Abstract
Background and Aims
We investigated the association between significant liver fibrosis, determined by AST-to-platelet ratio index (APRI), and all-cause mortality among HIV-infected patients prescribed antiretroviral therapy (ART) in Zambia
Methods
Among HIV-infected adults who initiated ART, we categorized baseline APRI scores according to established thresholds for significant hepatic fibrosis (APRI ≥1.5) and cirrhosis (APRI ≥2.0). Using multivariable logistic regression we identified risk factors for elevated APRI including demographic characteristics, body mass index (BMI), HIV clinical and immunologic status, and tuberculosis. In the subset tested for hepatitis B surface antigen (HBsAg), we investigated the association of hepatitis B virus co-infection with APRI score. Using Kaplan-Meier analysis and Cox proportional hazards regression we determined the association of elevated APRI with death during ART.
Results
Among 20,308 adults in the analysis cohort, 1,027 (5.1%) had significant liver fibrosis at ART initiation including 616 (3.0%) with cirrhosis. Risk factors for significant fibrosis or cirrhosis included male sex, BMI <18, WHO clinical stage 3 or 4, CD4+ count <200 cells/mm3, and tuberculosis. Among the 237 (1.2%) who were tested, HBsAg-positive patients had four times the odds (adjusted odds ratio, 4.15; 95% CI, 1.71–10.04) of significant fibrosis compared HBsAg-negatives. Both significant fibrosis (adjusted hazard ratio 1.41, 95% CI, 1.21–1.64) and cirrhosis (adjusted hazard ratio 1.57, 95% CI, 1.31–1.89) were associated with increased all-cause mortality.
Conclusion
Liver fibrosis may be a risk factor for mortality during ART among HIV-infected individuals in Africa. APRI is an inexpensive and potentially useful test for liver fibrosis in resource-constrained settings.
Keywords: HIV/AIDS, Africa, liver disease, AST-to-platelet ratio index, FIB-4, hepatitis B virus
Introduction
Liver disease has become a leading cause of death for HIV-infected patients on antiretroviral therapy (ART) in upper-income settings [1,2]. However, in sub-Saharan Africa (SSA), less is known regarding the epidemiology of liver disease, largely due to limited availability of diagnostic tests for viral hepatitis and for hepatic fibrosis. Although hepatitis B virus (HBV) is an endemic cause of liver disease in SSA, few HIV treatment programs have implemented routine screening for HBV because of cost [3]. Liver biopsy – the gold standard diagnostic test for staging liver disease – is generally unavailable in SSA; it is also invasive and prone to sampling error.
As an alternative to liver biopsy to stage hepatic fibrosis, non-invasive tests have been recently developed and validated. Two indirect blood fibrosis markers, AST-to-platelet ratio index (APRI) and FIB-4, as well as an ultrasound-based imaging modality, called transient elastography, were included in the 2014 World Health Organization (WHO) guidelines for management of hepatitis C virus (HCV) in resource-limited settings [4]. These tests have been validated in HCV, HIV-HCV co-infection, HBV, and HIV-HBV co-infection, and have been used in published studies of HIV mono-infection in North America, Asia, and Europe [5–9]. Using thresholds reported in the WHO guidelines, the accuracy of APRI and FIB-4 ranges from 40–90%, depending on the population and the threshold selected. To date these tests have not been widely investigated in SSA, particularly in relationship to patient outcomes. In a large cohort of HIV-infected Zambian adults, we used the APRI score to estimate the prevalence of hepatic fibrosis and cirrhosis. We then investigated the association of APRI at the time of ART initiation with subsequent all-cause mortality.
