Abstract
BACKGROUND
Neural tube defects (NTDs) remain the second most common cause of congenital malformations. Myelomeningocele (MM), the most common NTD compatible with survival, results from genetic and environmental factors. Epidemiologic studies and murine models support the hypotheses that obesity, diabetes and hyperglycemia confer increased risk of NTDs. Presence of wildtype facilitated glucose transporter, Glut2, in mouse embryos has been shown to increase risk for NTDs in hyperglycemic pregnancy.
METHODS
The GLUT2 gene of 96 MM patients was amplified, sequenced and compared to the reference sequence (NM_000340). Variants previously unreported in the single nucleotide polymorphisms database (dbSNP) were considered novel. Allele frequencies of reported SNPs were compared to reference populations using Fisher's exact test.
RESULTS
Analysis revealed three novel variants: a substitution in the core promoter region (c.-331c>t), a substitution (c.-182g>a) in the 5′-untranslated region (UTR), and a single base pair deletion (c.1441delT) in the coding sequences. Polymorphic alleles for 10 SNPs were also identified. Seven SNPs are significantly associated with MM in the Mexican American patients tested (p<0.05) and two of the seven remained significant after Bonferroni correction.
CONCLUSION
We identified three novel variants and seven SNPs associated with MM. The novel variants in the core promoter and in the 5′-UTR could affect GLUT2 mRNA transcription and stability and translation efficiency. The c.1441delT variant is predicted to alter the reading frame and prematurely terminate translation of the GLUT2 protein at the C-terminus, affecting GLUT2 protein function. Presence of GLUT2 variants may disrupt GLUT2 activity and influence MM susceptibility.
Keywords: myelomeningocele, facilitated glucose transporter 2, novel variants, single nucleotide polymorphism, association, neural tube defects
INTRODUCTION
Birth defects are the leading cause of death among infants under one year of age in the United States (Detrait et al., 2005). Neural tube defects (NTDs) are the second most commonly observed defects after congenital heart defects, occurring with an incidence of 1-2/1,000 American live births (Copp et al., 2003; Boulet et al., 2008). NTDs, including anencephaly and spina bifida, are malformations of the brain and spinal cord resulting from failure of the neural tube to close during the fourth week of embryogenesis (Blencowe et al., 2010). Myelomeningocele (MM) is the most common form of spina bifida and occurs when the caudal neural tube fails to close during embryogenesis, resulting in herniation of the spinal cord and meninges through the defect (Au et al., 2010). MM is the most severe form of open NTD compatible with survival and carries a significant burden of disease as greater than 95% of patients have associated conditions that may include hydrocephalus, learning difficulties, motor disabilities and loss of bladder or bowel control (Sandler, 2010). The severity of symptoms is dependent upon the spinal nerves involved and the degree of damage incurred. In the first year of life, children with NTDs average five surgeries as well as a higher mortality rate over the general population. Only 1% of children born with an open NTD are completely free of handicap, requiring multiple specialists to coordinate care as well as significant cost burden to the families and healthcare system (Detrait et al., 2005; Sandler, 2010).
While the etiology of NTDs remains incompletely understood, it is recognized that there is a multifactorial inheritance pattern inclusive of genetic and environmental influences that affect the developing embryo at a critical time during embryogenesis (Botto et al., 1999; Mitchell, 2005). Following mandated folic acid supplementation of grain products by the US Food and Drug Administration in 1998, as well as attention to periconceptual maternal folate status, there has been a significant reduction in the incidence of NTDs worldwide (Honein et al., 2001; CDC, 2004; Boulet et al., 2008). However, these efforts have not completely eradicated disease, suggesting a role for other pathways in the pathogenesis of NTDs (Honein et al., 2001; Mitchell, 2005; Davidson et al., 2008).
Epidemiologic studies and animal models support the hypotheses that obesity, diabetes and hyperglycemia confer increased risk for NTD (Chang and Loeken 1999; Fine et al., 1999; Li et al., 2007; Blomberg and Kallen, 2010; McMahon et al., 2013). Maternal obesity and maternal diabetes are well-established risk factors for NTDs that may alter regulation of glucose homeostasis during critical times of embryo development, although the mechanism remains unclear (Shaw et al., 1996; Hendricks et al., 2001). Mothers of Mexican American descent are of particular interest because they have significantly higher rates of diabetes, maternal obesity and NTD-affected pregnancies than other ethnic groups in the US (Shaw et al., 1996; Hendricks et al., 2001; Rasmussen et al., 2008).
The GLUT family of transmembrane proteins facilitates glucose and other monosaccharides transport across eukaryotic cells membrane (reviewed by Mueckler and Thorens, 2013). These proteins are encoded by the GLUT genes (also known as Solute Carrier family 2A, SLC2) and all members of the family are facilitative transporters. Currently, there are 14 human GLUT genes, and they are classified based on sequence similarity. They differ in localization and time of expression within the body, substrate specificity and regulation by insulin (reviewed by Mueckler and Thorens, 2013). The physiologic role of Class I transporters (GLUT 1-4) have been described in greater detail compared to other classes (reviewed by Mueckler and Thorens, 2013).
