (Table 4).
Table 4.
Correlations among fatigue, A1C, depression, diabetes symptoms and diabetes distress in the controlled (above black) vs uncontrolled glucose group (below black)
| Fatigue | A1C | Depression (yes) |
DM Symptoms |
DM Distress |
|
|---|---|---|---|---|---|
| Fatigue | 0.015 | 0.221* | 0.635** | 0.500** | |
| A1C | 0.156 | −0.048a | −0.058b | 0.142 | |
| Depression (yes) | 0.239* | 0.334a** | 0.188 | 0.212 | |
| DM Symptoms | 0.732** | 0.349b** | 0.383** | 0.305** | |
| DM Distress | 0.267* | 0.205 | 0.084 | 0.422** |
p < 0.05,
p < 0.01.
significant each group difference
significant each group difference
In the uncontrolled glucose group, A1C was positively associated with self-reported depression (r = 0.334, p = 0.005) and diabetes symptoms (r = 0.349, p = 0.003). In both groups, diabetes symptoms were correlated with diabetes distress (r = 0.422 and 0.305, respectively; p < 0.01). However, only in the uncontrolled glucose group were diabetes symptoms associated with self-reported depression (r = 0.383, p = 0.001; Table 4).
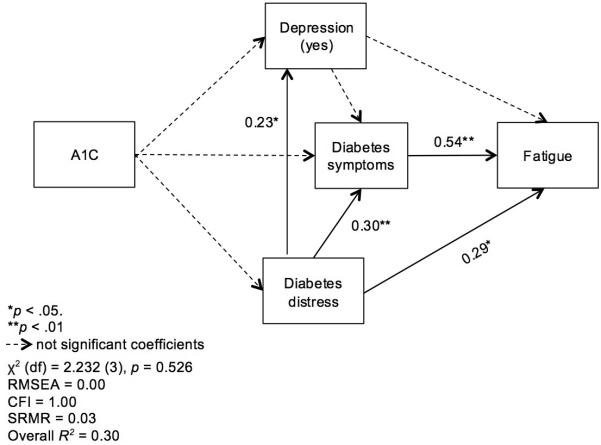
We estimated path analysis models by A1C group. In the model from the controlled glucose group (A1C ≤ 7%, n = 70), we found neither direct nor indirect effect of A1C on fatigue. Fatigue was directly influenced by both diabetes symptoms (β = 0.54, p < 0.001) and diabetes distress (β = 0.29, p = 0.002). Diabetes symptoms were also significantly influenced by diabetes distress (β = 0.30, p = 0.003). The indirect effect of diabetes distress on fatigue was validated (β = 0.19, p < 0.004; Figure 1).
Figure 1.
Path analysis of fatigue adjusted for gender in the controlled glucose group (A1C ≤ 7%, n = 85)
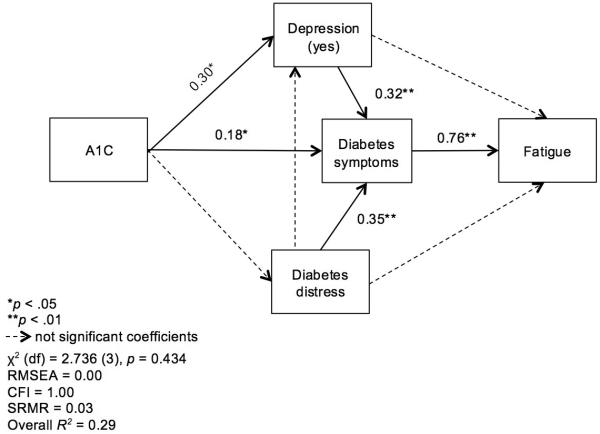
In the model of the uncontrolled glucose group (A1C > 7%, n = 85), again no direct effect of A1C on fatigue was found. Fatigue was directly influenced only by diabetes symptoms (β = 0.76, p < 0.001), which were significantly influenced by A1C (β = 0.18, p = 0.044), self-reported depression (β = 0.32, p = 0.007) and diabetes distress (β = 0.35, p = 0.001). Self-reported depression was positively influenced by A1C (β = 0.30, p = 0.013). The indirect effect of A1C on fatigue was confirmed (β = 0.12, p = 0.018). The indirect effect of diabetes distress on fatigue was confirmed (β = 0.12, p = 0.0), as was the indirect effect of depression on fatigue (β = 0.67, p = 0.002; Figure 2).