Materials and methods
We analyzed routine program data from a well-characterized cohort of HIV-infected adults receiving antiretroviral therapy in public-sector facilities in Zambia’s capital Lusaka [10]. We included HIV-infected patients who were 16+ years old and initiated ART during 2006–2008 when both aspartate aminotransferase (AST) and platelet count were used in routine ART monitoring. Patients were eligible for ART if they had WHO stage 4 disease, a CD4+ count of <200 cells/mm3, or WHO stage 3 disease with a CD4+ count <350 cells/mm3. During the period of the study, first-line ART regimens comprised stavudine or zidovudine plus lamivudine with either nevirapine or efavirenz. In July 2007, tenofovir became the preferred first-line nucleotide reverse transcriptase inhibitor and stavudine and zidovudine were gradually phased out [11]. Patients were excluded from analysis if the AST or platelet count results from the time of ART initiation were not available. The remaining patients comprised the analysis cohort.
At our reference laboratory, the upper limit of normal was 38 U/L for AST and 33 U/L for ALT. When liver disease was suspected based on physical examination or serum transaminases, patients were screened for HBV co-infection using a hepatitis B surface antigen serologic assay (HBsAg; Access 2 Analyzer, Beckman Coulter Inc.). Testing for HBV DNA and hepatitis B e antigen were not available. As it is rare in Zambia [12], hepatitis C virus was not tested for in this cohort. Tuberculosis (TB) co-infection was documented when a patient initiated ART during treatment with anti-tuberculosis medications. After initiation of ART, follow-up visits for clinical examinations and drug refills took place at 2, 6, and 12 weeks, and then every 3–6 months, based upon clinical status. All-cause mortality was ascertained by report of a family member, clinic staff member, or community health worker. Specific causes of death were not available.
To assess liver disease at the time of ART initiation, we calculated each patient’s APRI score using its formula as follows: {AST (in U/L)/upper limit of normal} / platelet count (expressed as platelets × 109/L) X 100 [5]. In our primary analysis, we dichotomized APRI at ≥1.5, an established threshold for significant hepatic fibrosis (Metavir stages F2–F4). We also considered APRI ≥2.0, a validated threshold for cirrhosis (Metavir stage F4).
We compared the characteristics of patients excluded from analysis for missing AST or platelet results with those in the analysis cohort using Wilcoxon rank sum tests for continuous variables and Chi square tests for categorical ones. Among patients in the analysis cohort, using logistic regression, we modeled possible factors associated with significant fibrosis at ART initiation including age, sex, body mass index (BMI), WHO clinical stage, CD4+ count, and tuberculosis treatment status. Using Kaplan-Meier analysis and Cox proportional hazards regression, we examined the association between significant fibrosis and all-cause mortality during ART. Cox models were adjusted for confounders that predicted mortality in prior analyses of this cohort as follows: age, sex, WHO clinical stage, BMI, CD4+ count, and ART regimen. These analyses were then repeated for cirrhosis (i.e., APRI ≥2.0).
Although HBsAg testing was not routine in the cohort, among tested patients, we explored the association between HBV co-infection and APRI score using logistic regression adjusted for age, sex, BMI, WHO clinical stage, and CD4+ count. Tuberculosis is a common co-infection in our program and its treatment may cause elevated serum transaminases and increased APRI scores. In a sensitivity analysis, we repeated models after exclusion of TB patients to investigate the robustness of the primary results.
Although the focus of this analysis was on APRI, we also calculated FIB-4 scores using its formula as follows: {age (in years)*AST (in U/L)} / {platelet count (109/L)*ALT (in U/L)1/2} [13]. We dichotomized FIB-4 at ≥3.25, a validated threshold for significant liver fibrosis. Using the kappa statistic, we assessed the level of concordance between APRI and FIB-4 at thresholds for significant fibrosis.
We also investigated APRI thresholds that accurately exclude the presence of significant fibrosis (APRI ≤0.5) and cirrhosis (APRI ≤1.0). Using Kaplan-Meier analysis we assessed the association of APRI score below these thresholds with subsequent survival. We used Stata version 12 (Statacorp, College Station, Texas) for statistical analysis. The Biomedical Research Ethics Committee of University of Zambia School of Medicine (Lusaka, Zambia) and the Institutional Review Board of University of North Carolina at Chapel Hill (Chapel Hill, North Carolina, USA) approved our use of de-identified program data without patient informed consent.
