Abstract
Background
The functional lumen imaging probe (FLIP) measures luminal cross-sectional area and pressure during volumetric distension. By applying novel customized software to produce FLIP topography plots, organized esophageal contractility can be visualized and analyzed. We aimed to describe the stimulus thresholds and contractile characteristics for distension-induced esophageal body contractility using FLIP topography in normal controls.
Methods
Ten healthy controls were evaluated during endoscopy with FLIP. During stepwise bag distension, simultaneous intra-bag pressure and luminal diameter measurements were obtained and exported to a MatLab program to generate FLIP topography plots. The distension volume, intra-bag pressure, and maximum esophageal body diameters were measured for the onset and cessation of repetitive antegrade contractions (RACs). Contraction duration, interval, magnitude, and velocity were measured at 8 and 3-cm proximal to the EGJ.
Results
Eight of ten subjects demonstrated RACs at a median onset volume of 29 ml (IQR, 25 - 38.8), median intra-bag pressure of 10.7 mmHg (IQR, 8.6 - 15.9), and median maximum esophageal body diameter of 18.5 mm (IQR, 17.5 - 19.6). Cessation of RACs occurred prior to completion of the distension protocol in three of the eight subjects exhibiting RACs. Values of the RAC-associated contractile metrics were also generated to characterize these events.
Conclusions
Distension-induced esophageal contractions can be assessed utilizing FLIP topography. RACs are a common finding in asymptomatic controls in response to volume distention and have similar characteristics to secondary peristalsis and repetitive rapid swallows.
Keywords: esophageal motility; functional lumen imaging probe; impedance planimetry, secondary peristalsis
Introduction
The functional lumen imaging probe (FLIP) is a commercially available device for the assessment of esophageal geometry during volumetric distention (EndoFLIP™, Crospon Inc, Galway, Ireland). By utilizing multiple closely-spaced impedance planimetry channels within a balloon, the FLIP measures luminal cross-sectional areas at discreet locations along the length of the measurement segment during controlled volumetric distension. When combined with measurement of the intra-bag pressure, distensibility can be calculated. The FLIP has primarily been used within the esophagus to evaluate the distensibility of the esophagogastric junction (EGJ) and to inform treatment in achalasia patients (1-5). Demonstration of abnormal distensibility of the esophageal body utilizing the FLIP in eosinophilic esophagitis patients has also shown promise in its ability to prognosticate food impaction risk or the need for dilation therapy (6, 7).
We recently developed customized software to convert high-resolution impedance planimetry into FLIP topography plots in order to display data as a space-time-luminal diameter continuum during volumetric distention (8). Our initial analysis focused on defining regional differences along the length of the esophagus in distensibility by filtering out vascular and respiratory activity and also cancelling out esophageal contractions in order to focus on the passive mechanical properties of the esophageal wall. However, in the process of that data analysis we observed esophageal contractions occurring in response to distension that we suspected to be the equivalent of contractions previously described (sometimes termed reactivity) using single measure impedance planimetry (9-11). Additionally, we observed propagating contractions that appeared to be a form of peristalsis elicited by volumetric distention.
Since the essential function of the esophagus is to transport and clear bolus from the esophagus, we hypothesized that the FLIP could help define the normal contractile response to volumetric distention and that this could have clinical relevance as a new measure of esophageal function. The FLIP is uniquely suited for this evaluation as it provides simultaneous measurement of luminal diameters and pressure during controlled distension through the body of the esophagus and EGJ. Initial reports on FLIP utilized an assembly with an 8-cm FLIP balloon. However, the recent introduction of a 16-cm balloon has allowed for the concurrent evaluation of the EGJ and the esophageal body with the advantage of improved spatial resolution. Hence, the aim of this experiment was to define the esophageal contractile response to volumetric distention in healthy control subjects using FLIP topography. In addition, we sought to quantify FLIP measures of contractility in a format similar to manometry.
Methods
Subjects
Ten asymptomatic, healthy volunteers (ages 20-49; 6 female) were studied. These subjects have been previously described (12). After a ≥ 6-hour fast, all subjects underwent upper endoscopy that excluded hiatal hernia, esophagitis, stricture, and/or mucosal changes suggestive of eosinophilic esophagitis. None of the subjects had a history of malignancy or gastrointestinal surgery. Informed consent was obtained from each subject and the study protocol was approved by the Northwestern University Institutional Review Board.
