Abstract
Background
Individuals with anorexia nervosa (AN) override the drive to eat, forgoing immediate rewards in favor of longer-term goals. We examined delay discounting and its neural correlates in AN before and after treatment to test a potential mechanism of illness persistence.
Methods
Inpatients with AN (n=59) and healthy controls (HC, n=39) performed a delay discounting task at two timepoints. A subset (n=30 AN, n=22 HC) participated in fMRI scanning during the task. The task consisted of a range of monetary choices with variable delay times, yielding individual discount rates—the rate by which money loses value over time.
Results
Before treatment, the AN group showed a preference for delayed over earlier rewards (i.e., less steep discount rates) compared with HC; after weight restoration, AN did not differ from HC. Underweight AN showed slower response times for earlier versus delayed choices; this reversed with treatment. Underweight AN showed abnormal neural activity in striatum and dorsal anterior cingulate; normalization of behavior was associated with increased activation in reward regions (striatum and dorsal anterior cingulate) and decision-making regions (dorsolateral prefrontal cortex and parietal cortex).
Conclusion
The undernourished state of AN may amplify the tendency to forgo immediate rewards in favor of longer-term goals. The results suggest that behavior that looks phenotypically like “excessive self-control” does not correspond with enhanced prefrontal recruitment. Rather, the results point to alterations in cingulo-striatal circuitry that offer new insights on the potential role of abnormalities in decision-making neural systems in the perpetuation of AN.
Keywords: Imaging, Anorexia Nervosa, Eating Disorders, Delay Discounting, Decision-Making, Longitudinal
Introduction
Anorexia nervosa (AN) is a serious disorder with a mortality rate six times as high as expected among young women(1). Despite ongoing research, the neurobiology of AN remains poorly understood. One defining characteristic of AN is the ability to resist the drive to eat. Individuals with AN demonstrate a capacity to forgo receipt of food reward—to override biological hunger cues and “postpone” eating. This common feature of AN is examined in the current study using a behavioral task that quantifies delay discounting, a measure of one aspect of self-control.
The clinical phenomena seen in AN have been understood as manifestations of excessive self-control, dating back to early descriptions by Hilde Bruch of “iron determination”(2). In a recent study using a monetary delay discounting task, we measured preferences between smaller-but-immediate rewards versus larger-but-delayed rewards, providing an estimate for individual discount rates—reflecting how rapidly a reward loses subjective value as a function of how long one must wait to receive it(3). Individuals with AN had discount rates that were significantly less steep than their healthy peers (i.e., they preferred larger-but-delayed rewards)(4), suggesting greater “self-control” or “patience.” This result was provocative in part because it is uncommon to find heightened self-control in this task in a psychiatric population, and because discount rates have been shown to have behavioral correlates and thus ecological validity(5). Steeper discounting (i.e., less patience) is associated with poorer self-control as evidenced by increased tendencies toward impulse shopping and gambling(6), and lower achievement later in life(7; 8). These established links between discount rate and behavior have implications for AN, where the core disturbances are maladaptive eating behaviors.
Measuring self-control in a monetary reward paradigm has advantages in AN, as the reward value of food is uncertain(9). Disrupted decision-making around money is not part of the AN diagnosis, therefore, the presence of an abnormality in delay discounting of monetary reward may indicate an attribute that extends beyond eating-related abnormalities and that can provide clues to underlying neurobiology. In AN, the tendency to choose delayed monetary rewards suggests a disposition that may contribute to persistent maladaptive eating choices. Perhaps brain function is altered in AN in a way that makes it easier to resist the temptation of short-term reward—resisting both monetary smaller-sooner rewards and food rewards such that the waiting for future weight loss is less of a burden. Paradigms with non-food rewards have previously shown reward processing abnormalities supporting this avenue of investigation in AN(10).