A recent article reviewed that GLUT2 has physiologic roles in different organ systems such as liver, intestine, kidney, pancreatic islet beta cells, and cells in the central nervous system (Thorens 2014). GLUT2 is expressed early in embryonic life and primarily in postnatal hepatocytes, pancreatic β cells and intestinal cells (Hogan et al., 1991; reviewed by Mueckler and Thorens, 2013). It is the major glucose transporter of hepatocytes and is essential for glucose uptake, but not release, in hepatocytes. GLUT2 is unique among other glucose transporters in that it has a low affinity for glucose and a high capacity for glucose transport (reviewed by Mueckler and Thorens, 2013). . A large scale genome-wise association study discovered that the common allele of GLUT2 SNP rs11920090 confers risk of fasting glucose-raising in non-diabetic humans (Dupuis et al., 2010). McCulloch et al. (2011) demonstrated GLUT1 and GLUT3 not GLUT2 are the principal glucose transporters in human islet and beta cells and proposed that elevation of fasting plasma glucose levels was driven by the GLUT2 variants in metabolic tissues other than the islet beta cells. Guillemain et al. (2000) demonstrated the cytoplasmic loop between transmembrane domains 6 and 7 of GLUT2 is capable of translocating into the cell nucleus and potentially function in glucose signal transduction. Animal models have shown that expression of Glut2 during early embryogenesis is a risk factor for NTDs in pre-gestational diabetic pregnancies because its high efficiency for glucose transport during times of maternal hyperglycemia adversely affects the developing embryo (Li et al., 2007). It is not known whether GLUT2 in human embryonic cells behave in a similar fashion.
Several genes known to play a role in glucose homeostasis have been identified and studied to determine their function as well as their relation to NTDs. Genetic studies have evaluated and linked single nucleotide polymorphisms (SNPs) of glucose transporters with risk of NTDs (Davidson et al., 2008; Cormier et al., 2011; Suazo et al., 2013; Connealy et al., 2014). To our knowledge, current research and data are limited to GLUT1 and GLUT3 (Davidson et al., 2008; Cormier et al., 2011; Suazo, et al., 2013; Connealy et al., 2014). Lupo et al. (2012) found no association for minor alleles of three GLUT2 tagged SNPs with human NTDs in single allele analyses but in a later report described NTDs risk associated with a combination of minor alleles of maternal ENPP1 rs1044498 and fetal GLUT2 rs6785233 that is 12.2 kbp upstream from GLUT2 (Lupo et al., 2014). Similar but less significant risk for the minor allele of GLUT2 rs5400 was also reported by Lupo et al. (2014). The objective of our study was to profile the GLUT2 gene and describe genetic variations in subjects with the MM phenotype by reporting both novel variations as well as population frequencies of previously reported variants.
MATERIALS AND METHODS
Patient Samples
Children with MM in our study were chosen from a cohort of non-syndromic (Au et al., 2008) myelomeningocele patients enrolled in ongoing genetic studies in our laboratory. Patients with MM and their parents were enrolled after informed consent into the study between 1996 and 2006 across three sites (University of Texas Houston, University of California Los Angeles, and Toronto, Canada). The characteristics of the study population are described in detail elsewhere (Au et al., 2008). The study was approved by the Institutional Review Board at the University of Texas Health Science Center at Houston. Ninety six patients with MM were tested, including 48 Caucasians of European descent and 48 Hispanics of Mexican descent.
Parental DNA was also collected to study inheritance patterns of novel variations. The environmental information on potential non-genetic influences such as diet, pregestational diabetes, and Body Mass Index for the mother during the pregnancy of the affected child was not collected. With 192 chromosomes to be examined, rare variants could be detected with an approximately 0.5% population frequency (1 of 192). Additionally, patients born post-folate fortification in 1998 were chosen to enrich for folate-resistant cases. The SNPs and novel variation allele frequency was compared with ethnically-matched populations published in the single nucleotide polymorphism database (dbSNP).
DNA extraction
Genomic DNA was extracted from blood lymphocytes using the Puregene DNA extraction kit (Gentra Systems Inc, Minneapolis, Minnesota). Parental saliva samples were collected in some cases when blood samples were not available and the saliva DNA was prepared using the Oragene DNA preparation kit (DNA Genotek; Kanata, Ontario, Canada) following manufacturer's protocols. Working DNA was prepared from whole genome amplification using the GenomiPhi kit following standard protocols (GE Healthcare, Piscataway, NJ).
PCR amplification
PCR and nested-sequencing primers were designed for each of the eleven GLUT2 exons, including the flanking regions of up to 100 base pairs to account for the splicing donors and acceptors (see online supplemental table for primer sequences). Primers were synthesized by Integrated DNA Technologies USA (Commercial Park, Coralville, IA). GLUT2 exons were amplified by PCR with MyTaq Hot Start DNA Polymerase (Bioline USA Inc, Taunton, MA) using the MJ Research PTC-100 Thermal Cycler (MJ Research, Waltham, MA). Sizes of PCR products were examined by electrophoresis in a 1.6% agarose gel. Excess primers and nucleotides were then removed from amplified products using exonuclease I and shrimp alkaline phosphatase treatment (United States Biochemicals, Affymetrix, Cleveland, OH) prior to sequencing.
Sequencing
Sanger sequencing was performed using the BigDye Terminator Protocol (Applied Biosystems, Foster City, CA) with nested-sequencing primers. The sequencing products were resolved on the ABI3100 Genetic Analyzer (Life Technologies Inc, Grand Island, NY).
