Abstract
Background
Transoral robotic-assisted oncologic surgery of the head and neck offers promising functional results. Nonetheless, the efficacy of oncologic surgery remains critically dependent on obtaining negative margins. We aimed to integrate a miniaturized high resolution fiberoptic microendoscope (HRME), which provides real time histological assessment, with the da Vinci robotic system (Intuitive Surgical Inc., Sunnyvale, CA).
Methods
Three patients undergoing transoral robotic surgery were prospectively enrolled. Optical imaging of the oropharynx was performed intraoperatively with the robotic-assisted HRME.
Results
All patients underwent the procedure successfully with no complications. The HRME was successfully integrated with the Da Vinci Robotic system. Several sites of the oropharynx and associated malignancy were imaged, which correlated with the standard histopathological analysis.
Conclusions
Transoral robotic-assisted high resolution microendoscopic imaging of the oropharynx is a safe and technically feasible approach, providing a real time histological assessment and may serve as a valuable aid in oncologic surgery.
Keywords: Transoral robotic surgery, optical imaging, HRME, oropharynx, microendoscopy, squamous cell carcinoma
Introduction
Recently, the role of transoral robotic surgery (TORS) in the management of oropharyngeal squamous cell carcinoma (OPSCC) has gained acceptance due to its excellent functional and oncologic outcomes, obviating the need for traditional transcervical and transmandibular open approaches in selected cases.(1-6) Consequently, surgical treatment has gained importance as a component of the multidisciplinary care for patients with oropharyngeal squamous cell carcinoma (OPSCC). Obtaining clear (negative) margins remains indispensable for a positive oncologic outcome, necessary to maximize survival and reduce recurrence.(7, 8) Failure to do so constitutes an adverse prognostic factor, requiring subsequent adjuvant therapy, which has an impact on patient’s quality of life.(9, 10) In contrast, extensive resection can lead to serious functional deficiencies and treatment associated morbidity – mortality. Hence, the capacity to define tumoral margins with a high degree of accuracy is critical for maximizing the efficacy of surgical treatment and the patient’s subsequent quality of life. Technical aspects of transoral robotic surgery make this goal difficult to achieve, notably, the inability to palpate the tumor due to lack of haptic feedback and issues related to access and orientation of specimen inherent to the retractor system, optical cavity, and robotic instrumentation. Current standard of care involves careful margin assessment with “frozen section” analysis and surgeon controlled inking of specimens at the time of surgery. (11, 12) Although intraoperative “frozen section” analysis of surgical margins is a valuable adjunct to oncologic surgery, the method is costly, time-consuming, and discrepancies between frozen section margins and final pathology are common. (13-15)
Image-guided oncologic surgery is an emerging area of research, where several optical imaging modalities have been proposed to improve intraoperative delineation of tumor margins(16). The ability to establish an immediate, real-time microendoscopic diagnosis that is consistent with the histologic diagnosis is the ultimate objective of the field. The goal of microendoscopic imaging is multifold. Not only does it enable prediction of histology, it allows real-time visualization of the epithelium at a subcellular level of resolution. The high-resolution microendoscope (HRME) is a novel noninvasive imaging modality that utilizes a flexible fiberoptic probe to obtain images in situ and in real time of tissue stained with a topical fluorescent nuclear contrast agent, allowing visualization of epithelial architecture and cellular morphology,(17-20) Furthermore, we have previously described and validated this device for the detection of head and neck squamous cell carcinoma (HNSCC) ex vivo, revealing a sensitivity and specificity of 98% and 92% respectively.(21, 22)
We aimed to integrate the miniaturized fiberoptic probe of the HRME with the Da Vinci robotic system (Intuitive Surgical Inc., Sunnyvale, CA) to evaluate the feasibility of intraoperative optical imaging during TORS.
Materials and Methods
Three patients diagnosed with tonsillar OPSCC that were to undergo transoral robotic surgery were prospectively enrolled under a Mount Sinai Hospital (09-2057) and Rice University (09-166E) Institutional Review Board-approved study. Inclusion criteria for the study consisted of age ≥ 18 years, regardless of sex, race, or ethnicity, with biopsy proven squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx and larynx that were to undergo surgical resection. Written informed consent was obtained from all patients before surgery. Patients were deemed appropriate for TORS after evaluation by the multidisciplinary tumor board at Mount Sinai Head and Neck Cancer Center.