Neurobiologically, fronto-striatal reward and fronto-parietal control networks are implicated in delay discounting, including the medial prefrontal cortex, ventral striatum, dorsolateral prefrontal cortex (dlPFC) and inferior parietal lobule(11–14). The increased patience in delay discounting seen in AN raises the question as to whether individuals with AN may exhibit functional abnormalities in these decision-making systems. Existing data in healthy adults suggest that choosing the larger-later rewards is associated with activity in the dlPFC(11; 15), a region identified with self-control processes(16; 17). Neuroimaging studies in AN have pointed to potential abnormalities in regions relevant for delay discounting (striatum and dorsal anterior cingulate)(10; 18–20), yet this hypothesis has not been investigated.
Clarification of the neurocognitive underpinnings of AN is critical for developing new treatment targets for this potentially severe illness. In this study, we aimed to examine delay discounting behavior before and after treatment, along with the associated neural systems. We hypothesized that individuals with AN would show less steep discounting both before and after treatment, as compared with healthy peers, suggesting a possible underlying trait consistent with excessive self-control and that this would be associated with greater activity in the dlPFC.
METHODS AND MATERIALS
Participants
Participants were individuals with AN presenting to the Columbia Center for Eating Disorders/ New York State Psychiatric Institute (NYSPI) and healthy controls (HC)(Table 1). Eligible patients were between 16 and 45 years old, met DSM-5 (American-Psychiatric-Association 2013) criteria for AN, restricting (AN-R) or binge-purge (AN-BP) subtype, and were receiving inpatient treatment. Individuals were excluded if they had an estimated IQ less than 80, history of a neurological, bipolar, or psychotic disorder, substance abuse in the last 6 months, or if they were pregnant. Anxiety or depressive disorders, which commonly co-occur, were not an exclusion when AN was the primary diagnosis(21).
Table 1.
Demographic and Clinical Characteristics of Participants.
| Table 1. Clinical Characteristics | |||||||
| HC (n=39) | AN (n=59)* | ||||||
| Mean ± SD | Mean ± SD | t | df | p | |||
| Time 1 | n=39 | n=54** | |||||
| Age (years) | 24.7±7.6 | 25.0±7.5 | −0.17 | 91 | 0.87 | ||
| BMI (kg/m2) | 21.7±1.9 | 16.6±1.5 | −3.13 | 62.5 | <0.005 | ||
| Education (years) | 15.1±3.0 | 14.1±2.1 | 1.72 | 63.9 | 0.09 | ||
| Eating Disorder Examination | 0.08±0.10 | 3.1±1.4 | −15.3 | 53.7 | <0.001 | ||
| WTAR estimated IQ | 108.5±11.8 | 107.9±8.0 | 0.26 | 56.6 | 0.79 | ||
| Time 2 | n=31 | n=43 | |||||
| Days between sessions | 58.5±35.4 | 52.6±15.6 | 0.87 | 38.8 | 0.39 | ||
| BMI (kg/m2) | 21.9±2.0 | 20.4±0.7 | −2.76 | 50.3 | <0.01 | ||
| EDE | 0.1±0.1 | 2.1±1.3 | −13.9 | 42.7 | <0.001 | ||
| Caucasian | N | (%) | N | (%) | X2 | df | p |
| 27 | 69.2 | 53 | 89.8 | 2.5 | 1 | 0.11 | |
| Female | 37 | 94.9 | 57 | 96.6 | 0.20 | 1 | 0.65 |
| Subset of the above participants who also provided fMRI data | |||||||
| HC (n=21) | AN (n=25)* | ||||||
| Mean ± SD | Mean ± SD | t | df | p | |||
| Time 1 | n=21 | n=23 | |||||
| Age (years) | 20.7±2.8 | 19.3±2.5 | 1.6 | 42 | 0.10 | ||
| BMI (kg/m2) | 21.4±1.8 | 16.8±1.4 | 3.1 | 39.9 | <0.005 | ||
| Education (years) | 14.1±2.2 | 13.2±2.0 | 1.4 | 40.6 | 0.17 | ||
| Eating Disorder Examination | 0.08±0.11 | 3.09±1.58 | −9.1 | 22.5 | <0.001 | ||
| WTAR estimated IQ | 109.3±10.2 | 104.7±8.1 | 1.6 | 34 | 0.13 | ||
| Time 2 | n=16 | n=18 | |||||
| Days between sessions | 53.3±30.2 | 45.4±11.8 | 1.0 | 19.1 | 0.34 | ||
| BMI (kg/m2) | 21.7±1.8 | 20.2±0.6 | 3.3 | 31.8 | <0.005 | ||
| EDE | 0.1±0.1 | 2.3±1.4 | −9.6 | 17.1 | <0.001 | ||
WTAR=Weschler Test of Adult Reading, EDE=Eating Disorder Examination, BMI=Body Mass Index
At Time 1, AN-R n=25 and AN-BP n=29. At Time 2, AN-R n=22 and AN-BP n=21.