Functional lumen imaging probe system and study protocol
The FLIP assembly consisted of a 240-cm long, 3-mm outer diameter catheter with an infinitely compliant bag (up to a distension volume of 60 mL) mounted on the distal 18 cm of the catheter. The bag housed 17 ring electrodes spaced 1 cm apart and a solid-state pressure transducer positioned at the distal end of the bag to provide simultaneous measurement of 16 channels of CSA and intra-bag pressure. The bag was tapered at both ends to assume a 16-cm long cylindrical shape that formed the impedance planimetry segment. The impedance planimetry segment had a minimum-to-maximum range of measureable CSA within the infinitely compliant range of 21 - 380 mm2; assuming the lumen cross-sections are circular, this corresponded to a diameter of 5.2 - 22 mm. Values greater than 380 mm2 (22-mm diameter) could be measured, but mechanical properties of the bag would be engaged above this limit. Measurements from the impedance planimetry electrode pairs and the pressure transducer were sampled at 10 Hz with the data acquisition system and transmitted to the recording unit.
Subjects underwent upper endoscopy in the left lateral decubitus position. Moderate sedation with 5-10 mg midazolam and 50-200 μg fentanyl was administered during the procedure. The FLIP probe was placed transorally and positioned with the distal 1-3 impedance sensors distal to the EGJ as confirmed by demonstration of a waist in the impedance planimetry segment at a bag distension volume of 20-30 ml. The endoscope was withdrawn before initiation of the FLIP study protocol. Simultaneous CSAs and intra-bag pressures were measured during 5 ml step-wise distensions beginning with 5 ml and increasing to 60 ml. Each distension volume was maintained for 10 - 20 seconds. The recording unit was set to stop infusing and display an alarm message if the intra-bag pressure exceeded 60 mmHg to avoid unintended dilation.
Data analysis
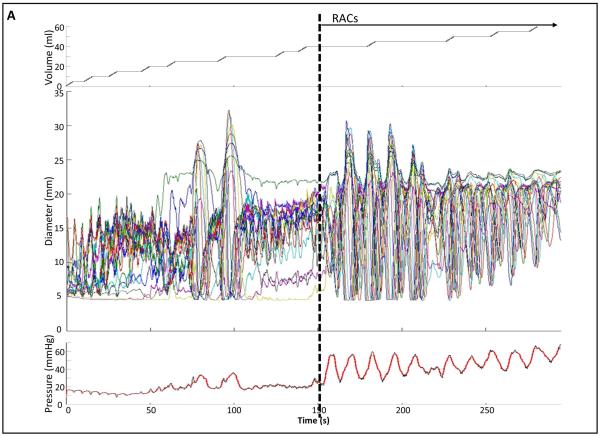
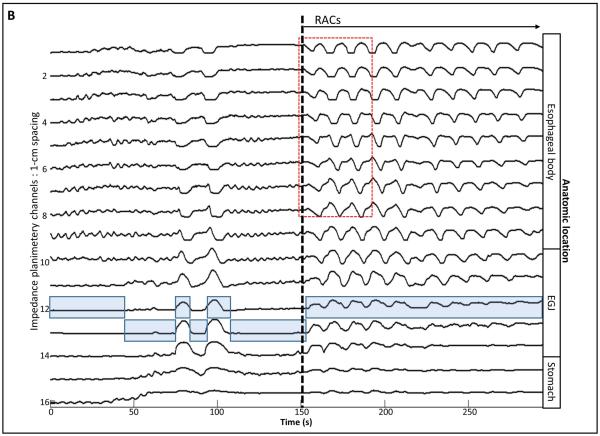
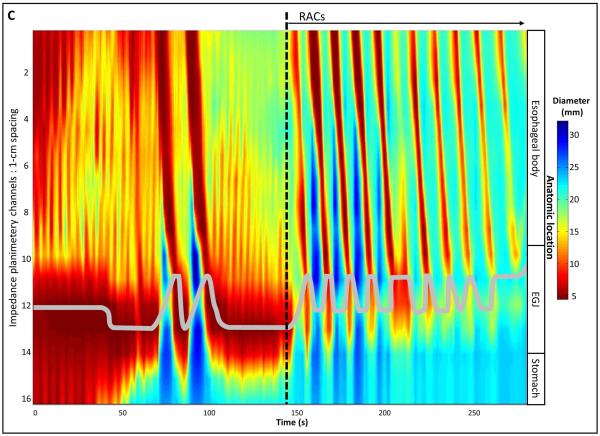
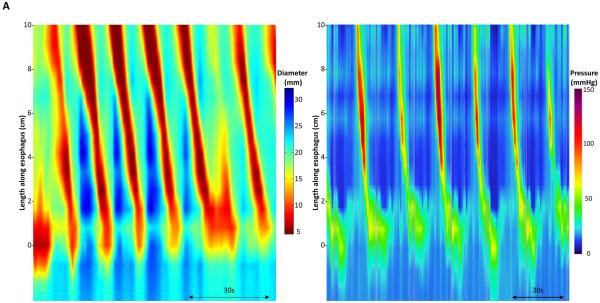
Data including distension volume, intra-bag pressure, and 16 channels of CSA measurements (via impedance planimetry) for all studies were exported to MATLAB (The Math Works, Natick, MA, USA) for further analysis using a customized MATLAB programs. This program filtered vascular and respiratory artifact, and generated tracings of each channel’s measured luminal diameter and also generated topography plots of the interpolated luminal diameters (Figure 1). Additionally, the program identified the EGJ-midline by searching for the minimal CSA of the distal six impedance planimetry channels.