Results
From 1 July 2006 to 30 Jun 2008, 27,309 HIV-infected adults initiated ART across 22 public-sector facilities in Lusaka. After the exclusion of those with missing AST and/or platelet counts at baseline, 20,308 (74.3%) patients remained in the analysis cohort. Patients with available laboratory results were more likely to be male (40.7% vs. 35.9%), have WHO stage 3 or 4 disease (65.8% vs. 57.5%), CD4+ count <100 cells/mm3 (34.3% vs. 27.6%), and tuberculosis co-infection (15.4% vs. 10.8%) compared to those with missing results (all P<0.001). Within the analysis cohort median age was 34 years (interquartile range [IQR], 29–40) and 59% were women (Table 1). The median APRI score was 0.35 (IQR, 0.23–0.57). According to APRI score 1,027 (5.1%) had significant fibrosis (APRI ≥1.5) including 616 (3.0%) had cirrhosis (APRI ≥2.0) at ART initiation.
Table 1.
Demographic, clinical, and treatment characteristics of the cohort, by the degree of hepatic fibrosis based on the AST-to-platelet ratio index
| APRI <1.5 No significant fibrosis (n=19,281) |
APRI ≥1.5 Significant fibrosis (n=1,027) |
P | |
|---|---|---|---|
| Age, years | 34 (29–40) | 34 (30–39) | <0.001 |
| Male sex | 7,771 (40) | 621 (59) | <0.001 |
| WHO clinical stage | |||
| 1 or 2 | 6,734 (35) | 180 (18) | <0.001 |
| 3 or 4 | 12,441 (65) | 846 (82) | |
| Body mass index | |||
| >25 | 1,679 (9) | 40 (4) | <0.001 |
| 18–25 | 11,868 (63) | 551 (56) | |
| <18 | 5,201 (28) | 402 (40) | |
| Tuberculosis | 2,899 (15) | 238 (23) | <0.001 |
| CD4+ count, cells/mm3 | |||
| >200 | 6,326 (34) | 146 (15) | |
| 100–200 | 6,024 (33) | 260 (27) | <0.001 |
| <100 | 6,089 (33) | 568 (58) | |
| ART regimen | |||
| NRTI | |||
| D4T+3TC | 6,962 (36) | 447 (44) | <0.001 |
| AZT+3TC | 5,702 (30) | 214 (21) | |
| TDF+XTC | 6,490 (34) | 360 (35) | |
| NNRTI | |||
| NVP | 13,528 (70) | 554 (54) | <0.001 |
| EFV | 5,753 (30) | 473 (46) | |
All values are median (IQR) or number (%).
Abbreviations: APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; WHO, World Health Organization; NRTI, nucleotide reverse transcriptase inhibitor; NNRTI, non-nucleotide reverse transcriptase inhibitor; D4T, stavudine; 3TC, lamivudine; XTC, either lamivudine or emtricitabine; AZT, zidovudine; TDF, tenofovir; NVP, nevirapine; EFV, efavirenz; ART, antiretroviral therapy
In multivariable analysis, men were twice as likely as women to have APRI ≥1.5 (adjusted odds ratio [AOR], 1.99; 95% confidence interval [CI], 1.72–2.28). Other factors associated with significant fibrosis included BMI <18 (AOR, 1.41; 95% CI, 1.00–1.99), WHO clinical stage 3 or 4 (AOR, 1.85; 95% CI, 1.54–2.22), tuberculosis co-infection (AOR, 1.18; 95% CI, 1.00–1.39), and CD4+ count <100 cells/mm3 (AOR, 3.44; 95% CI, 2.84–4.18; Table 2).
Table 2.