EDE scores were significantly different between AN-R and AN-BP (3.7±1.1 vs 2.6±1.5, respectively, p=0.004).
Three individuals' data were excluded at Time 1, based on the behavioral algorithm. These individuals were included at Time 2. Two subjects participated only at Time 2.
At Time 1, AN-R n=13 and AN-BP n=10. At Time 2, AN-R n=9, and AN-BP n=9.
EDE scores did not differ between subtypes.
**Two individuals data were excluded at Time 1, based on the behavioral algorithm. These individuals were included at Time 2. One HC and one subject with AN had fMRI data that was excluded due to motion, their behavioral data were included in analyses.
A subset of these individuals were recruited to participate during an fMRI scan (n=48) if they were 16-25 years old, female, with no contraindication to MRI, and not taking psychotropic medication. Medications are not routinely used for AN on the inpatient unit, due to lack of evidence of utility(22).
HC were matched for age, sex, and ethnicity and were included if they had no current or past psychiatric illness, no significant medical illness, no psychotropic medications, and a BMI in the normal range (18-25 kg/m2). This study was approved by the NYSPI Institutional Review Board, and after complete description of the study to the participants, written informed consent was obtained (ClinicalTrials.gov NCT00325520).
Procedures
Height and weight were measured on a beam balance scale (Detecto). Participants were administered the Eating Disorders Examination semi-structured interview(23), and the Wechsler Test of Adult Reading(24). Testing occurred twice. Individuals with AN were tested within 1 week of hospital admission (Session 1), and after weight restoration to a BMI of 19.5 kg/m2(Session 2). Time between sessions was group-matched.
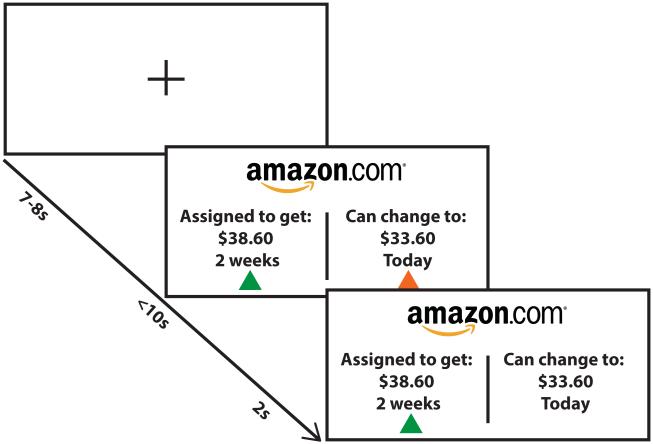
Delay Discounting Task (Figure 1)
Figure 1. Delay discounting task design.
Individuals are presented with a choice between a smaller amount of money available sooner (SS) and a larger amount available later (LL). Amounts ranged from $15 to $85 and time of delivery for SS choices was either Now or in 2 weeks, and the time of delivery for LL was 2 or 4 weeks after the SS. Outside the scanner, there was no time limit for responding. In the fMRI version, there was a fixation cross between trials. All task parameters (i.e. monetary values, time differences) were the same inside and outside the scanner.