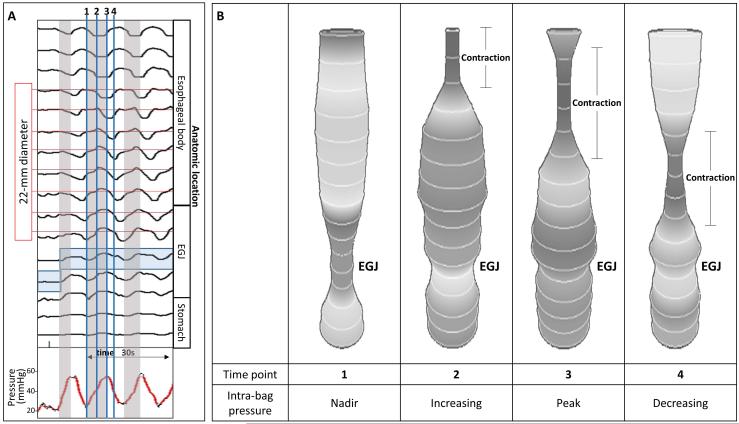
Figure 1. Example of FLIP topography.
Data output generated by the customized program over the course of the study protocol from a single subject are displayed: 1A) Distension volume (top), 16-channels of luminal diameters (middle), and intra-bag pressure (bottom), 1B) 16 channels of diameter changes are represented as line tracings as the measurement channels are now axially located along the esophagus, and 1C) topographic representation of interpolated diameter changes using a color scale. The esophagogastric (EGJ) midline is represented by the superimposed blue boxes in 1B and gray line in 1C. The initiation of repetitive, antegrade contractions (RACs) is illustrated with vertical dashed lines. In this subject, RACs onset occurs at a distension volume of 40-ml and continues through the end of the study protocol. The section within the red box (1B) is enlarged in Figure 2. EGJ – esophagogastric junction. Figure used with permission from the Esophageal Center at Northwestern.
Esophageal contractions were identified by a transient decrease of ≥5 mm in luminal diameter detected in ≥2 adjacent axial impedance planimetry channels. A threshold of 5 mm was established to avoid measurement of residual vascular and respiratory fluctuations. Contractions were classified as antegrade or retrograde and considered repetitive when ≥3 occurred consecutively without interruption. Presence or absence of repetitive antegrade contractions (RACs) as well as RAC cessation were recorded as dichotomous variables. Distension volume, intra-bag pressure, and maximum esophageal body diameter (measured 2-10 cm proximal to the EGJ midline) were measured at the times of onset and cessation of RACs.
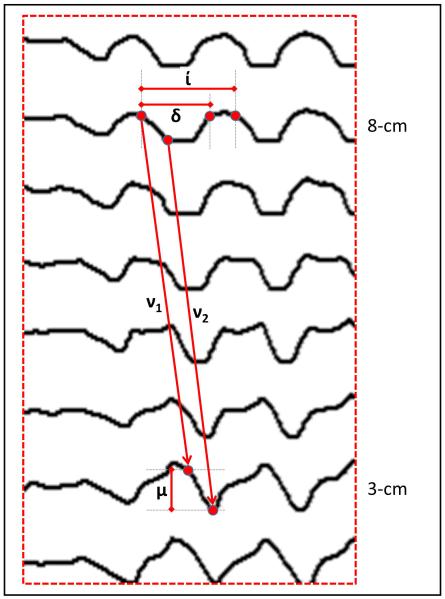
RAC characteristics were further analyzed at loci corresponding to 3 and 8-cm proximal to the EGJ (5 and 10 impedance planimetry channels above the EGJ-midline) with measurements of contraction duration, interval, and magnitude, as illustrated in Figure 2. Additionally, contraction wave velocities were measured using the slope of the lines both from the start of contractions (v1) and from the nadir diameters (v2) (Figure 2). Finally, the contraction-associated change in intra-bag pressure was measured as the difference in nadir and peak pressure associated with a contraction (Figure 3). These metrics were applied to the first five RACs (initial RACs) and, if present, to RACs continuing at 50 ml distension (late RACs).