Imaging System
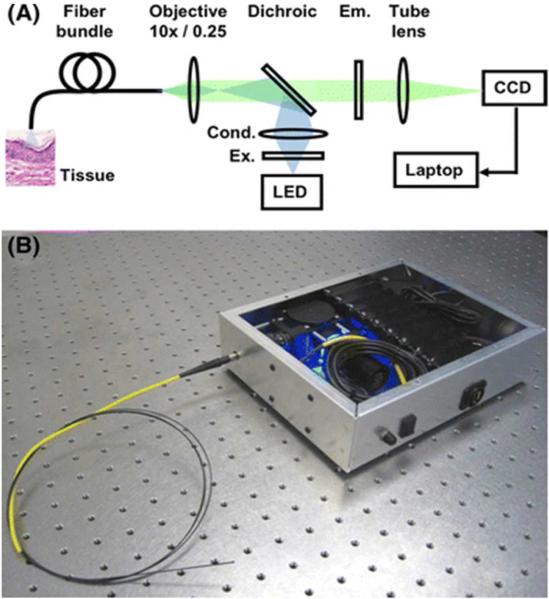
Technical details of the HRME have been described in detail by Muldoon et al. (17, 18) The HRME device essentially operates as a fluorescence microscope coupled to a fiberoptic imaging probe (Fig.1). This probe consists of a 1 mm outer diameter fiber bundle (Fujikura, FIGH-30-850N), which is comprised of 30,000 optical fibers with a center-to-center spacing of 4 μm that allow a circular field of view of 720 μm, displaying images in real time at 12 frames per second. The cost of the device is less than $2500, with a probe that can be sterilized and reused. (23)After topical application of a fluorescent contrast (Proflavine hemisulfate 0.01%) agent, the probe is placed in contact with the mucosal surface, transmitting an image of the tissue back through the fiberoptic probe to a charged-coupled device (CCD) camera which is connected to a laptop computer for image capture and storage. A 0.01% solution of proflavine (Sigma-Aldrich, St Louis, MO) was used as the fluorescent contrast agent to label cell nuclei, in accordance to previous studies conducted by our group.(21, 22)
The HRME, shown in schematic (A) and fully assembled (B).
TORS
The TORS surgical technique has been described previously. (24)Succinctly, the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) consists of a surgeon’s console, a surgical cart, a manipulator unit with two laterally placed instrument arms and a centrally located endoscopic arm holding the three dimensional camera. The patient is placed in the supine position on an operating room table. Nasotracheal intubation allows for an unobstructed view of the oropharynx. Oral cavity retraction is achieved by using the Crowe Davis retractor, Dingman retractor (Omega Health Care, London, England) or the FK retractor (Gyrus Company, Maple grove, MN) and the da Vinci robot is positioned at a 30- to 45-degree angle to the operating table. The 0- or 30-degree high-magnification three-dimensional camera is inserted into the oral cavity followed by the positioning of the dual robotic arms. Imaging was performed using a 5mm 5Fr introducer instrument (Intuitive Surgical Inc., Sunnyvale, CA) that allows for flexible fiber delivery and manipulation of the HRME probe (Fig.2).

The fiberoptic probe of the HRME inserted through a 5FR Introducer (Intuitive Surgical Inc., Sunnyvale, CA) proximally (A), distally (B) and fully integrated in the robotic arm (C).
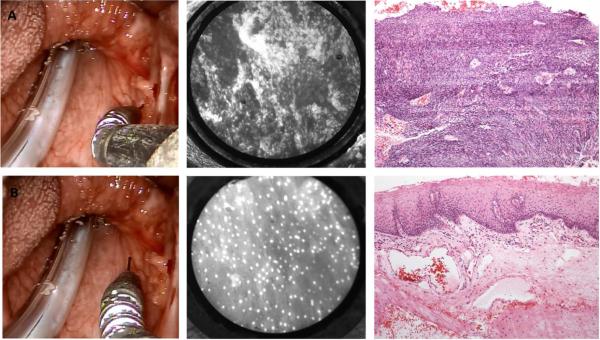
Using a cotton-tipped applicator prior to resection, proflavine hemisulfate was topically applied to the mucosal surface of previously determined tumor and adjacent normal sites of the oropharynx by clinical examination. Subsequently, the fiberoptic probe was introduced via a 5mm 5Fr introducer and placed robotically-assisted in gentle contact with the mucosa. HRME images were immediately acquired (Fig 3). Sample sections from each imaged site were obtained using a four millimeter punch biopsy immediately after imaging, then placed in formalin and submitted for histopathologic processing and analysis by a board-certified pathologist who used standard criteria and was blinded to the corresponding HRME image.
Transoral robotic-assisted microendoscopic imaging of the oropharynx with corresponding representative HRME and H&E histopathologic images from the oropharynx of squamous cell carcinoma (A) and benign mucosa (B).
HRME images were analyzed to identify imaging features of benign and malignant mucosa which correlate with histopathological diagnosis, including nuclear size and shape, nuclear density, and overall cellular architecture.