Analysis of Sequencing Data
Patient sequences were manually compared to the GLUT2 reference sequence (NM_000340) to identify single nucleotide polymorphisms (SNPs) and novel variations. Each sequencing result was examined by two or more individuals in the laboratory. Variants not previously reported in dbSNP were considered novel and were further confirmed by sequencing from both directions using PCR product amplified from a fresh preparation of genomic DNA following the standard laboratory protocol. When available, genomic DNA from parents of patients with novel GLUT2 variants was sequenced from both directions to determine whether variants that occurred were de novo or inherited. Ethnically-matched reference populations were used for comparison in cases of identified single nucleotide polymorphisms (SNPs). The Caucasian reference population includes data from two sources: (1) the 1000 genomes project (http://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/) which includes Caucasians from Utah, and (2) the NHLBI Exome Sequencing Project (http://evs.gs.washington.edu/EVS/). The Mexican American reference population includes Mexican Americans recruited from Los Angeles, CA (MXL) used in the 1000 Genomes Project in the single nucleotide polymorphism database (dbSNP). In addition, collaborators at The University of Texas School of Public Health at Houston provided the Mexican American reference population (MEX). There are no known reports of NTDs in the reference populations used.
Statistical Analysis
Fisher's exact test was performed to compare the allele frequencies of SNPs between the MM patient cohort and the ethnically-matched unaffected reference population. A two-tailed p-value of <0.05 was considered significant for association of the SNP to the MM phenotype. The Bonferroni test was used to resolve the effects of multiple testing (Dunn, 1961).
RESULTS
Identification of novel GLUT2 variants that may alter GLUT2 activity
We identified three novel variants each in a different MM patient after examining 11 exons and flanking sequences (~3160 bp) of the GLUT2 gene of 96 MM patients, yielding a novel variant occurrence rate of approximately 3% (Table 1). Each of the novel variants were confirmed with sequencing of PCR products from both strands. The first novel variant (c.-331c>t) was found within the core promoter region 15 bp downstream from a CCAATT site and 22 bp upstream of the transcription start site of exon 1 in a Caucasian MM patient. The variant c.-331c>t was also found in the father's DNA. Overall, it is observed in approximately 1% (1/96) of our total patient cohort. The second novel variant (c.-182g>a) identified within exon 1 is a single base pair substitution 182 bases upstream from the translation initiation codon ATG in one Caucasian patients and the variant was also found in the father's DNA. This variant is found in approximately 1% (1/96) of our study patient samples.
Table 1.
Novel SNPs identified across the GLUT2 gene in patients with MM.
| Chr3 location | A1/A2 | cdna Variant | significance | Caucasian MM A2/A1 | Mexican MM A2/A1 | GERP/PhyloP |
|---|---|---|---|---|---|---|
| 170744790 | C/T | c.−331c>t | Core Promoter | 1/95 | 0/96 | −0.79/−0.3486 |
| 170744641 | G/A | c.−182g>a | 5'-UTR | 1/95 | 0/96 | 3.91/1.33 |
| 170715821 | T/- | c.1446dlT | p.F482Lfs*31 | 0/96 | 1/95 | 1.51/1 |
Note: A1, reference allele; A2, variant allele; MM, myelomeningocele; SNP, single nucleotide polymorphism; GLUT2, glucose transporter 2 gene. GERP/PhyloP, Genomic Evolutionary Rate Profiling score/Phylogenetic P-Values
A third novel variant (c.1446delT) is a deletion of a T base located within the coding region in exon 11 in a Mexican American patient and the variant is not found in the parents’ DNAs. This single nucleotide deletion in the coding frame is predicted to cause a shift of the translation reading frame (Figure 1). The allele frequency observed across our study cohort was approximately 1% (1/96).
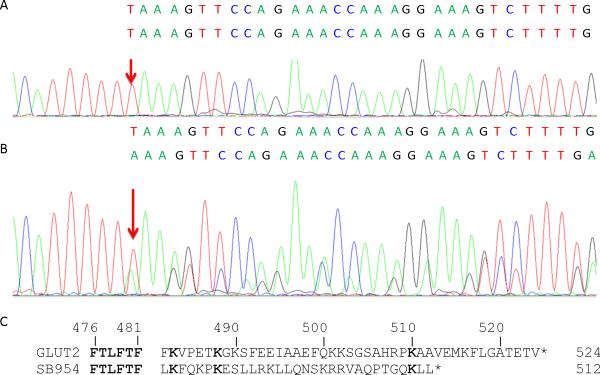
Figure 1.
A MM patient carries a c.1446delT mutation. Sanger sequencing result of a reference allele c.1446T (arrow) is shown on the top panel A, and the sequencing result for the patient with the variant allele c.1446delT (arrow) is shown on the middle panel B. The variant sequence with c.1446delT is aligned under the reference sequence showing results of overlapping nucleotides in B. Panel C shows the predicted consequences of the c.1446dlT mutation (p.F482Lfs*31) resulting in frame shifting at codon 482 and addition of 31 amino acids unrelated to GLUT2 protein. Identical amino acids are bolded. The GLUT2 mutant protein loses 44 amino acids of the carboxy-terminus of the normal glucose transporter GLUT2.