Figure 2.
Path analysis of fatigue adjusted for gender in the uncontrolled glucose group (A1C > 7%, n = 70)
DISCUSSION
Fatigue has long been thought to be related to aberrant glucose homeostasis in patients with diabetes. We found, however, that this relationship is not direct and differs by level of glucose control. Fatigue was indirectly related to A1C; and only in those subjects with poor glucose control. The relationship between A1C and fatigue was mediated through diabetes symptoms, depression and diabetes distress. In both groups, the number and severity of diabetes symptoms were the strongest predictors of fatigue, regardless of blood glucose control. Diabetes symptoms, however, were only associated with A1C in those subjects with poor glucose control. In prior studies about the relationship between poor glucose control and diabetes symptoms, Warren et al. (2003) reported that diabetes symptoms (including fatigue) were strongly related to acute blood glucose levels at or above 270 mg/dl (15.1 mmol/L), but only modestly associated with A1C (Warren et al. 2003). Later, in a population-based cohort trial of symptom differences between adults with normal glucose tolerance, impaired glucose tolerance and overt diabetes, diabetes symptoms (measured using the DSC-R) increased as glucose metabolism worsened (Adriaanse et al. 2008). This relationship was attenuated by depression. The average A1C in the diabetes group was 7.7% (SD 1.7) so we do not know how patients with higher A1C levels might be affected. Arbuckle et al. (2009) also reported a linear relationship between diabetes symptoms and A1C levels to 7.9% (Arbuckle et al. 2009). However, after A1C levels rose above 7.9%, the symptom burden started to decrease (Arbuckle et al. 2009), suggesting there might be a plateau effect in the relationship between diabetes symptoms and glucose control. Findings from both these studies run contrary to ours, where diabetes symptoms were only associated with A1C in the uncontrolled glucose group. While the relationship between A1C and diabetes symptoms remains unclear, we found that the number and severity of diabetes symptoms were the strongest predictors of fatigue in the controlled glucose group and the key mediating variable between A1C and fatigue in the uncontrolled glucose group.
Diabetes symptoms have previously been associated with diabetes distress (Brod et al. 2006) and were also associated with diabetes distress across all groups in our study. In bivariate analyses, diabetes distress was associated with fatigue in the total sample, but this relationship was stronger in the controlled glucose group (r = 0.50) than in the uncontrolled glucose group (r = 0.27). When we examined the relationship between diabetes distress and fatigue in the path models, diabetes distress was directly linked with fatigue in the controlled glucose group, but not the uncontrolled glucose group. Diabetes distress was indirectly linked to fatigue in both models and mediated by diabetes symptoms. It is unclear why distress and fatigue were directly related only in the path model of patients with controlled glucose levels.
Few papers have addressed the association between diabetes distress and fatigue. In cross-sectional analyses, Fritschi et al. (2012) reported a moderate relationship between diabetes distress and fatigue (r = 0.445, p < 0.01), but in further regression analyses of fatigue predictors, diabetes distress was no longer significant in a final model that included depressive symptoms, BMI and diabetes symptoms (Fritschi et al. 2012). In our current study, among the controlled glucose group, depression was not related to fatigue nor any other variable in the model. Distress, however, was related to both diabetes symptoms and fatigue, suggesting that diabetes distress is a dynamic construct that co-varies with other stressful factors, such as symptoms. There is mounting belief that much of the depression seen in diabetes is really diabetes emotional distress (Fisher et al. 2010, Zagarins et al. 2012).
Fatigue is one of the most prevalent symptoms of depression and often included among the items in measures of depression. A four-year cohort study of 151 healthy employees with the presence of severe fatigue at baseline showed that the influence of depression on fatigue was strong and the impact increased over time (Huibers et al. 2007). In our study, depression was only modestly associated with fatigue in the total sample and the uncontrolled glucose groups. In the path models, depression had an indirect link with fatigue but was mediated by diabetes symptoms and found only in the uncontrolled glucose group of the study. This relationship between depression and diabetes symptoms has been reported previously, (Lustman et al. 1988, Ciechanowski et al. 2003, Franks et al. 2010) supporting our findings.
