Abstract
Purpose
Adolescent sleep restriction (SR) is common and can lead to overeating. Here we test whether lengthening sleep via early bedtimes affects dietary intake differently for adolescents accustomed to a later sleep phase (“night owls”) versus an earlier sleep phase (“morning larks”).
Methods
Using a randomized cross-over design, 67 adolescents changed bedtimes to create 5-night periods of SR (6.5 hours in bed) versus healthy sleep (HS; 10 hours in bed). Caloric intake was measured via validated interviews. Phase preference was based on participants' pre-manipulation sleep.
Results
Actigraphy verified that the manipulation altered sleep regardless of phase preference. Phase preference moderated the effect of the manipulation on cumulative caloric intake (p=.01-.03). Night owls showed little effect, but morning larks reduced their evening intake during HS.
Conclusions
An “early to bed” approach confers little dietary benefit for night owls, but may have a protective effect for adolescents who gravitate towards earlier bedtimes.
Introduction
Sleep experts have increasingly pointed toward chronic sleep restriction (SR) as a contributor to obesity during adolescence, and have called for interventions to lengthen adolescent sleep [1]. Although most studies linking adolescent sleep and diet have used correlational data, we recently published experimental findings that show a causal link between SR and the eating behavior of adolescents [2, 3]. Specifically, healthy adolescents consumed ∼10% more calories after several nights of SR than when well-rested.
Sleep timing also appears to be important. Adolescents with a later circadian preference (“night owls”) tend to have poorer quality diets than those with an earlier circadian preference (“morning larks”), though studies disagree on how much this is attributable to differences in sleep duration versus metabolic factors [4, 5]. Metabolic processes follow circadian patterns, and experimentally inducing a misalignment between adults' internal clocks and external stimuli can cause metabolic disturbances [6]. If a similar effect is present in adolescents, it would have implications for public health interventions to lengthen adolescents' sleep. For example, an early-to-bed approach might have more dietary benefits for morning larks than for night owls (because early bedtimes mismatch their internal clocks), even if both sleep longer.
Here we re-examine data from our previously-published experiments to test whether interventions that primarily target bedtimes might have a differential effect on caloric intake depending on adolescents' circadian preferences. We hypothesized that adolescents who entered our study with a preference for an earlier sleep phase would show a greater response to our bedtime-based sleep manipulation than those who entered with a later sleep phase.
Methods
Procedures were approved by the local Institutional Review Board and explained verbally and in writing to participants and their parents. Data were collected during summer breaks to prevent an adverse effect of SR on school performance.
Participants
As previously detailed [2, 3], participants were recruited from flyers posted throughout a regional care network. We enrolled typically-developing 14-17 year-olds without a history of neurological illness/injury, current psychiatric diagnosis, intellectual disability, or marked obesity (BMI>30).
Three-Week Sleep Protocol
During the baseline week, participants self-selected their bedtimes, but were asked to awaken daily at a time that would allow them to come to our office for an 8:30 am visit. That wake time was maintained throughout the study (no “sleeping in” on weekends) to minimize shifts in circadian rhythm. Adolescents who successfully completed the baseline then changed their bedtimes to match two 5-night sleep conditions, in randomly counterbalanced order: SR (6.5 hours in bed) versus Healthy Sleep (HS; 10 hours in bed). The conditions were separated by a 2-night washout with the same sleep instructions as the baseline. Two nights of adequate sleep fully normalizes caloric intake in sleep-deprived adults[7] and eliminated carryover effects in our prior papers [2, 3]. Adolescents were asked to refrain from napping and to limit caffeine intake.
Outcome Measures
All sleep occurred at home and was monitored via objective actigraphy (MicroMotionlogger Sleep Watch, Ambulatory Monitoring, Inc.). Assessments were conducted the morning immediately after SR and HS. Actigraphy data were reviewed with the teen and parent to verify accuracy, then passed through a validated algorithm [8] to determine sleep onset, offset, and duration. Initial sleep phase preference was operationalized as the midpoint of each adolescent's sleep period during the pre-manipulation baseline.