GLUT2 SNPs reveal association with MM
We identified heterozygous variants for ten previously reported SNPs located within the 11 GLUT2 exons and flanking intronic region (~3160 bp) of the 96 MM patients we sequenced (Table 2). Up to 130 SNPs (dbSNP138) were recorded within the regions we sequenced and we observed that 120 SNPs are homozygous for the common reference alleles in the MM patients. The ten previously reported SNPs were then evaluated for association to the MM phenotype by comparing the rare allele frequencies with the ethnically-matched reference populations. We found seven of the ten SNPs (rs7637863, rs5402, rs5404, rs2292621, rs2292622, rs5406, and rs5398) have a nominally significantly lower rare allele frequency in Mexican American MM patients than the MXL reference population (Table 3) (p-value < 0.05). Both rs5402 and rs5406 appear to be significant after correction for multiple testing of 10 SNPs by Bonferroni method (p< 0.005). Five of seven significant SNPs (rs5402, rs5404, rs2292621, rs2292622, and rs5406) are linked in a single Linkage Disequilibrium (LD) block (D′=1 and r.2 >0.95) in the MXL reference population (1000 Genomes Project) (Table 4). The other two significant SNPs (rs7356034 and rs5398) form another LD block.
Table 2.
Properties of 10 SNPs in GLUT2 gene with observed heterozygote variants in subjects with MM.
| #base (NCBI.37) | SNP ID | Function | Protein Variant | Cdna Variant | Conservation Score PhastCons | Conservation Score GERP | Grantham Score |
|---|---|---|---|---|---|---|---|
| 3:170732599 | rs7356034 | Intron 2 | NA | c.109-79C>T | NA | −1.45 | NA |
| 3:170732426 | rs7637863¶ | missense | p.P68L | c.203C>T | 0.008 | 3.71 | 98 |
| 3:170732300 | rs5400¶§ | missense | p.T110I | c.329C>T | 0.981 | 6.08 | 89 |
| 3:170727739 | rs5402 | intron 4 | NA | c.496+8A>T | 0 | −5.81 | NA |
| 3:170724955 | rs5404§ | synonymous | p.T198T | c.594G>A | 0.003 | −11.5 | NA |
| 3:170723668 | rs2292621 | intron 6 | NA | c.775+64G>A | NA | 0.471 | NA |
| 3:170723664 | rs2292622 | intron 6 | NA | c.775+68T>C | NA | 0.42 | NA |
| 3:170723276 | rs5406§ | intron 6 | NA | c.776-15C>T | 0.093 | 2.59 | NA |
| 3:170716937 | rs76362149¶ | missense | p.A363S | c.1087G>T | 1 | 4.96 | 99 |
| 3:170715830 | rs5398§ | synonymous | p.F479F | c.1437C>T | 0.113 | −3.68 | NA |
NIEHS SNP Function Prediction FuncPred (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.htm) predicts that the SNP is located within a transcription factor binding site or linked to another SNPs located within a transcription binding site or splicing enhancer/silencer.
NIEHS SNP Function Prediction FuncPred predicts missense change in a SNP is possibly/probably damaging. The degree of conservation for the common alleles are reported in Phastcons score for vertebrates, and/or GERP (Genomic Evolutionary Rate Profiling score), and/or Grantham score for change involve a codon.
Table 3.
Allele frequencies for 10 SNPs of GLUT2 in patients with MM
| CAUC | MEX | CAUC/MEX | |||||
|---|---|---|---|---|---|---|---|
| GLUT2 SNP | A1/A2 | All MM | MM | NHLBI ESP EA/CEU* | MM | 1KGP MXL | p-values |
| rs7356034 | C/T | 23.37% (43/141) | 30.00% (27/63) | 28.24% (48/122)* | 17.02 (16/78) | 31.82% (41/89) | 0.78/0.0138 |
| rs7637863¶ | C/T | 0.5% (1/191) | 0.00% (0/96) | 0.49% (42/8558) | 1.04% (1/95) | 0.00% (0/130) | 1.00/0.24 |
| rs5400§¶ | C/T | 14.29% (22/154) | 17.71% (17/79) | 15.39% (1147/7453) | 6.25% (6/90) | 18.18% (23/107) | 0.226/0.011 |
| rs5402 | A/T | 10.97% (17/155) | 14.58% (14/82) | 11.90% (914/7682) | 4.26% (4/90) | 18.94% (24/106) | 0.241/0.0015 |
| rs5404§ | G/A | 8.75% (14/160) | 11.46% (11/85) | 11.31% (874/7726) | 3.13% (3/93) | 9.85% (13/117) | 0.612/0.065 |
| rs2292621 | G/A | 14.97% (25/167) | 16.67% (16/80) | 12.35% (21/149)* | 7.28% (7/89) | 18.18% (23/107) | 0.359-/0.0227 |
| rs2292622 | T>C | 13.10% (22/168) | 16.67% (16/80) | 12.35% (21/149)* | 7.29% (7/89) | 18.18% (23/107) | 0.359/0.0227 |
| rs5406§ | C>T | 11.70% (22/188) | 18.48% (17/75) | 13.31% (1145/7445) | 5.21% (5/91] | 18.18% (23/107) | 0.164/0.0049 |
| rs76362149 | G/T | 0.56% (1/177) | 1.04% (1/95) | 0.12% (10/8590) | 0.00% (0/96) | 0.00% (0/130) | 0.106/1.00 |
| rs5398§ | C/T | 35.38% (46/130) | 30.85% (29/65) | 42.55% (2567/6033) | 19.79% (19/77) | 32.58% (42/88) | 0.821/0.0448 |
A1, reference allele; A2, variant allele; CEU, Centre d'Etude du Polymorphisme; CAUC, Caucasian American; MEX, Mexican-American; MM, myelomeningocele; ND, no data for reference population; significant p-values using Fisher's exact test (2 tailed) are bolded; SNP, single-nucleotide polymorphism; GLUT2, glucose transporter 2 gene.