Full methods can be found in the supplemental methods. Participants made binary choices between amounts of money, adapted from McClure et al(11). In each trial, they chose between an amount of money that was available sooner, (“smaller-sooner,” SS) or a larger amount of money available after a delay (“larger-later,” LL). In half the trials, the SS was available on the day of participation (NOW); in the other half, the SS was available in 2 weeks (NOT-NOW). The reward was either cash or an Amazon gift-card, counterbalanced across blocks. Participants were instructed that there were no right or wrong answers and to choose the option they truly preferred because at the end of the experiment they would be paid according to their choice on one of the trials. After finishing participation, one trial was selected by a random number generator, and the participant was paid according to their preference on that trial (e.g., if they had selected an SS of $24 today over an LL of $36 in 4 weeks, they received $24 that day).
fMRI Data Acquisition
Imaging was performed on a 1.5T Philips Intera scanner, with an 8-channel head coil. High-resolution T1-weighted anatomical images were acquired using an SPGR sequence (TR=25sec, TE=3.7ms, angle=30°, FOV=256mm, 256x204 matrix, 128 slices, voxel size 1x1x1mm). The task was performed during 4 functional runs using an EPI sequence (TR=2000ms, TE=40ms, FOV=192mm, 64x63 matrix, 33 axial slices, voxel size 3x3x4mm, 180 TRs). Trials advanced with participants’ selections, and were not synchronized with TRs.
fMRI Data Preprocessing
fMRI data were preprocessed and analyzed using the Analysis of Functional Neuroimages software package (AFNI)(25). Functional scans were corrected for slice acquisition using sinc-interpolation. Volume registration using 6-parameter rigid-body transformation, to account for head motion, and normalization into Talairach space using 12-parmeter affine transformation were performed in a single interpolation step. Data were resampled to 3mm isotropic voxels. Data were iteratively smoothed to achieve a final full-width half-max Gaussian kernel of 6mm. Signal intensity was normalized by individual voxel to percent signal change.
Data Analysis
Behavioral
Clinical characteristics were compared using Student's t-tests for independent samples, with Welch correction for unequal variances, and Mann-Whitney-Wilcox tests for ordered measures. For each individual, a hyperbolic discount rate (k) was estimated from their choice data, per Session. Fitting procedures, alternative discount models, and a generalized linear mixed-effects model approach to analyzing the choice and response-time data are described in the Supplemental Methods. Participants for whom the fitted model was not better than random choice were excluded. Log-transformed discount rates, log(k), were analyzed using a linear mixed-effects model, with main predictors of interest Diagnosis, Session, and their interaction and with random intercepts and Session slopes for each participant. This method models individual variability and is robust to missing data, and was selected due to the different sample sizes across different stages of analysis (Supplemental Methods). The p-value of the interaction term was determined using conditional F tests with Kenward-Roger adjustments of degrees of freedom (Supplemental Methods)(26). The interaction was examined using post-hoc tests of Diagnosis for Session 1 and 2, and tests of Session for AN and HC. Lower values of log(k) indicate less steep discount rates, or a greater preference for the LL reward. Response-time analysis was performed using the same parameters in a linear mixed-effects model, in addition to choice (SS or LL) and the absolute difference in subjective value between the SS and LL options, as determined by subject specific discount rates (k).
fMRI
Single-subject analysis on preprocessed data was done using a general linear model (GLM). Each subject had a design matrix with 22 regressors: baseline, trend, and quadratic signal to capture shifts in signal change for each of the 4 runs(12), motion parameters(6), and 4 trial-specific regressors that reflected participants’ choices (SS-NOW, SS-NOT-NOW, LL-NOW, LL-NOT-NOW). These trial regressors were convolved with a duration-modulated (by trial response time) block hemodynamic-response function. Trials with greater than 2mm of motion (as well as the preceding and following trial) were censored, and scans with greater than 10% of trials censored were excluded from further analysis. This analysis was repeated using an amplitude-modulated regressor for the difference in subjective value between the two presented options (Supplemental Methods).