Caloric intake was measured at each visit using the USDA Multiple Pass Method, previously validated against doubly labeled water[9] and weighed diet diaries.[10] Trained, condition-blinded interviewers interviewed adolescents regarding food consumed over the previous 24 hours, using handouts to help estimate portion sizes. To minimize reactance effects, a variety of neurobehavioral outcomes were assessed during visits, with dietary interviews comprising ∼10% of the time. Caloric intake was tallied in five 4-hour blocks (6-10 am, 10-2 pm, 2-4 pm, 4-10 pm, 10-2 am; intake from 2-6 am was negligible) and then modeled as a cumulative function to allow for simultaneous examination of both overall intake and its relative timing.
Results and Discussion
Of 88 randomized participants, 3 had unclear baseline sleep phase due to actigraph failure, 4 dropped out, 9 were nonadherent to the sleep protocol, and 5 were outliers who reported a single-day intake >4000 calories. The final 67 participants (61% female) were 15.7±1.0 years old and did not differ from dropped participants in age or sex. During baseline, they averaged 7.00±0.98 hours of sleep (12:06 am - 7:06 am). They averaged 2.52 hours more sleep during HS (8.96±0.78) than SR (6.44±0.58), p<.0001; the size of that gap was uncorrelated with their initial sleep phase preference, r=-.18, p>.05. As planned, adolescents had earlier sleep onset during HS (10:15 pm) than SR (12:44 am), p<.0001, but rise times were similar (7:12 vs 7:09 am; p>.05).
Linear mixed models tested for differences in caloric intake as a function of sleep condition and time of day (both entered as repeated measures), testing the potential moderating effect of several between-subjects factors: sleep phase preference, order of conditions, race (Caucasian vs. non-Caucasian), age, or sex. As shown in Table 1, when entered individually, sleep phase preference and race moderated the relationship between time and sleep condition. However, phase preference remained the only significant moderator in analyses that concurrently entered both.
Table 1. Effect of potential moderators of the impact of the sleep manipulation on dietary intake.
| Potential Moderator Variable | (A) Each Moderator Entered Individually | (B) All Moderators Entered Simultaneously | (C) Circadian Preference and Race Entered Simultaneously |
|---|---|---|---|
| Circadian Preference | F(4,103)=3.47, p=.01 | F(4,97.4) = 3.17, p=.02 | F(4,101) = 2.87, p=.03 |
| Race (Caucasian vs. Non-Caucasian) | F(4,103)=2.90, p=.03 | F(4,97.4) = 2.24, n.s. | F(4,101) = 2.29, n.s. |
| Order of Experimental Conditions | F(4,103) = 1.16, n.s. | F(4,97.4) = 1.11, n.s. | |
| Sex | F(4,104) = 1.60, n.s. | F(4,97.4) = 2.26, n.s. | |
| Age | F(4,103) = 0.52, n.s. | F(4,97.4) = 0.44, n.s. |
Note: n.s. = not significant (p>.05). Values reflect tests of the moderator-by-time-by-condition interactions, when tested with no other moderators in the model (column A), when all moderators were entered in the model at once (column B), and in a “trimmed” model (column C) that entered only the two variables found individually to be significant.
As illustrated in Figure 1, the caloric intake of adolescents who entered the study as night owls was unaffected by the sleep manipulation, with intake steadily rising through the evening before leveling off. Adolescents who entered the study as morning larks showed a similar intake pattern during SR. However, starting in the evening hours, morning larks ate less when in the HS condition. This protective effect of longer sleep emerged before their sleep onset, suggesting a circadian effect and not simply diminished opportunity to eat. Although we did not assess metabolic markers, our findings fit with a metabolic model derived from the adult literature, in which sleep promotes metabolic health primarily if it matches individuals' internal clocks [6]. We speculate that correlations of adolescent chronotypes with diet [4, 5] reflect not just differences in sleep duration, but also the mismatch between early school start times with the internal clocks of adolescent night owls.
Figure 1.