Depression was directly related to A1C among those in the uncontrolled glucose group only. In prior studies, the evidence supporting a direct relationship between glucose (measured variably, e.g. A1C level, fasting) and depression is inconsistent. Some have reported a positive relationship between depression and glucose metabolism (Bouwman et al. 2010) and depression and glucose control, (Lustman et al. 2005) while others reported no relationship between depression and glucose control (Ciechanowski et al. 2003, Fisher et al. 2010, Zagarins et al. 2012). We collected self-reported data about depression and operationalized it dichotomously as either having or not having been diagnosed with depression. While the use of self-reported depression is subject to reliability issues, these findings do suggest that depression may have synergistic and negative associations with diabetes symptoms and thus indirectly cause higher complaints of fatigue in those who have poor blood glucose control.
The complex interplay among blood glucose control, diabetes symptoms, diabetes distress, depression and fatigue is not well understood. Other personal and biological factors likely influence these relationships and may better explain the high levels of fatigue seen in patients with T2DM. Many of these factors were proposed in a multidimensional fatigue model, which suggested that a variety of neuroendocrine biomarkers mediate the relationship between physiological and psychological factors and fatigue in diabetes (Payne 2004). In the model, it is hypothesized that fatigue occurs as a result of physiological (e.g., disease process, endocrine system) and psychological (e.g., depression, stressors) patient characteristics through physical biomarkers such as cytokines and cortisol (Payne 2004). In fact, recent evidence suggests that biomarkers associated with inflammation are the link between diabetes and fatigue (Lasselin et al. 2012).
Limitations
There are several limitations to our study. The data were gathered cross-sectionally, so we cannot infer causality. Further longitudinal studies may confirm dynamic effects of blood glucose control on fatigue. Measuring depression by a self-reported, dichotomous measure raises the potential for social desirability bias and reported bias that may influence the construct validity even though the dichotomous nature of measurement was considered in the data analyses. Additionally, using self-reported data may have led to missing or incomplete data.
Future observational research in fatigue and diabetes should include data about the number and types of diabetes complications drawn from clinic or hospital medical records. Additional study of the role of inflammation in fatigue is also warranted. There are likely many possible intervention targets and these are likely to vary from patient to patient. Only by understanding all the key contributors to fatigue will we be able to gain an understanding of this symptom.
CONCLUSIONS AND NURSING IMPLICATIONS
Fatigue is a common complaint among people with T2DM and is influenced by a variety of factors, including blood glucose control, diabetes symptoms, depression and diabetes distress, especially in those with poor diabetes control. Fatigue may be a barrier to diabetes self-management tasks that are key in maintaining blood glucose control (Wenzel et al. 2005), thus potentiating the risks for diabetes complications, their related symptoms and ultimately more fatigue. Nurses caring for patients with diabetes should prioritize teaching their patients to find means of preventing or minimizing diabetes symptoms, getting depression diagnosed and treated early, reducing the burden of diabetes distress and attaining or maintaining good blood glucose control. Nursing interventions might include ensuring patients are maintaining routine diabetes-related clinic visits and laboratory testing or helping patients to manage the timing self-care tasks around periods when symptoms and fatigue are worst.
Summary Statement.
Why is this research or review needed?
Fatigue is pervasive among patients with Type 2 diabetes, but is not well understood.
Fatigue in diabetes has long been thought to be related to uncontrolled blood glucose levels. However, data from cross-sectional studies have not shown any evidence of this relationship.
What are the key findings?
In Type 2 diabetes, fatigue is related to glucose control only in those with A1C levels > 7%. This relationship was mediated by diabetes symptoms, depression and diabetes distress.
In those with A1C levels ≤ 7%, there was no relationship between glucose control and fatigue.
In all patients, the burden of Type 2 diabetes symptoms were the strongest predictors of fatigue.
How should the findings be used to influence policy/practice/research/education?
Future research in the area of diabetes related fatigue should include assessment of diabetes symptoms, psychological factors such as depression and diabetes distress, along with measures of blood glucose control.