Figure 2. FLIP topography metrics.
Metrics were measured at the impedance planimetry channels at 5 and 10 cm above the EGJ midline to represent the esophageal body 3 and 8 cm proximal to the EGJ. Contraction duration (δ) was defined as the time from the time of initial decrease in diameter to the time of diameter return to baseline. Contraction interval (í) was the duration between the start of consecutive, repetitive contractions. Contraction magnitude (μ) was the change in diameter from baseline to the nadir diameter. Contraction wave velocity was measured: 1) as the slope of the line from the onset of the contraction (v1), and 2) from the onset of the minimal contraction diameters (v2). Figure used with permission from the Esophageal Center at Northwestern.
Figure 3. Contraction-associated intra-bag pressure changes.
A) Intra-bag pressure (bottom) and 16-channel diameter changes of the first three repetitive antegrade contractions (RACs) in the same subject depicted in Figures 1 and 2. The gray-shaded boxes indicate times during which the intra-bag pressure is increasing. The 22-mm diameter limit is indicated by the horizontal red lines. The shaded blue box represents the esophagogastric (EGJ) midline. The blue vertical lines indicate the time points depicted in Figure 3B. B) Screen shot images taken during the FLIP study representing the bag diameters at time points related to contraction-associated intra-bag pressures. The increase in pressure occurred during periods of decreasing diameter (contraction) in the proximal impedance channels (representing the mid-esophageal body), but before the contraction proximal to the EGJ (where diameters increased, often to greater than 22 mm, as intra-bag pressure increased). Increasing pressure also coincided with increasing diameter within the EGJ. Figure used with permission from the Esophageal Center at Northwestern.
Statistical analysis
Numerical results were expressed as median and interquartile range (IQR). Intra-subject comparison of contraction metrics was made between the initial and late RACs to assess for changes during the course of the protocol using the Wilcoxon signed ranks test. Measures were considered statistically different at a p-value <0.05.
Results
Repetitive contraction thresholds
Repetitive antegrade contractions were observed in 8/10 (80%) subjects; RAC onset occurred at a median (IQR) distension volume of 29 ml (25-35.8), intra-bag pressure of 10.7 mmHg (8.6-15.9), and maximum esophageal body diameter of 18.5 mm (17.5-19.6). All subjects demonstrating RACs exhibited a preceding period of disorganized esophageal contractility with or without single or paired propagating contractile waves similar to the example in Figure 1. Similar disorganized esophageal contractility was observed in the two subjects that did not develop RACs. Three of the eight subjects with RACs had cessation of RACs prior to the end of the distension protocol. One subject had onset/cessation of RACs at distension volumes of 20/30 ml, pressures of 9.6/10.2 mmHg and maximum esophageal body diameters of 17.9/21.4 mm. The second had RAC onset/cessation at volumes of 30/60 ml, pressures of 8.38/21.1, and maximum esophageal body diameters of 17.7/21.8 mm. The third subject had RAC onset/cessation at volumes of 35/40 ml, pressures of 13.9/15.8, and maximum esophageal body diameters of 18.2 /19.7mm.
Contraction characteristics
Metrics for contractile properties were obtained for a total of 66 RACs: 39 initial (first five) RACs from eight subjects and 27 late (at the 50-ml distension volume) RACs from six subjects. Initial and late RACs were compared to assess for changes in metrics during the course of the distension protocol (Table 1). In the six subjects that exhibited both, the late RACs demonstrated decreased contraction duration at 8-cm, but not 3-cm proximal to the EGJ. There was also decreased contraction magnitude for late contractions, although this marginally missed achieving significance at the site 3-cm proximal to the EGJ. The contraction intervals and velocities were unchanged between initial and late RACs.
Table 1.