Factors associated with having either significant hepatic fibrosis or cirrhosis, based on the AST-to-platelet ratio index, at ART initiation in Zambia
| APRI ≥1.5 (Significant fibrosis) | Crude OR (95% CI) |
Adjuste OR (95% CI) |
|---|---|---|
| Age, per 10-year increase | 1.03 (0.97–1.10) | 0.95 (0.88–1.03) |
| Male sex | 2.22 (1.96–2.52) | 1.98 (1.72–2.28) |
| BMI | ||
| >25 | Reference | Reference |
| 18–25 | 1.93 (1.40–2.66) | 1.25 (0.90–1.74) |
| <18 | 3.21 (2.32–4.44) | 1.41 (1.00–1.99) |
| WHO clinical stage 3 or 4 | 2.50 (2.12–2.93) | 1.82 (1.52–2.18) |
| Tuberculosis | 1.71 (1.48–1.99) | 1.20 (1.02–1.41) |
| CD4+ count, cells/mm3 | ||
| >200 | Reference | Reference |
| 100–200 | 1.87 (1.52–2.29) | 1.84 (1.49–2.27) |
| <100 | 4.03 (3.36–4.85) | 3.44 (2.84–4.18) |
| APRI ≥ 2.0 (Cirrhosis)
| ||
| Age, per 10-year increase | 0.98 (0.89–1.07) | 0.89 (0.81–0.99) |
| Male sex | 2.22 (1.89–2.61) | 2.04 (1.70–2.43) |
| BMI | ||
| >25 | Reference | Reference |
| 18–25 | 1.95 (1.27–2.98) | 1.26 (0.81–1.97) |
| <18 | 3.67 (2.39–5.63) | 1.59 (1.01–2.50) |
| WHO clinical stage 3 or 4 | 2.50 (2.03–3.07) | 1.76 (1.39–2.22) |
| Tuberculosis | 1.82 (1.51–2.19) | 1.26 (1.03–1.55) |
| CD4+ count, cells/mm3 | ||
| >200 | Reference | Reference |
| 100–200 | 1.91 (1.46–2.50) | 1.87 (1.42–2.46) |
| <100 | 4.28 (3.37–5.45) | 3.47 (2.70–4.46) |
Abbreviations: AST, aspartate aminotransferase; ART, antiretroviral therapy; OR, odds ratio; BMI, body mass index; WHO, World Health Organization
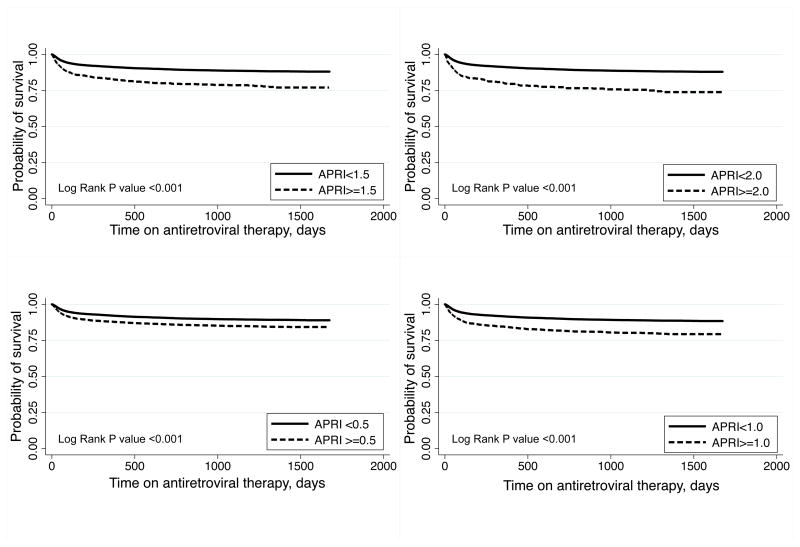
During a total of 49,058 person-years on ART, 2,238 (11.0%) of patients died for an all-cause mortality rate of 45.6 per 1000 person-years. Stratified by APRI score at ART initiation, the crude mortality rates per 1000 person-years were 39.3 for APRI <0.5, 49.9 for APRI 0.5–1.0, 74.9 for APRI 1.0–1.5, 71.1 for APRI 1.5–2.0, and 116.4 for APRI >2.0. In Kaplan-Meier analysis, significant fibrosis and cirrhosis were associated with increased mortality (both Log Rank P<0.001; Figure 1). When compared to patients with APRI <1.5, those with APRI ≥1.5 had an increased hazard of death (adjusted hazard ratio [AHR], 1.41; 95% CI, 1.21–1.64), adjusted for age, sex, BMI, WHO clinical stage, CD4+ count, and ART regimen (Table 3). Those with APRI ≥2.0 also experienced increased mortality (AHR, 1.57; 95% CI, 1.31–1.89) compared to those with APRI <2.0.