CEU population consists of the Utah residents with northern and western European ancestry. The NHLBI ESP EA (National Heart, Lung, and Blood Institute Exome Sequencing Project) European Americans. MXL population consists of Americans of Mexican ancestry living in Los Angeles in the 1000 Genomes Project (1KGP).
NIEHS SNP Function Prediction FuncPred (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.htm) predicts the SNP is located within a transcription factor binding site or linked to another SNP located within a transcription binding site or splicing enhancer/silencer.
NIEHS SNP Function Prediction FuncPred predicts missense change in SNP is possibly/probably damaging.
Table 4.
Pairwise correlation coefficient r2 values for eight SNPs in GLTU2 gene region for MXL in 1000GENOMES:phase_1
| SNP | rs7356034 | rs5400 | rs5402 | rs5404 | rs2292621 | rs2292622 | rs5406 | rs5398 |
|---|---|---|---|---|---|---|---|---|
| rs7356034 | 1 | |||||||
| rs5400 | 0.476 | 1 | ||||||
| rs5402 | 0.501 | 0.951 | 1 | |||||
| rs5404 | 0.234 | 0.492 | 0.468 | 1 | ||||
| rs2292621 | 0.476 | 1 | 0.951 | 0.492 | 1 | |||
| rs2292622 | 0.476 | 1 | 0.951 | 0.492 | 1 | 1 | ||
| rs5406 | 0.476 | 1 | 0.951 | 0.492 | 1 | 1 | 1 | |
| rs5398 | 0.966 | 0.46 | 0.484 | 0.226 | 0.46 | 0.46 | 0.46 | 1 |
Note: all SNPs have D' of 1. MXL -1000 Genomes Project Mexican American in Los Angeles
We examined the haplotypes of the seven SNPs in LD blocks using Haploview 4.2. The analysis revealed that the haplotype consisting of the rare alleles of all seven SNPs (TTTACTT) is observed significantly less often among the Mexican American MM patients (4.2%) than the MXL reference population (17.5%) as shown in Table 5. Similar results were observed when SNPs in the two LD blocks were independently analyzed. A permutation test suggests the differences observed are significant and remain significant after Bonferroni correction for the number of haplotypes tested.
Table 5.
Haplotype analysis of seven SNPs showing significant differences between Mexican American MM patients and reference population MXL in 1000GENOMEs
| Haplotype block | frequency | MEX MM | MXL | Permutation p value |
|---|---|---|---|---|
| rs5400, rs5402, rs2292621, rs2292622, and rs5406 block | ||||
| CAGTC | 86.3% | 92.7% (89/96) | 81.7% (103/126) | 0.0288 |
| TTACT | 11.9% | 4.2% (4/96) | 17.5% (22/126) | 0.0040 |
| rs7356034 and rs5398 block | ||||
| CC | 72.5% | 79.1% (76/96) | 67.4% (85/126) | 0.0803 |
| TT | 24.8% | 15.6% (15/96) | 31.7% (40/126) | 0.0036 |
| rs7356034, rs5400, rs5402, rs2292621, rs2292622, rs5406 and rs5398 block | ||||
| CCAGTCC | 72.1% | 78.1% (75/96) | 67.5% (85/126) | 0.1084 |
| TCAGTCT | 12.4% | 11.5% (11/96) | 13.5% (17/126) | 0.3705 |
| TTTACTT | 11.9% | 4.2% (4/96) | 17.5% (22/126) | 0.0013 |
| CCAGTCT | 1.8% | 3.2% (3/96) | 0.8 % (1/126) | 1.0000 |
LD block (D'=1 and r2 ≥0.88) consisting allele of haplotypes of the seven significant SNPs shown in Table 4 were analyzed using Haploview 4.2 between Mexican American (MEX) MM patients and MXL reference population. Significant differences were observed showing MM patients have a higher frequency of the LD block consisting of the common alleles of all five SNPs and lower frequency of the LD block consisting of the rare alleles of all five SNPs. Bonferroni test for haplotypes suggests the rare allele haplotype remains significant with p≤0.00625 (bolded).
None of these SNPs showed statistically significant association in the Caucasian MM patient population when compared to the European American cohort in the NHLBI exome sequencing project or the CEU cohort in the 1000 Genomes Project.
DISCUSSION
Expression of facilitated glucose transporters plays a critical role to maintain glucose homeostasis and expression of some members of facilitated glucose transporters is tightly regulated by the status of glucose availability (reviewed by Cura & Carruthers 2012). A murine model study has demonstrated presence of wildtype Glut2 is a risk factor for NTDs in hyperglycemic pregnancies and loss of Glut2 protected against NTD development in embryos (Li et al., 2007). However, a most recent study of zebrafish demonstrated embryos treated with GLUT2 morpholino resulted in developmental defects in the hindbrain and tail (Marin-Juez et al. 2014). In humans, how GLUT2 activity affects neural tube development remains to be elucidated. Sequences of ~3,160 bp of the 11 exons and flanking regions of the GLUT2 gene for 96 MM patients were examined and three novel variants were identified in this study. We observed an overall novel mutation rate of 9.9 × 10−6 per base (3/3,160 × 96) and a de novo mutation rate of 3.3 × 10−6 per base within or adjacent to the GLUT2 gene among MM patients. The rate of novel variants we observed is 2 orders higher than the estimated general human intergenerational mutation rate of approximately 2.5 × 10−8 per base per generation (Nachman and Crowell. 2000).