Results
Initial assessment of the technical feasibility of this robotic-assisted imaging method proved to be successful in all three cases. Several factors were evaluated, including contrast agent delivery, exposure and visualization of the tumor, maneuverability of the fiberoptic probe integrated with the robotic arm, image quality, motion artifact, length of the procedure and complications. Delivery of the contrast agent to the oropharyngeal mucosa was performed using cotton tipped applicators and was straight forward. However this required manipulation of the robotic arms which was time consuming, encouraging the need for a robotic contrast delivery system. Adequate exposure was achieved in all cases with proper visualization of the tumor and manipulation of the HRME probe was comparable to working with any other robotic instrument. As expected, the robot provided for excellent manipulation and precision of the fiberoptic probe, virtually eliminating any motion artifact and obtaining satisfactory image quality. Current available robotic instrumentation limited the size of the fiberoptic probe and consequently the field of view. No complications were reported and the length of the procedure was 4, 5 and 7 minutes in the three cases.
Figure 3 shows representative HRME images obtained intraoperatively and the corresponding histopathology of benign mucosa and invasive cancer of the oropharynx.
In all cases, HRME images obtained during surgery showed features that were consistent with histologic assessment and could be used to discriminate between benign mucosa and invasive cancer. Images of benign mucosa are characterized by nuclei of consistent, regular size which are evenly spaced. This contrasts with images of malignant mucosa, where enlarged, crowded nuclei distort the cellular architecture, corresponding to increased cellularity found in cancerous tissue.
Discussion
Although histopathologic evaluation remains the gold standard for discriminating between benign and malignant mucosa, the applications of a real time evaluation that can closely approximate histologic resolution are vast. With growing interest in minimally invasive surgical techniques in the head and neck, such as transoral robotic surgery (TORS)(1), advanced imaging modalities are likely to play an increasingly important role in accurately delineating margins, targeting biopsies and guiding minimally invasive surgery. The HRME is a simple, low-cost, portable and easily trainable diagnostic tool that has the ability to obtain real-time microscopic information (17, 21, 23), which can be successfully delivered via robotic instrumentation to the difficult to access anatomy of the upper aerodigestive tract.
A limitation of this technology is that it is restricted to the superficial mucosa. While excellent at determining benign vs. malignant tissue at the surface of the tumor, limited depth of penetration of roughly 50 μm makes it difficult to detect submucosal tumor spread and images may be incorrectly classified as normal, when tumor extends in a submucosal manner. The strong affinity of the proflavine contrast agent, for keratin can mask the underlying nuclei in heavily keratinized tissue, limiting the ability to interpret the images obtained. Additionally, imaging of the deep muscle margins with this technology remains unexplored and is the subject of ongoing investigation by our research group. Strategies, which permit greater depth of penetration and the interrogation of deep margins, are active areas of ongoing research.
Future research in alternative targeted contrast agents that highlight specific markers overexpressed in head and neck squamous cell carcinoma, such as epidermal growth factor receptor (EGFR) or human papilloma virus (HPV), may allow for selective visualization of cancer cells with optical imaging technology.(25, 26)
Another issue to consider, specific to minimal-access surgery, is that the field-of-view of the microendoscope is inherently limited by the size of the probe’s distal end and this, by the diameter of the instrument utilized for its delivery. Smaller probes result in a smaller field-of-view and can make it difficult for users to obtain a broad sense of tissue morphology. This may be addressed by current algorithms for real-time video mosaicing that has emerged as an effective technique to increase the acquired image size.(27)
In conclusion, we demonstrated that a novel, low cost microendoscopy device can be safely and successfully used to acquire high-quality, high-resolution images of cellular morphology and architecture in real time of the oropharynx during transoral robotic surgery. With advances in optical technology and novel delivery systems, this innovative technique may serve as a valuable adjunct to ablative oncologic surgery, potentially improving tumoral margin discrimination and oncologic outcomes.