Summary of repetitive antegrade contraction (RAC) metrics. IQR – interquartile range.
| All RACs | Initial RACs | Late RACs | ||||||
|---|---|---|---|---|---|---|---|---|
| Location* | Median | IQR | Median | IQR | Median | IQR | P-value | |
| Duration (s) | 8 cm | 5.4 | 4.9 - 6.2 | 5.9 | 5.0 - 6.5 | 5.4 | 4.9 - 7.3 | 0.04 |
| 3 cm | 4.9 | 4.2 - 5.6 | 5.0 | 4.2 - 6.2 | 4.6 | 4.3 - 6.2 | 0.35 | |
| Interval (s) | 8 cm | 8.7 | 6.3 - 10.2 | 8.3 | 6.1 - 9.3 | 9.9 | 8.2 - 12.0 | 0.23 |
| 3 cm | 8.6 | 6.8 - 10 | 8.8 | 6.7 - 9.9 | 10.0 | 8.2 - 11.6 | 0.89 | |
| Magnitude (mm) | 8 cm | 9.9 | 6.9 - 12.8 | 10.9 | 6.8 - 12.8 | 9.4 | 7.7 - 11.9 | 0.08 |
| 3 cm | 9.4 | 8.2 - 11.2 | 11.1 | 8.4 - 14.2 | 7.9 | 7.0 - 10.2 | 0.04 | |
| Velocity1 (cm/s) | 1.6 | 1.4 - 2.4 | 1.8 | 1.4 - 2.4 | 1.5 | 1.1 - 1.7 | 0.14 | |
| Velocity2 (cm/s) | 2.0 | 1.7 – 4.0 | 1.9 | 1.4 - 4.0 | 1.8 | 1.5 - 2.4 | 0.23 | |
|
Pressure change
(mmHg) |
20.8 | 7.9 - 22.7 | 18.4 | 6.0 - 22.2 | 22.6 | 18.4 - 28.1 | 0.23 | |
|
Peak pressure
(mmHg) |
42.2 | 21.1 - 53.4 | 34.8 | 15.9 - 45.8 | 53.9 | 45.5 - 62.1 | 0.03 | |
Measurement location proximal to the esophagogastric junction. 1Wilcoxon signed ranks test was used to compare initial versus late RACS (p-value).
Contraction-associated pressures
Median intra-bag pressure increases associated with RACs was 20.8 mmHg, (IQR 7.9-22.7 mmHg). Comparison of pressure changes associated with initial RACs (18.4 mmHg, IQR, 6.0-22.2 mmHg) and late RACs (22.6 mmHg, IQR, 18.4-28.1 mmHg) did not demonstrate a significant difference (p = 0.225). The median peak pressures observed during all RACs was 42.2 mmHg (IQR, 21.1-53.4 mmHg); the median peak intra-bag pressures measured with initial RACs (34.8 mmHg, IQR, 15.9-45.8) were lower than those with late RACs (53.9 mmHg, IQR, 45.5-62.1) (p = 0.028). The timing of pressure changes relative to regional contraction is depicted in Figure 3. The bag diameter increased to greater than 22 mm (the diameter limit below which the bag compliance was infinite) in six of the eight subjects with RACs; hence, pressure increases in these subjects likely represent some contribution of the bag properties.
Discussion
Using the FLIP with a 16-cm length balloon spanning the distal esophageal body and EGJ and incorporating a specialized program to generate FLIP topography plots, we observed organized, repetitive antegrade contractions (RACs) in the esophageal body of healthy control subjects during volumetric distension. Because FLIP topography localizes the EGJ throughout the study, we were able to characterize these contractions based on their location relative to the EGJ. The majority, though not all, of the subjects demonstrated RACs during the study protocol. We generated threshold values for development of distension-induced RACs, as well as developed and report RAC-related metrics including contraction duration, magnitude, and interval, for normal controls. Our results suggest that these antegrade contractions represent a peristaltic response to sustained volumetric distention, likely secondary peristalsis.
Although this is the first report utilizing FLIP topography to assess peristalsis, impedance planimetry has previously been used to study esophageal contractile in response to distension. These prior studies evaluated unsedated subjects using a device housing a single impedance planimetry measurement channel and pressure sensor within a bag that was 4-5 cm in length and 30-50 mm in diameter, along with proximal and distal pressure sensors (9, 10, 13, 14). The bag was positioned within the esophagus and study protocols involved bag pressure-graded distension. Although these methodological differences limit comparisons with our study, the threshold pressures reported for induction of secondary peristalsis (means of 11.5 and 13.9 mmHg) (9, 13) were similar to the median pressure we report for RAC onset (10.7 mmHg). A diameter threshold for inducing secondary peristalsis was not reported in previous studies. However, the FLIP findings we report (median diameter 18.5 mm) was similar to previous studies using the combination of manometry and intra-esophageal balloon distension reporting induction of secondary peristalsis with balloon diameters of 20-25 mm (15, 16).