In this study, we discovered two novel single base pair substitutions and one single base pair deletion in the coding sequences of GLUT2. One of the two substitution variants c.-331c>t is located 22 bp upstream of the transcription start site and 15bp downstream from a CCAAT sequence in the GLUT2 core promoter. In general the CCAAT box is accompanied by conserved sequences providing signals for the RNA polymerase complex to bind and initiate mRNA transcription. The c.-331c is the third nucleotide in a putative transcription factor target E-box (CACRTG) and is conserved throughout all primates. The effect of the novel variant c.-331c>t on transcription factor binding and/or transcription initiation of GLUT2 mRNA is not known and needs to be experimentally tested. The second novel variant, c.-182g>a, is located within the 5′-untranslated region (UTR) and is highly conserved among vertebrates. The c.-182g>a variant may affect mRNA folding and protein translation initiation and/or mRNA stability. In either case, changes of GLUT2 mRNA transcription/translation during embryogenesis could favor MM formation. Additional factors may facilitate MM development since these variants are inherited from one of their respective unaffected parent.
An observed deletion mutation in exon 11 (c.1446delT) is interesting because it results in a shift of reading frame at codon 482 (p.F482Lfs*31) to produce a GLUT2 with a C-terminus containing 31 foreign amino acids and truncating the C-terminal 43 amino acids of normal GLUT2. GLUT1 and GLUT2 are both Class I facilitated glucose transporters with similarities in monomeric and oligomeric structures and functions (Reviewed by Cura and Carruthers 2012). It has been shown that the C-terminal domain of GLUT2 plays an important role in regulating substrate transport (Katagiri et al., 1992; Thorens et al., 1996). In 2007, Blodgett and colleagues demonstrated the C-terminal domain of GLUT1 is required for inhibition of glucose transport by ATP (Blodgett et al., 2007). With the C-terminal domain replaced, the p.F482Lfs*31 GLUT2 mutant is expected to have an altered GLUT2 function and may also affect formation of oligomers of normal and mutant GLUT2.
In the study, we observed seven of the ten informative SNPs of GLUT2 have significantly less frequent rare alleles among Mexican American patients compared to the reference population. Rare alleles of two SNPs (rs5402 and rs5406) remain significant after Bonferroni correction for multiple testing suggesting a significant protective effect to MM and implying a risk associated with the common alleles. The common allele of GLUT2 SNP rs11920090 conferring risk of fasting glucose-raising in non-diabetic humans (Dupuis et al., 2010) is in complete LD with SNP rs5400 and rs5400 is in complete LD with the two SNPs (rs5402 and rs5406) found significantly associated with MM risk in this study. The patient population significantly associated with GLUT2 variants in this study is Mexican-American children, an ethnicity that maintains the highest risk for NTDs in the US. Incidentally, obesity and diabetes are known risk factors associated with birth defects and Mexican American women have high rates of overweight and obesity (45.8%; CDC, 2013) and diagnosed diabetes (13.9%) in the US (CDC, 2014). Lupo et al. (2012) did not find association of GLUT2 SNPs in single allele analyses but observed NTD risk associated with interaction of minor alleles of the maternal SNP rs1044498 in ENPP1 and the fetal SNPs rs6785233 and rs5400 in GLUT2 (Lupo et al., 2014). The sample size of our study is not large enough for maternal-fetal SNP-SNP interaction analyses. In this single allele analysis study, we observed the minor alleles of multiple GLUT2 SNPs not tested by Lupo et al. (2012, 2014) have protective effects with MM among Mexican Americans. Future studies using a large sample size and multi-ethnic NTDs subjects are warranted.
The most significantly associated SNP, rs5402, is located eight bases downstream from exon 4 of GLUT2 near the splice donor site (gtaag) preceding a dinucleotide CA repeat (rs373165390). The rare allele T of rs5402 has been reported by Matsutani et al. (1992) linking to shorter lengths of the CA repeat (14 and 15 copies) and the common allele C linking to 16 to 24 copies of CA repeats (Fig. 2). Several studies have demonstrated the length of intronic CA repeats near splice sites affect the stability and/or splicing of mRNA (Hui et al., 2005; Yang et al., 2013). In addition, heterogeneous nuclear ribonucleoprotein (hnRNP) L has been shown to target the CA repeats (rs373165390) observed in GLUT2 repressing splicing at the exon 4 splice donor site leading to skipping of exon 4 in the GLUT2 mRNA (Heiner et al., 2010). The biologic effect of the rare allele T of rs5402 and/or shorter CA repeats to GLUT2 mRNA stability remains to be examined. It is noted that human GLUT2 produces three mRNA isoforms (NM_000340, NM_001278658 and NM_001278659). Exon 4 of isoform 3 (NM_001278659) is skipped (alternatively spliced) resulting in a peptide without the N-terminal 173 amino acids consisting of transmembrane domains 1-4 of the GLUT2 protein. The function of the GLUT2 isoform-3 peptide is not known, but is expected to be quite different from the full-length GLUT2 protein as there are four missing transmembrane domains. The second most significant SNP rs5406 c>t allele is located 15 nucleotides upstream of GLUT2 exon 7 and may be part of the splice acceptor regulatory sequence. No known functional significance for alleles of rs5406 has been reported. It is possible that the SNPs rs5400, rs2292621 and rs2292622 are significant because they are in the same LD block with rs5402 and rs5406.