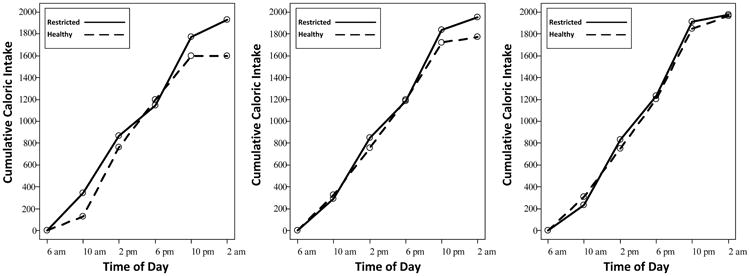
Cumulative caloric intake as a function of experimental condition and time of day within three circadian preferences. Although circadian preference was modeled as a continuous variable in analyses, for illustration purposes this figure presents findings discretely for morning larks (left panel), intermediate circadian preference (middle panel), and night owls (right panel). These values correspond to the following fall-asleep and rise time times at baseline: morning larks, 11:12 pm/6:48 am; average midsleep group, 12:06 am/7:06 am; night owls, 1:03 am/7:27 am.
Limitations of this study include the use of midsleep as a proxy for circadian phase; a dim light melatonin onset protocol would have offered more precision. Because our protocol focused on earlier bedtimes to lengthen sleep, it also remains unclear whether waking later might have a differential benefit for night owls [4]. Finally, present data do not speak to whether other outcomes, such as mood or attention, are differentially affected by SR among adolescents with earlier versus later sleep phase preferences.
On a practical level, our SR condition was similar to the sleep duration of many adolescents on school nights, which experts believe is insufficient [1]. Suggestions for lengthening sleep range from earlier bedtimes to later rise times (e.g., later school start). Present findings suggest that an “earlier to bed” approach (e.g., via sleep hygiene education, parental limit-setting) can lengthen sleep in night owls, but without an impact on dietary behaviors. Instead, the dietary benefit appears most prominent among adolescents who are more accustomed to earlier bedtimes. When lengthening adolescents' sleep, approaches that focus on earlier bedtimes without allowing later awakening have potential health benefits, but these may be incomplete.
Implications and Contribution.
Experts have suggested that lengthening adolescents' sleep could protect against overeating, but the best approach remains unclear. Findings indicate that approaches focused on earlier bedtimes may have an incomplete effect, benefiting primarily those who already prefer an earlier sleep phase, and failing to change the dietary intake of “night owls.”
Acknowledgments
Funding support from the National Institutes of Health, grant numbers R01 HL092149 and UL1 RR026314, though the NIH was not directly involved in study design, data collection, analyses, interpretation, or manuscript preparation. Present findings were presented orally at the SLEEP 2014 meeting in Minneapolis, MN. None of the authors have any conflicts of interest to report. The initial draft of the paper was prepared by Dr. Beebe. Dr. Rausch assisted with data analyses, interpretation, and manuscript preparation and revision. Ms. Noe and Drs. Zhou and Simon assisted with data collection, interpretation and manuscript revision.
Abbreviations
- SR
Sleep Restriction
- HS
Healthy Sleep
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owens JG Adolescent Sleep Working, and A. Committee on. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–32. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beebe DW, et al. Dietary intake following experimentally restricted sleep in adolescents. SLEEP. 2013;36:827–34. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon SL. Field J. Miller LE. DiFrancesco M. Beebe DW. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0115434.e0115434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golley RK, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond) 2013;37(4):546–51. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 5.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes (Lond) 2015;39(1):39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 6.Gonnissen HK, et al. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr. 2012;96(4):689–97. doi: 10.3945/ajcn.112.037192. [DOI] [PubMed] [Google Scholar]
- 7.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36(7):981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–4. doi: 10.1016/S0002-8223(96)00293-3. [DOI] [PubMed] [Google Scholar]
- 10.Klesges RC, et al. Validation of the 24-hour dietary recall in preschool children. J Am Diet Assoc. 1987;87(10):1383–5. [PubMed] [Google Scholar]