Differences in devices, protocols, and metrics may limit the comparison of absolute values of contractile metrics reported in this FLIP study with previous studies of secondary peristalsis assessed using a single impedance planimetry sensor and/or manometry. However, our measured contraction magnitudes and durations were comparable to analogous measures from most previous studies (9, 10, 13). Reported differences generally arose in studies that identified variations in contraction parameters with increasing distension. Discrepant with our findings, longer (manometrically measured) contraction durations (up to 13 s) and greater contraction magnitudes (up to 26 mm) were reported to occur with increased distension (9, 10, 13, 14, 17). The reduced contraction magnitude at higher distension volumes observed in our study is likely related to using non-compressible fluid, as opposed to compressible air, for distension. The peristaltic velocities we measured (median 1.6-2.0 cm/s) were slightly slower than previous reports of secondary peristalsis, with means or medians ranging from approximately 2.2–3.5 cm/s (15, 18-20). Finally, one study previously reported contraction frequencies (analogous to our contraction interval) increasing to slightly greater than 6 contractions/minute, very similar to the contraction interval of 9–10 s seen in our study. However, while they reported increased contraction frequencies at higher balloon pressures, our contraction interval remained constant throughout the distension protocol. It is also worth noting that the contraction interval of 8–10 seconds is similar to the duration of deglutitive inhibition reported with multiple swallows (21, 22). However, previous reports of the effect of peristaltic inhibition on secondary peristalsis are inconsistent (23, 24).
While the potential for assessing peristalsis with FLIP is appealing, the current FLIP assembly and protocol are designed to assess distensibility resulting in some limitations. One limitation is in the assessment of the intra-bag pressure. With the current device, the single pressure sensor was positioned in the stomach. Hence, while the intra-bag pressure is accurate when the EGJ was open, this location may limit accuracy when the EGJ is closed, such as early in the distension protocol at low distension volumes. Another limitation related to a single distal pressure sensor is an inability to determine regional differences in mechanical wall state (e.g. auxotonic contraction, auxotonic relaxation, isometric contraction, passive dilatation), as occur during the four phases of esophageal bolus transit and peristalsis (9, 13, 25). Incorporating additional pressure sensors and using a greater bag diameter may help overcome these limitations in future iterations of the FLIP device optimized for the assessment of esophageal function.
Another limitation of our study protocol was the potential effect of midazolam and fentanyl on RACs. Although these may alter the magnitude and propagation of contractions, there was no obvious effect and further work will be required to see if there are dose-dependent changes in the contraction magnitude or propagation. Additionally, since the patient was sedated, we cannot confidently exclude the possibility of some primary peristalsis occurring. Incorporation of a swallow-detection device, such as surface electromyography (EMG) sensors, could help in that regard.
Despite the limitations related to pressure measurement and sedation, the application of FLIP topography represents a novel method for the assessment of esophageal function in response to distension that may complement esophageal manometry, which does not typically evaluate secondary peristalsis. In addition to providing a method for assessment of esophageal sphincter and wall distensibility, FLIP topography allows one to assess whether RACS occur and at what threshold pressure, diameter, and volume. We speculate that certain disease states may be associated with a loss of RAC generation and that this could have diagnostic and/or therapeutic implication. Manometrically-assessed secondary peristalsis was previously reported to differ between normal controls, reflux patients, and patients with non-obstructive dysphagia (16, 19, 20, 26). Additionally, several pharmacologic agents, including topical lidocaine, baclofen (a GABA-agonist), and mosapride (a 5-HT4 agonist) (27-29) have been reported to alter thresholds for eliciting secondary peristalsis. Other studies have reported that erythromycin (a motilin agonist) may increase contraction frequency and magnitude of secondary peristalsis (but not primary peristalsis) and butylscopolamine (an anticholinergic agent) may decrease contraction frequency and magnitude in secondary peristalsis (14, 17). Opposed to the primary methods of inducing secondary peristalsis in patient studies using infused air or water boluses (which may disperse causing potentially inconsistent and/or undetermined degrees of esophageal distension), the FLIP offers a method to control and objectively-measure esophageal distension. Hence, the response to esophageal distension may have clinically important implications and FLIP topography may represent a more user-friendly method to assess it during sedated endoscopy.
In conclusion, although FLIP topography requires refinement of the device and study protocol to overcome current methodological limitations, it represents an interesting and appealing method for esophageal function assessment. With some alterations to the FLIP device and optimization of the distension protocol, the FLIP and FLIP topography may provide a method to simultaneously assess distensibility of the lower esophageal sphincter and esophageal body, while also assessing distention-induced peristalsis. We hypothesize that RACs represent a unique metric of peristaltic function that may identify motor dysfunction in patients with dysphagia and GERD. Future work will focus on optimizing the FLIP design to better characterize motor function and to determine whether abnormalities in RACs are present in disease states.