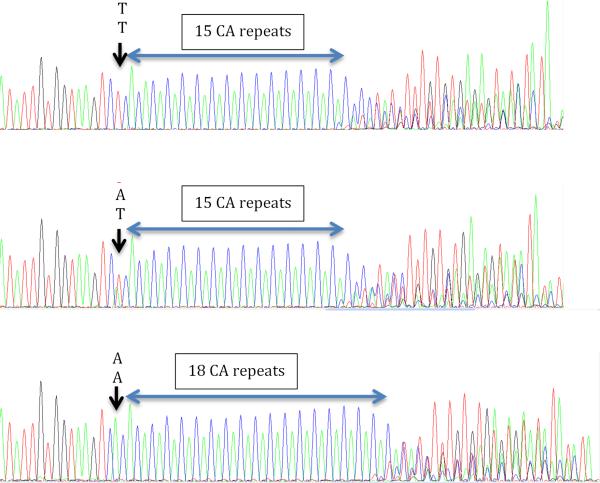
Figure 2.
The rare allele T of rs5402 is associated with shorter CA repeat in MM patients. Top panel shows homozygote TT alleles of rs5402 in one MM patient with 15-17 CA repeats. The middle panel shows heterozygote AT alleles of rs5402 in another patient with 15-17 CA repeats. The bottom panel shows another patient with homozygote AA alleles of rs5402 with 18-20 CA repeats.
There are several strengths of our study. First, we focused our cohort to MM patients born post-folate fortification in order to enrich for folate-resistant cases to study association of variants in the GLUT2 gene. We analyzed patient and parental sequences using Sanger sequencing, the gold standard for novel DNA variant discovery. Other advantages of using Sanger sequencing are that it allows for interrogating core promoter, exon flanking intronic regions, UTRs, and regions with high GC content, which newer modalities such as massively parallel shotgun sequencing of the exome will miss. One other strength of this study is that we had parental DNA available from affected children in whom new variants were found to determine the origin of these variants.
The major limitations of our study include a small sample size and low minor allele frequency of SNPs both limiting the power of the study. Despite the strengths of Sanger sequencing, it is a high cost and time-consuming technique and is limited to examining a small number of target sequences at a time.
In conclusion, this study provides evidence that rare alleles of glucose transporter gene GLUT2 SNPs confer protective effect and the common allele may confer increased risk for the MM phenotype. Based on our findings and in conjunction with other studies that have demonstrated genetic associations of rare variant alleles to dys-regulated glucose transport by adult tissues, there remains a need to further elucidate the genetic basis of pathways other than the folate pathway, which may contribute to the pathogenesis of NTDs.
Supplementary Material
ACKNOWLEDGEMENT
Patients and family members enrolled into this project were supported by NICHD P01 HD35946-06A2 (Hope Northrup and Kit Sing Au) and the Shiners Hospital for Children grant #850 (Hope Northrup and Kit Sing Au). This study is also supported by the endowment fund to Professor Kathleen A. Kennedy (Richard W. Mithoff Professor of Pediatrics, Division of Perinatal and Neonatal Medicine at the University of Texas Medical School at Houston) (Jaclyn E. Ruggiero). The authors report no conflicts of interest.
This work was supported by grants from the National Institutes of Health (P01 HD35946-06A2) and the Shriners Hospital for Children (project 8580). Additional support was provided by Dr.
Kathleen Kennedy, Richard W. Mithoff Professor, Division of Neonatology, Department of Pediatrics, The University of Texas Medical School at Houston.
REFERENCES
- Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16:6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au KS, Tran PX, Tsai CC, et al. Characteristics of a spina bifida population including North American Caucasian and Hispanic individuals. Birth Defects Res Part A Clin Mol Teratol. 2008;82:692–700. doi: 10.1002/bdra.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Modell B, et al. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 39 Suppl. 2010;1:i110–121. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett DM, De Zutter JK, Levine KB, et al. Structural basis of GLUT1 inhibition by cytoplasmic ATP. J Gen Physiol. 2007;130:157–168. doi: 10.1085/jgp.200709818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg M, Kallen B. Maternal obesity and morbid obesity: the risk for birth defects in the offspring. Birth Defects Res Part A Clin Mol Teratol. 2010;88:35–40. doi: 10.1002/bdra.20620. [DOI] [PubMed] [Google Scholar]
- Botto L, Moore CA, Khoury MJ, et al. Neural Tube Defects. N Engl J Med. 1999;341:1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res Part A Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Spina bifida and anencephaly before and after folic acid mandate – United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Health United States, 2012. Table 68. Healthy weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States, selected years 1960–1962 through 2007–2010. 2013:220. http://www.cdc.gov/nchs/data/hus/hus12.pdf.
- Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its burden in the United States, 2014. U.S. Department of Health and Human Services; Atlanta, GA: 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- Chang TI, Loeken MR. Genotoxicity and diabetic embryopathy: impaired expression of developmental control genes as a cause of defective morphogenesis. Semin Reprod Endocrinol. 1999;17:153–165. doi: 10.1055/s-2007-1016222. [DOI] [PubMed] [Google Scholar]
- Connealy BD, Northrup H, Au KS. Genetic variations in the GLUT3 gene associated with myelomeningocele. Am J Obstet Gynecol. 2014;211:305.e1–8. doi: 10.1016/j.ajog.2014.05.013. Epub 2014 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Cormier CM, Au KS, Northrup H. A 10 bp Deletion Polymorphism and 2 New Variations in the GLUT1 Gene Associated with Myelomeningocele. Reprod Sci. 2011;18:463–468. doi: 10.1177/1933719110388293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cura AJ, Carruthers A. The role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism and homeostasis. Compr Physiol. 2012;2:863–914. doi: 10.1002/cphy.c110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CM, Northrup H, King TM, et al. Genes in glucose metabolism and association with spina bifida. Reprod Sci. 2008;15:51–58. doi: 10.1177/1933719107309590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrait ER, George TM, Etchevers HC, et al. Human Neural Tube Defects: Developmental Biology, Epidemiology, and Genetics. Neurotoxicol Teratol. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn OJ. Multiple Comparisons Among Means. J Am Stat Assoc. 1961;56:52–64. [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EL, Horal M, Chang TI, et al. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–2462. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- Guillemain G, Loizeau M, Pinçon-Raymond M, et al. The large intracytoplasmic loop of the glucose transporter GLUT2 is involved in glucose signaling in hepatic cells. J Cell Sci. 2000;113:841–847. doi: 10.1242/jcs.113.5.841. [DOI] [PubMed] [Google Scholar]
- Heiner M, Hui J, Schreiner S, et al. HnRNP L-mediated regulation of mammalian alternative splicing by interference with splice site recognition. RNA Biol. 2010;7:56–64. doi: 10.4161/rna.7.1.10402. [DOI] [PubMed] [Google Scholar]
- Hendricks KA, Nuno OM, Suarez L, et al. Effects of hyperinsulinemia and obesity on risk of neural tube defects among Mexican Americans. Epidemiology. 2001;12:630–635. doi: 10.1097/00001648-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Hogan A, Heyner S, Charron MJ, et al. Glucose transporter gene expression in early mouse embryos. Development. 1991;113:363–72. doi: 10.1242/dev.113.1.363. [DOI] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- Hui J, Hung LH, Heiner M, et al. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J. 2005;24:1988–1998. doi: 10.1038/sj.emboj.7600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Asano T, Ishihara H, et al. Replacement of intracellular C-terminal domain of GLUT1 glucose transporter with that of GLUT2 increases Vmax and Km of transport activity. J Biol Chem. 1992;267:22550–22555. [PubMed] [Google Scholar]
- Li R, Thorens B, Loeken R. Expresssion of the gene encoding the high-Km glucose transporter 2 by the early postimplantation mouse embryo is essential for neural tube defects associated with diabetic embryopathy. Diabetologia. 2007;50:682–689. doi: 10.1007/s00125-006-0579-7. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Canfield MA, Chapa C, et al. Diabetes and Obesity-Related Genes and the Risk of Neural Tube Defects in the National Birth Defects Prevention Study. Am J Epidemiol. 2012;176:1101–1109. doi: 10.1093/aje/kws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo PJ, Mitchell LE, Canfield MA, et al. Maternal-fetal metabolic gene-gene interactions and risk of neural tube defects. Mol Genet Metab. 2014;111:46–51. doi: 10.1016/j.ymgme.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Juez R, Rovira M, Crespo D, et al. GLUT2-mediated glucose uptake and availability are required for embryonic brain development in zebrafish. J Cerebral Blood Flow & Metab. 2014 doi: 10.1038/jcbfm.2014.171. Epub 2014 October 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsutani A, Hing A, Steinbrueck, et al. Mapping the human liver/islet glucose transporter (GLUT2) gene within a genetic linkage map of chromosome 3q using a (CA)n dinucleotide repeat polymorphism and characterization of the polymorphism in three racial groups. Genomics. 1992;13:495–501. doi: 10.1016/0888-7543(92)90116-a. [DOI] [PubMed] [Google Scholar]
- McMahon DM, Liu J, Zhang H, Torres ME, Best RG. Maternal obesity, folate intake, and neural tube defects in offspring. Birth Defects Res Part A Clin Mol Teratol. 2013;97:115–122. doi: 10.1002/bdra.23113. [DOI] [PubMed] [Google Scholar]
- Mitchell LE. Epidemiology of Neural Tube Defects. Am J Med Genet C Semin Med Genet. 2005;135C:88–94. doi: 10.1002/ajmg.c.30057. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch LJ, van de Bunt M, Braun M, et al. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: Implications for understanding genetic association signals at this locus. Mol Genet Metab. 2011;104:648–653. doi: 10.1016/j.ymgme.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genet. 2000;156(1):297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Chu SY, Kim SY, et al. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Sandler AD. Children with spina bifida: key clinical issues. Pediatr Clin North Am. 2010;57(4):879–892. doi: 10.1016/j.pcl.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA. 1996;275:1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- Suazo J, Pardo R, Castillo S, et al. Family-based association study between SLC2A1, HK1, and LEPR polymorphisms with myelomeningocele in Chile. Reprod Sci. 2013;10:1207–1214. doi: 10.1177/1933719113477489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2014 doi: 10.1007/s00125-014-3451-1. Epub 2014 November 25. [DOI] [PubMed] [Google Scholar]
- Thorens B, Deriaz N, Bosco D, DeVos A, Pipeleers D, Schuit F, Meda P, Porret A. Protein kinase A-dependent phosphorylation of GLUT2 in pancreatic cells. J Biol Chem. 1996;271:8075–8081. doi: 10.1074/jbc.271.14.8075. [DOI] [PubMed] [Google Scholar]
- Yang W, Ni L, Silveyra P, et al. Motifs within the CA-repeat-rich region of Surfactant Protein B (SFTPB) intron 4 differentially affect mRNA splicing. J Mol Biochem. 2013;2:40–55. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.