Figure 1.
Association of AST-to-platelet ratio index (APRI) at antiretroviral therapy initiation with survival among HIV-infected Zambians
Table 3.
Association of hepatic fibrosis at ART initiation with all-cause mortality among HIV-infected Zambian adults
| Crude AHR (95% CI) |
Adjusted AHR (95% CI) |
|
|---|---|---|
| Hepatic fibrosis level | ||
| APRI <1.5 (No significant fibrosis) | Reference | Reference |
| APRI ≥ 1.5 (Significant fibrosis) | 2.08 (1.80–2.40) | 1.41 (1.21–1.64) |
| Age, per 10-year increase | 1.11 (1.06–1.16) | 1.10 (1.05–1.16) |
| Male sex | 1.46 (1.34–1.58) | 1.28 (1.17–1.40) |
| WHO clinical stage 3 or 4 | 2.29 (2.06–2.54) | 1.61 (1.44–1.81) |
| Body mass index | ||
| >25 | Reference | Reference |
| 18–25 | 1.67 (1.35–2.06) | 1.30 (1.05–1.62) |
| <18 | 3.75 (3.03–4.64) | 2.20 (1.76–2.74) |
| CD4+ count, cells/mm3 | ||
| >200 | Reference | Reference |
| 100–200 | 1.36 (1.20–1.54) | 1.30 (1.14–1.47) |
| <100 | 2.66 (2.39–2.97) | 2.04 (1.81–2.29) |
| ART regimen | ||
| NRTIs | ||
| D4T+3TC | Reference | Reference |
| AZT+3TC | 0.43 (0.39–0.49) | 0.51 (0.45–0.57) |
| TDF+XTC | 0.77 (0.70–0.85) | 0.82 (0.74–0.91) |
| NNRTI | ||
| NVP | Reference | Reference |
| EFV | 1.16 (1.06–1.26) | 0.85 (0.77–0.94) |
Abbreviations: ART, antiretroviral therapy; WHO, world health organization; AHR, adjusted hazard ratio; NRTI, nucleotide reverse transcriptase inhibitor; NNRTI, non-nucleotide reverse transcriptase inhibitor; D4T, stavudine; 3TC, lamivudine; XTC, either lamivudine or emtricitabine; AZT, zidovudine; TDF, tenofovir; NVP, nevirapine; EFV, efavirenz
Within the analysis cohort, only 237 (1.2%) patients were tested for HBsAg. Among that subset, 49 (20.7%) were HBsAg-positive, and those with positive tests had four times the odds (AOR, 4.15; 95% CI, 1.71–10.04) of significant fibrosis compared with HBsAg-negative individuals, adjusted for age, sex, CD4+ count, and tuberculosis co-infection.
The median FIB-4 score in the cohort was 1.06 (IQR, 0.71–1.64) and 1,315 patients (6.5%) had significant fibrosis determined by FIB-4 ≥3.25. At thresholds for significant fibrosis, 96.0% of patients were staged concordantly by FIB-4 and APRI (Kappa=0.636; P<0.001; Table 4). Similar patient characteristics were associated with FIB-4 ≥3.25 as observed in the APRI analysis. In Cox survival models, patients with FIB-4 ≥3.25 experienced increased all-cause mortality during ART (AHR, 1.44; 95% CI, 1.25–1.65).