Key Messages.
Propagating, esophageal contractions induced during esophageal distension can be visualized using customized output generated from the functional lumen imaging probe (FLIP): FLIP topography.
The aim of this study was to define the esophageal contractile response to volumetric distention in healthy control subjects using FLIP topography.
The study used a novel analysis technique generated with a customized MATLAB program to identify and analyze esophageal contractions during volumetric esophageal distension with the FLIP in 10 normal subjects.
Repetitive, antegrade, contractions induced by esophageal distension were observed in the majority (8/10) of the normal subjects. All ten subjects demonstrated some degree of unorganized esophageal contractions (reactivity) during distension.
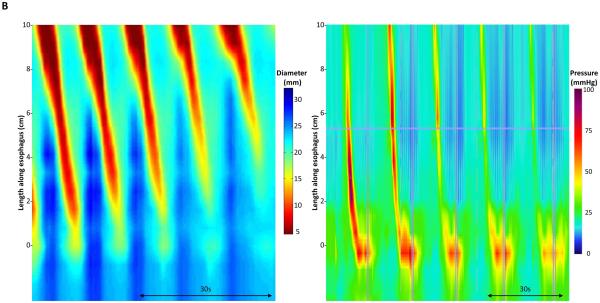
Figure 4. Esophageal motility assessment with FLIP topography and esophageal pressure topography (EPT).
Topographic plots generated using interpolated esophageal diameter (Left: FLIP topography) and pressures (Right: EPT, generated using high-resolution manometry) of two subjects: Figure 4A is the same subject represented in Figures 1-3; Figure 4B is another subject. The proximal portion of the EPT plot has been removed for comparative display. Though the FLIP topography plots represent distension-induced contractions and the EPT plots represent the peristaltic response to voluntary swallows of 5-ml water, the similarities in motility between FLIP topography and EPT can be appreciated. Figure used with permission from the Esophageal Center at Northwestern.
Acknowledgements
This work was supported by T32 DK101363 (JEP) and R01 DK079992 (JEP) from the Public Health service.
Funding: No funding declared
Footnotes
Conflict of interest:
John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
John E. Pandolfino, Zhiyue Lin, and Peter J. Kahrilas: Patent filed for FLIP topography methodology with Crospon, Inc. through Northwestern University
Dustin A. Carlson, Melinda Rogers, Chen Yuan Lin: None
References
- 1.McMahon BP, Frokjaer JB, Drewes AM, Gregersen H. A new measurement of oesophago-gastric junction competence. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(5):543–6. doi: 10.1111/j.1365-2982.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 2.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143(2):328–35. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Pandolfino JE, de Ruigh A, Nicodeme F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(6):496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Hirano I, Boris L, Nicodeme F, Lin Z, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surgical endoscopy. 2014 doi: 10.1007/s00464-014-3733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Boris L, Nicodeme F, Lin Z, Hungness ES. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surgical endoscopy. 2014;28(10):2840–7. doi: 10.1007/s00464-014-3563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140(1):82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicodeme F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, Gonsalves N, Kwasny MJ, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(9):1101–7. doi: 10.1016/j.cgh.2013.03.020. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Z, Kahrilas PJ, Xiao Y, Nicodeme F, Gonsalves N, Hirano I, Pandolfino JE. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therapeutic advances in gastroenterology. 2013;6(2):97–107. doi: 10.1177/1756283X12470017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orvar KB, Gregersen H, Christensen J. Biomechanical characteristics of the human esophagus. Digestive diseases and sciences. 1993;38(2):197–205. doi: 10.1007/BF01307535. [DOI] [PubMed] [Google Scholar]
- 10.Patel RS, Rao SS. Biomechanical and sensory parameters of the human esophagus at four levels. The American journal of physiology. 1998;275(2):G187–91. doi: 10.1152/ajpgi.1998.275.2.G187. Pt 1. [DOI] [PubMed] [Google Scholar]
- 11.Mujica VR, Mudipalli RS, Rao SS. Pathophysiology of chest pain in patients with nutcracker esophagus. The American journal of gastroenterology. 2001;96(5):1371–7. doi: 10.1111/j.1572-0241.2001.03791.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin Z, Nicodeme F, Boris L, Lin CY, Kahrilas PJ, Pandolfino JE. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP) Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(11):e765–71. doi: 10.1111/nmo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao SS, Hayek B, Summers RW. Impedance planimetry: an integrated approach for assessing sensory, active, and passive biomechanical properties of the human esophagus. The American journal of gastroenterology. 1995;90(3):431–8. [PubMed] [Google Scholar]