In the clinic setting, health care providers should address all diabetes-related symptoms, including depression and distress in their patients who complain of fatigue as this is a known barrier to accomplishment of diabetes self-management tasks.
Acknowledgements
The authors thank Kevin Grandfield, UIC Department of Biobehavioral Health Science Publication Manager, for editorial assistance.
Funding: This work was supported in part by the National Institutes of Health/National Institute for Nursing Research K99 R00 NR012219 and F31 NR009751 (CF)
Footnotes
Conflict of interest statement:No conflict of interest has been declared by the authors.
References
- Acock AC. Discovering Structural Equation Modeling Using Stata. Stat Press; College Station, TX: 2013. [Google Scholar]
- Adriaanse MC, Pouwer F, Dekker JM, Nijpels G, Stehouwer CD, Heine RJ, Snoek FJ. Diabetes-related symptom distress in association with glucose metabolism and comorbidity: the Hoorn Study. Diabetes Care. 2008;31(12):2268–70. doi: 10.2337/dc08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Arbuckle RA, Humphrey L, Vardeva K, Arondekar B, Danten-Viala M, Scott JA, Snoek FJ. Psychometric evaluation of the Diabetes Symptom Checklist-Revised (DSC-R)--a measure of symptom distress. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009;12(8):1168–75. doi: 10.1111/j.1524-4733.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- Bode BW, Irvin BR, Pierce JA, Allen M, Clark AL. Advances in haemoglobin A1c point of care technology. J Diabetes Sci Technol. 2007;1(3):405–11. doi: 10.1177/193229680700100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman V, Adriaanse MC, van 't Riet E, Snoek FJ, Dekker JM, Nijpels G. Depression, anxiety and glucose metabolism in the general dutch population: the new Hoorn study. PLoS One. 2010;5(4):e9971. doi: 10.1371/journal.pone.0009971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod M, Skovlund SE, Wittrup-Jensen KU. Measuring the impact of diabetes through patient report of treatment satisfaction, productivity and symptom experience. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2006;15(3):481–91. doi: 10.1007/s11136-005-1624-6. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Structural equation modeling with AMOS: basic concepts, applications and programming. Routledge; New York: 2009. [Google Scholar]
- Chasens ER, Korytkowski M, Sereika SM, Burke LE, Drumheller OJ, Strollo PJ., Jr. Improving activity in adults with diabetes and coexisting obstructive sleep apnea. West J Nurs Res. 2014;36(3):294–311. doi: 10.1177/0193945913500567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25(4):246–52. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–8. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi C, Quinn L. Fatigue in patients with diabetes: A review. Journal of Psychosomatic Medicine. 2010;69(1):33–41. doi: 10.1016/j.jpsychores.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks MM, Lucas T, Stephens MAP, Rook KS, Gonzalez R. Diabetes Distress and Depressive Symptoms: A Dyadic Investigation of Older Patients and Their Spouses. Fam Relat. 2010;59(5):599–610. [Google Scholar]
- Fritschi C, Park C, Quinn L, Park H, Richardson A, Mermelstein R, Collins EG. Glucose, Physical Activity and Fatigue: Temporal Relationships in T2DM; Amedican Diabetes Association 74th Scientific SessionsDiabetes; San Francisco. 2014. [Google Scholar]
- Fritschi C, Park C, Quinn L, Park H, Richardson A, Mermelstein R, Collins E. Glucose, Physical Activity and Fatigue: Temporal Relationships in T2DM. Diabetes. 2014;63(Suppl. 1):A195. [Google Scholar]