Table 4.
Percentage agreement between APRI and FIB-4 scores among HIV-infected Zambian adults initiating ART
| FIB-4 | APRI, n (%) of patients
|
|
|---|---|---|
| ≤1.5 | >1.5 | |
| ≤ 3.25 | 18,735 (92.2) | 258 (1.3) |
| >3.25 | 546 (2.7) | 769 (3.8) |
Abbreviations: APRI, AST-to-platelet ratio index; ART, antiretroviral therapy
In a sensitivity analysis that excluded patients with tuberculosis, we observed that the mortality hazard ratio for significant fibrosis was similar to that of the primary analysis (data not shown). At lower thresholds, patients with APRI ≤0.5 and APRI ≤1.0 experienced reduced mortality in Kaplan-Meier analysis (both Log Rank P value <0.001; Figure 1).
Discussion
Based on APRI – an easy to calculate marker of liver disease – approximately 5% of HIV-infected Zambian adults had significant hepatic fibrosis or cirrhosis at ART initiation. These patients were at much higher risk for mortality, with increases of up to 50%. This highlights the need for liver disease screening at the time of ART initiation, along with targeted interventions designed to reduce morbidity and improve survival in HIV care centers in resource-constrained settings.
Although non-invasive tests are commonly used to stage liver disease in upper-income settings, this analysis is one of the first to investigate APRI and FIB-4 in an HIV treatment program in SSA. When the Zambian Ministry of Health scaled up ART, a strong commitment was made to laboratory monitoring which allowed us to investigate these markers in more than 20,000 patients receiving treatment. The large size of the cohort allowed us to identify factors associated with significant fibrosis and cirrhosis in our program. These results are likely similar to other cohorts in the region.
We also note several limitations. APRI and FIB-4 are imperfect surrogates of hepatic fibrosis and have not been validated in HIV-infected individuals in SSA. Although they have relatively high positive predictive value at thresholds for significant fibrosis and cirrhosis, sensitivity can be low [4]. In addition, ascertainment of death was incomplete in our program, and some deceased patients were likely misclassified as lost to follow-up. We do not believe this outcome misclassification would have altered our results because the likelihood that a death was documented was unrelated to APRI score. Lack of data on alcohol and herbal medicine use and low rates of HBsAg testing were other limitations to our analysis of fibrosis risk factors.
HBsAg-tested patients in our cohort were selected population according to clinical and laboratory triage; therefore, the strong association we observed between HBsAg-positivity and APRI cannot be generalized to all HIV-HBV co-infected individuals in this population. HIV infection causes thrombocytopenia; therefore, elevated APRI could have been caused by untreated advanced HIV disease and not liver disease. Evidence against this is a meta-analysis where APRI had similar diagnostic accuracy in HCV patients regardless of HIV status [14]. Finally we note that – although TB can cause granulomatous hepatitis [15] – anti-TB drugs could have confounded the observed association between APRI and mortality through elevation of AST. After exclusion of TB patients in a sensitivity analysis, we observed a similar association between elevated APRI and mortality.
Based on the APRI score, approximately 5% of HIV-infected patients in Zambia had significant fibrosis, similar to the 3–8% reported in published studies in upper-income settings [6,16,17]. FIB-4 results were similar, and we observed substantial agreement between FIB-4 and APRI, consistent with another report [17]. However, at thresholds used in our and other studies, the burden of significant fibrosis was underestimated with these markers. Liver disease might be more common in SSA compared with upper-income settings due to environmental factors such as aflatoxin ingestion and traditional herbal medication [18]. In Uganda and Nigeria 10% and 17% of HIV-infected adults had significant fibrosis based on transient elastography, a non-invasive fibrosis test that has greater sensitivity than APRI and FIB-4 [19,20]. Herbal medication use was associated with liver stiffness measurements in rural Uganda [18]. Together these data suggest that HIV-infected individuals in SSA may have a substantial burden of liver disease and further investigation in this field is necessary [21].