- 14.Liao D, Villadsen GE, Gregersen H. Distension-evoked motility analysis in human esophagus. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(5):407–12. doi: 10.1111/nmo.12081. e296-7. [DOI] [PubMed] [Google Scholar]
- 15.Paterson WG, Hynna-Liepert TT, Selucky M. Comparison of primary and secondary esophageal peristalsis in humans: effect of atropine. The American journal of physiology. 1991;260(1):G52–7. doi: 10.1152/ajpgi.1991.260.1.G52. Pt 1. [DOI] [PubMed] [Google Scholar]
- 16.Schoeman MN, Holloway RH. Secondary oesophageal peristalsis in patients with non-obstructive dysphagia. Gut. 1994;35(11):1523–8. doi: 10.1136/gut.35.11.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao D, Krarup AL, Lundager FH, Drewes AM, Gregersen H. Quantitative differences between primary and secondary peristaltic contractions of the esophagus. Digestive diseases and sciences. 2014;59(8):1810–6. doi: 10.1007/s10620-014-3070-1. [DOI] [PubMed] [Google Scholar]
- 18.Schoeman MN, Holloway RH. Stimulation and characteristics of secondary oesophageal peristalsis in normal subjects. Gut. 1994;35(2):152–8. doi: 10.1136/gut.35.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwakiri K, Hayashi Y, Kotoyori M, Tanaka Y, Kawami N, Sano H, Takubo K, Sakamoto C, et al. Defective triggering of secondary peristalsis in patients with non-erosive reflux disease. Journal of gastroenterology and hepatology. 2007;22(12):2208–11. doi: 10.1111/j.1440-1746.2006.04817.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen CL, Szczesniak MM, Cook IJ. Identification of impaired oesophageal bolus transit and clearance by secondary peristalsis in patients with non-obstructive dysphagia. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20(9):980–8. doi: 10.1111/j.1365-2982.2008.01140.x. [DOI] [PubMed] [Google Scholar]
- 21.Shi G, Pandolfino JE, Zhang Q, Hirano I, Joehl RJ, Kahrilas PJ. Deglutitive inhibition affects both esophageal peristaltic amplitude and shortening. American journal of physiology Gastrointestinal and liver physiology. 2003;284(4):G575–82. doi: 10.1152/ajpgi.00311.2002. [DOI] [PubMed] [Google Scholar]
- 22.Sifrim D, Janssens J. Secondary peristaltic contractions, like primary peristalsis, are preceded by inhibition in the human esophageal body. Digestion. 1996;57(1):73–8. doi: 10.1159/000201316. [DOI] [PubMed] [Google Scholar]
- 23.Bardan E, Xie P, Aslam M, Kern M, Shaker R. Disruption of primary and secondary esophageal peristalsis by afferent stimulation. American journal of physiology Gastrointestinal and liver physiology. 2000;279(2):G255–61. doi: 10.1152/ajpgi.2000.279.2.G255. [DOI] [PubMed] [Google Scholar]
- 24.Pandolfino JE, Shi G, Zhang Q, Kahrilas PJ. Absence of a deglutitive inhibition equivalent with secondary peristalsis. American journal of physiology Gastrointestinal and liver physiology. 2005;288(4):G671–6. doi: 10.1152/ajpgi.00388.2004. [DOI] [PubMed] [Google Scholar]
- 25.Takeda T, Nabae T, Kassab G, Liu J, Mittal RK. Oesophageal wall stretch: the stimulus for distension induced oesophageal sensation. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(6):721–8. doi: 10.1111/j.1365-2982.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 26.Rydberg L, Ruth M, Lundell L. Characteristics of secondary oesophageal peristalsis in operated and non-operated patients with chronic gastro-oesophageal reflux disease. European journal of gastroenterology & hepatology. 2000;12(7):739–43. doi: 10.1097/00042737-200012070-00004. [DOI] [PubMed] [Google Scholar]
- 27.Chen CL, Liu TT, Yi CH. Effects of lidocaine on esophageal secondary peristalsis in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(6):606–10. doi: 10.1111/j.1365-2982.2010.01494.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen CL, Liu TT, Yi CH. Control of esophageal distension-induced secondary peristalsis by the GABA(B) agonist baclofen in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23(7):612–e250. doi: 10.1111/j.1365-2982.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen CL, Liu TT, Yi CH, Orr WC. Effects of mosapride on esophageal secondary peristalsis in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23(7):606–e249. doi: 10.1111/j.1365-2982.2011.01714.x. [DOI] [PubMed] [Google Scholar]