Acknowledgements
This project was funded in part by the National Cancer Institute (NCI) Bioengineering Research Partnerships (BRP) Grant 2RO1CA103830-06A1
References
- 1.Genden EM, Kotz T, Tong CC, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121(8):1668–74. doi: 10.1002/lary.21845. [DOI] [PubMed] [Google Scholar]
- 2.Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009;31(3):283–9. doi: 10.1002/hed.20972. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein GS, Quon H, Newman HJ, et al. Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch Otolaryngol Head Neck Surg. 2012;138(7):628–34. doi: 10.1001/archoto.2012.1166. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MA, Weinstein GS, O'Malley BW, Jr., Feldman M, Quon H. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck. 2011;33(4):573–80. doi: 10.1002/hed.21500. [DOI] [PubMed] [Google Scholar]
- 5.Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. 2012;87(3):219–25. doi: 10.1016/j.mayocp.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmon J, Quon H, Gourin CG. The effect of transoral robotic surgery on short-term outcomes and cost of care after oropharyngeal cancer surgery. Laryngoscope. 2013 doi: 10.1002/lary.24358. [DOI] [PubMed] [Google Scholar]
- 7.Haque R, Contreras R, McNicoll MP, Eckberg EC, Petitti DB. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord. 2006;6:2. doi: 10.1186/1472-6815-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binahmed A, Nason RW, Abdoh AA. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol. 2007;43(8):780–4. doi: 10.1016/j.oraloncology.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 10.Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113(10):2704–13. doi: 10.1002/cncr.23898. [DOI] [PubMed] [Google Scholar]
- 11.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27(11):952–8. doi: 10.1002/hed.20269. [DOI] [PubMed] [Google Scholar]
- 12.Gandour-Edwards RF, Donald PJ, Wiese DA. Accuracy of intraoperative frozen section diagnosis in head and neck surgery: experience at a university medical center. Head Neck. 1993;15(1):33–8. doi: 10.1002/hed.2880150108. [DOI] [PubMed] [Google Scholar]
- 13.Black C, Marotti J, Zarovnaya E, Paydarfar J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107(12):2792–800. doi: 10.1002/cncr.22347. [DOI] [PubMed] [Google Scholar]
- 14.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110:1773–6. doi: 10.1097/00005537-200010000-00039. 10 Pt 1. [DOI] [PubMed] [Google Scholar]
- 15.Ord RA, Aisner S. Accuracy of frozen sections in assessing margins in oral cancer resection. J Oral Maxillofac Surg. 1997;55(7):663–9. doi: 10.1016/s0278-2391(97)90570-x. discussion 669-71. [DOI] [PubMed] [Google Scholar]
- 16.Keereweer S, Sterenborg HJ, Kerrebijn JD, Van Driel PB, Baatenburg de Jong RJ, Lowik CW. Image-guided surgery in head and neck cancer: current practice and future directions of optical imaging. Head Neck. 2012;34(1):120–6. doi: 10.1002/hed.21625. [DOI] [PubMed] [Google Scholar]
- 17.Muldoon TJ, Pierce MC, Nida DL, Williams MD, Gillenwater A, Richards-Kortum R. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15(25):16413–23. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muldoon TJ, Anandasabapathy S, Maru D, Richards-Kortum R. High-resolution imaging in Barrett's esophagus: a novel, low-cost endoscopic microscope. Gastrointest Endosc. 2008;68(4):737–44. doi: 10.1016/j.gie.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce MC, Vila PM, Polydorides AD, Richards-Kortum R, Anandasabapathy S. Low-cost endomicroscopy in the esophagus and colon. In: Am J Gastroenterol. United States. 2011:1722–4. doi: 10.1038/ajg.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy LL, Jiang N, Smouha E, Richards-Kortum R, Sikora AG. Optical imaging with a high-resolution microendoscope to identify cholesteatoma of the middle ear. Laryngoscope. 2013;123(4):1016–20. doi: 10.1002/lary.23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vila PM, Park CW, Pierce MC, et al. Discrimination of benign and neoplastic mucosa with a high-resolution microendoscope (HRME) in head and neck cancer. Ann Surg Oncol. 2012;19(11):3534–9. doi: 10.1245/s10434-012-2351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy LL, Vila PM, Park RW, et al. High-Resolution Optical Imaging of Benign and Malignant Mucosa in the Upper Aerodigestive Tract: An Atlas for Image-Guided Surgery. ISRN Minim Invasive Surg. 2012;2012 doi: 10.5402/2012/364285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regunathan R, Woo J, Pierce MC, et al. Feasibility and preliminary accuracy of high-resolution imaging of the liver and pancreas using FNA compatible microendoscopy (with video) Gastrointest Endosc. 2012;76(2):293–300. doi: 10.1016/j.gie.2012.04.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai SC, Sung CK, Jang DW, Genden EM. Transoral robotic surgery using a carbon dioxide flexible laser for tumors of the upper aerodigestive tract. Laryngoscope. 2008;118(12):2187–9. doi: 10.1097/MLG.0b013e31818379e4. [DOI] [PubMed] [Google Scholar]
- 25.Ke S, Wen X, Gurfinkel M, et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63(22):7870–5. [PubMed] [Google Scholar]
- 26.Soukos NS, Hamblin MR, Keel S, Fabian RL, Deutsch TF, Hasan T. Epidermal growth factor receptor-targeted immunophotodiagnosis and photoimmunotherapy of oral precancer in vivo. Cancer Res. 2001;61(11):4490–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Bedard N, Quang T, Schmeler K, Richards-Kortum R, Tkaczyk TS. Real-time video mosaicing with a high-resolution microendoscope. Biomed Opt Express. 2012;3(10):2428–35. doi: 10.1364/BOE.3.002428. [DOI] [PMC free article] [PubMed] [Google Scholar]