Predictors of significant hepatic fibrosis in our cohort included male sex, BMI <18, and low CD4+ count. Men were probably more likely to have fibrosis due to HBV and heavy alcohol use, two factors that are more common among men than women in our setting [22]. Along with alcohol and HCV, chronic HBV is the leading cause of cirrhosis and hepatocellular cancer worldwide. Consistent with our data, 30% of HIV-HBV co-infected patients in a U.S. cohort had significant fibrosis, based on the APRI score [16]. Although it is an important risk factor for chronic liver disease in upper-income settings, overweight/obesity is uncommon in our HIV program, and we did not find BMI >25 to be associated with fibrosis. Low BMI, which was associated with elevated APRI, is not an established cause of fibrosis, but it may increase fibrosis in the setting of alcoholic liver disease [23]. In settings like ours longitudinal studies are needed to better understand the impact of malnutrition on liver disease. Our finding that low CD4+ count was associated with fibrosis was consistent with at least one study in the U.S. [16] although the mechanism for this is poorly understood.
Our data suggest that elevated APRI score is associated with poor outcomes among HIV-infected individuals in Zambia. While we were unable to ascertain liver end points such as hepatocellular carcinoma or hepatic failure, we observed that patients with APRI ≥1.5 experienced increased all-cause mortality during ART, independent of other mortality risk factors. This supports published data from Europe and North America where liver disease has become a leading cause of mortality for HIV-infected patients receiving ART [1,2].
Non-invasive hepatic fibrosis tests such as APRI are needed in SSA because biopsy is invasive and rarely available outside of large urban hospitals [21]. The APRI score could be incorporated into HIV clinical care in resource-constrained settings as it relies on inexpensive and readily available blood tests and can be determined in real time by simple calculation, similar to the creatinine clearance for renal function. A triage strategy could be developed, utilizing both high and low APRI thresholds, to identify patients requiring further investigation or treatment. The score may also be useful to monitor patients at high risk for liver disease such as those with chronic viral hepatitis or excess alcohol consumption. In conclusion, our findings – that 1 in 20 Zambian HIV patients had significant hepatic disease and hepatic disease predicts mortality during ART – support the need for future research into the role of non-invasive fibrosis tests in HIV treatment programs in SSA.
Key Points.
Among 20,308 HIV-infected adults in Lusaka, Zambia, 5.1% had significant liver fibrosis (Metavir stage F2–F4) and 3.0% had cirrhosis (Metavir stage F4) determined by AST-to-platelet ratio index (APRI) at the time of antiretroviral therapy (ART) initiation.
Risk factors for significant fibrosis included male sex, body mass index <18, WHO clinical stage 3 or 4, CD4+ count <200 cells/mm3, tuberculosis, and hepatitis B virus co-infection.
Significant fibrosis and cirrhosis were associated with increased all-cause mortality during ART.
Non-invasive tests for liver fibrosis, particularly low-cost ones such as APRI, should be further investigated for routine use in HIV treatment programs in Africa.
Acknowledgments
Financial support:
Research reported in this publication was supported by the Fogarty International Center (K01TW009998 and R25TW009340) and the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK066144) of the National Institutes of Health, and by an Ambizione-PROSPER fellowship (PZ00P3_154730) from the Swiss National Science Foundation.
List of abbreviations
- ART
antiretroviral therapy
- SSA
sub-Saharan Africa
- HBV
hepatitis B virus
- APRI
AST-to-platelet ratio
- WHO
World Health Organization
- HCV
hepatitis C virus
- AST
aspartate aminotransferase
- HBsAg
hepatitis B surface antigen
- TB
tuberculosis
- BMI
body mass index
Footnotes
Conflict of interest:
M.W.F. serves as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck. All other authors report no conflicts of interest.