- Fritschi C, Quinn L, Hacker ED, Penckofer SM, Wang E, Foreman M, Ferrans CE. Fatigue in women with type 2 diabetes. Diabetes Educ. 2012;38(5):662–72. doi: 10.1177/0145721712450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedendorp MM, Tack CJ, Steggink E, Bloot L, Bazelmans E, Knoop H. Chronic fatigue in type 1 diabetes: highly prevalent but not explained by hyperglycemia or glucose variability. Diabetes Care. 2014;37(1):73–80. doi: 10.2337/dc13-0515. [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care. 2011;34(1):236–9. doi: 10.2337/dc10-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med. 1994;11(3):253–61. doi: 10.1111/j.1464-5491.1994.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Hessler D, Fisher L, Glasgow RE, Strycker LA, Dickinson LM, Arean PA, Masharani U. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care. 2014;37(3):617–24. doi: 10.2337/dc13-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlatky MA, Chung SC, Escobedo J, Hillegass WB, Melsop K, Rogers W, Brooks MM, Group BDS. The effect of obesity on quality of life in patients with diabetes and coronary artery disease. Am Heart J. 2010;159(2):292–300. doi: 10.1016/j.ahj.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Huibers MJH, Leone SS, van Amelsvoort LGPM, Kant I, Knottnerus JA. Associations of fatigue and depression among fatigued employees over time: a 4-year follow-up study. J Psychosom Res. 2007;63(2):137–42. doi: 10.1016/j.jpsychores.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd Guilford Press; New York ; London: 2011. [Google Scholar]
- Lasselin J, Laye S, Dexpert S, Aubert A, Gonzalez C, Gin H, Capuron L. Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain Behav Immun. 2012;26(8):1211–9. doi: 10.1016/j.bbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Little RR. Glycated haemoglobin standardization--National Glycohaemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41(9):1191–8. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE, Committee NS. The national glycohaemoglobin standardization program: a five-year progress report. Clin Chem. 2001;47(11):1985–92. [PubMed] [Google Scholar]
- Lustman P.J. anderson, R.J., Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19(2):113–22. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE, Carney RM. Depression and the reporting of diabetes symptoms. Int J Psychiatry Med. 1988;18(4):295–303. doi: 10.2190/lw52-jfkm-jchv-j67x. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JK. A neuroendocrine-based regulatory fatigue model. Biol Res Nurs. 2004;6(2):141–50. doi: 10.1177/1099800404268280. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–31. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 20022014 [Google Scholar]
- St John A, Davis TM, Goodall I, Townsend MA, Price CP. Nurse-based evaluation of point-of-care assays for glycated haemoglobin. Clin Chim Acta. 2006;365(1-2):257–63. doi: 10.1016/j.cca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore RL, Karter AJ, Huang ES, Moffet HH, Laiteerapong N, Schenker Y, Adams A, Whitmer RA, Liu JY, Miao Y, John PM, Schillinger D. Symptom burden of adults with type 2 diabetes across the disease course: diabetes & aging study. J Gen Intern Med. 2012;27(12):1674–81. doi: 10.1007/s11606-012-2132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborlane WV, Kollman C, Steffes MW, Ruedy KJ, Dongyuan X, Beck RW, Chase P, Fox LA, Wilson DM, Tsalikian E, Diabetes Research in Children Network Study, G. Comparison of fingerstick haemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes. 2005;6(1):13–6. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- Van der Does FE, De Neeling JN, Snoek FJ, Kostense PJ, Grootenhuis PA, Bouter LM, Heine RJ. Symptoms and well-being in relation to glycemic control in type II diabetes. Diabetes Care. 1996;19(3):204–10. doi: 10.2337/diacare.19.3.204. [DOI] [PubMed] [Google Scholar]
- Warren RE, Deary IJ, Frier BM. The symptoms of hyperglycaemia in people with insulin-treated diabetes: classification using principal components analysis. Diabetes Metab Res Rev. 2003;19(5):408–414. doi: 10.1002/dmrr.396. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Utz SW, Steeves R, Hinton I, Jones RA. ‘Plenty of sickness’: descriptions by African Americans living in rural areas with type 2 diabetes. Diabetes Educ. 2005;31(1):98–107. doi: 10.1177/0145721704273242. [DOI] [PubMed] [Google Scholar]
- Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med. 2012;35(3):299–304. doi: 10.1007/s10865-011-9359-z. [DOI] [PubMed] [Google Scholar]
- Sivo SA, Fan X, Witta EL, Willse JT. The Search for "Optimal" Cutoff Properties: Fit Index Criteria in Structural Equation Modeling. The Journal of Experimental Education. 2006;74(3):267–288. doi: 10.3200/JEXE.74.3.267-288. [Google Scholar]