References
- 1.Weber R, Sabin C, Friis-Moller N. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 2.Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. Journal of hepatology. 2005;42(6):799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 4.Organization WH. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 5.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 6.Dallapiazza M, Amorosa VK, Localio R, Kostman JR, Re VL. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC infectious diseases. 2010;10(1):116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. Journal of hepatology. 2005;43(1):78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Moodie EE, Pai NP, Klein MB. Is antiretroviral therapy causing long-term liver damage? A comparative analysis of HIV-mono-infected and HIV/hepatitis C co-infected cohorts. PLoS One. 2009;4(2):e4517. doi: 10.1371/journal.pone.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamadnejad M, Montazeri G, Fazlollahi A, Zamani F, Nasiri J, Nobakht H, et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. The American journal of gastroenterology. 2006;101(11):2537–2545. doi: 10.1111/j.1572-0241.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 10.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 11.Chi BH, Mwango A, Giganti MJ, Sikazwe I, Moyo C, Schuttner L. Comparative outcomes of tenofovir-based and zidovudine-based antiretroviral therapy regimens in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2011;58:475–481. doi: 10.1097/QAI.0b013e31823058a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapembwa KC, Goldman JD, Lakhi S, Banda Y, Bowa K, Vermund SH, et al. HIV, hepatitis B, and hepatitis C in Zambia. J Glob Infect Dis. 2011;3(3):269–274. doi: 10.4103/0974-777X.83534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterling RK, Lissen E, Clumeck N, Sola R, Correas MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 14.Nunes D, Fleming C, Offner G, O’brien M, Tumilty S, Fix O, et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;40(5):538–544. doi: 10.1097/01.qai.0000184856.31695.bf. [DOI] [PubMed] [Google Scholar]
- 15.Ocama P, Katwere M, Piloya T, Feld J, Opio KC, Kambugu A, et al. The spectrum of liver diseases in HIV infected individuals at an HIV treatment clinic in Kampala, Uganda. African health sciences. 2008;8(1) [PMC free article] [PubMed] [Google Scholar]
- 16.Price JC, Seaberg EC, Badri S, Witt MD, D’acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. Journal of Infectious Diseases. 2012;205(6):1005–1013. doi: 10.1093/infdis/jir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendeni M, Foca E, Gotti D, Ladisa N, Angarano G, Albini L, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without Hepatitis C and B infection. Clin Infect Dis. 2011;52(9):1164–1173. doi: 10.1093/cid/cir071. [DOI] [PubMed] [Google Scholar]
- 18.Auerbach BJ, Reynolds SJ, Lamorde M, Merry C, Kukunda-Byobona C, Ocama P, et al. Traditional herbal medicine use associated with liver fibrosis in rural Rakai, Uganda. PLoS One. 2012;7(11):e41737. doi: 10.1371/journal.pone.0041737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Boaz I, Ndyanabo A, et al. High prevalence of liver fibrosis associated with HIV infection: a cross-sectional study in rural Rakai, Uganda. Antiviral therapy. 2011;16(3):405. doi: 10.3851/IMP1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agbaji O, Ugoagwu P, Murphy R, Thio C, Ani C, Okafo C, et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus co-infection: Nigeria. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly P, Saloojee H, Chen JY, Chung RT. Noncommunicable diseases in HIV infection in low-and middle-income countries: gastrointestinal, hepatic, and nutritional aspects. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;67:S79–S86. doi: 10.1097/QAI.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musukuma K, Zanolini A, Mulenga LB, Sikazwe I, Bolton-Moore C, Mweemba A, et al. Increased hepatitis B virus co-infection screening following a change in Zambian HIV guidelines. Conference on Retroviruses and Opportunistic Infections; 2014; Boston, USA. 2014. [Google Scholar]
- 23.Bosma A, Seifert WF, Van Thiel-De Ruiter GCF, Van Leeuwen RE, Blauw B, Roholl P, et al. Alcohol in combination with malnutrition causes increased liver fibrosis in rats. Journal of hepatology. 1994;21(3):394–402. doi: 10.1016/s0168-8278(05)80319-8. [DOI] [PubMed] [Google Scholar]