Our main analysis of interest of the group data was performed using linear mixed-effects modeling using AFNI’s 3dLME function(27), which is robust to small amounts of missing data. Regression coefficients from the individual analysis estimated from fewer than 12 trials were excluded from the group analysis. The model included fixed-effect terms: Choice (SS or LL), Immediacy (NOW or NOT-NOW), Diagnosis (AN or HC), Session (1 or 2), and all their possible interactions, covariates (age and IQ), and a random intercept for each participant. For regions in which the Choice by Session by Diagnosis interaction term was identified as significant, we extracted the average SS and LL first-level regression coefficients of each individual for each session. These were used to further examine how these regions differed between groups through post-hoc t-tests.
RESULTS
Participants are described in Table 1. Of the initially enrolled 106 participants, we excluded 1 HC and 1 individual with AN when it was discovered during further screening that they did not meet inclusion criteria. Data from 6 participants (all AN) were excluded because their behavior was not distinguishable from random choice. The 6 excluded participants did not differ significantly from the included AN group in clinical characteristics, and are not included in any of the analyses below. The final sample included 98 participants (39 HC and 59 AN); of these, 30 HC and 37 AN provided task data at both Sessions. Mean duration of illness among AN was 8.6±6.9 years, with a history of 0-15 (mean=2.6) prior hospitalizations. There were 28 individuals with AN-R and 31 individuals with AN-BP in the full sample. There were no significant differences in clinical characteristics between the groups who completed one versus two sessions. As shown in Table 1, there was a small difference in BMI between AN and HC at Time 2, likely related to the narrow BMI range among weight-restored AN.
The age range for individuals who participated in the fMRI portion of the study was designed to be narrow in order to obtain a more homogeneous sample (22 HC, 26 AN). As such, they were younger than non-scanned participants (19.8±2.7 years versus 29.8±7.2 years, t96=8.97, p<0.0001), with no difference between AN and HC. All fMRI participants were female; two with AN were left-handed. There were no other significant differences between the fMRI and behavioral participants.
Behavioral Results
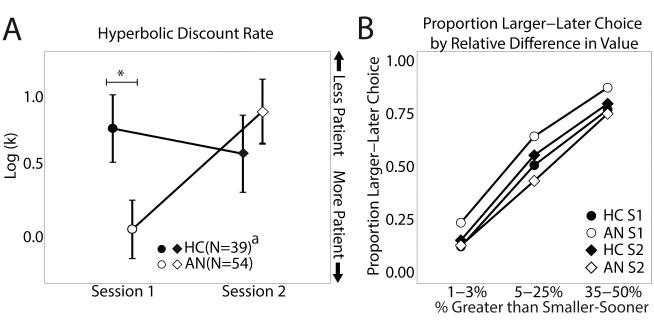
Results of the delay discounting task showed a significant effect of Session (F1,71.4=12.0, p=0.0009) and a Diagnosis by Session interaction effect (F1,71.4=19.45, p<0.0001) on the discount rates, log(k). Individuals with AN had a significantly lower mean discount rate than HC at Session 1(t91=2.25, p=0.027), a significant increase between Sessions (t36=−4.6, p<0.0001), and did not differ from HC at Session 2 (t72=−0.84, p=0.40)(Figure 2). There was no difference in HC between sessions (t29=1.22, p=0.23). The same pattern was seen when age and IQ were included in the linear mixed-effects model. Delay discounting results followed the same pattern, with no significant differences among the subsets in and out of the scanner(Supplemental Methods). Discount rate was not significantly associated with measures of illness severity (BMI, duration of illness, EDE, p-values >0.3). Other potential variables affecting value of reward (cash versus Amazon payment, time until discharge from the inpatient unit),were not associated with discount rates or changes in discount rate (p-values >0.2, Supplemental Methods).
Figure 2. Individuals with AN have lower discount rates than HC only when underweight.
(A) The log-transformed discount rates (per unit years) are shown for individuals with AN and HC at Sessions 1 and 2. Lower log-transformed discount rates indicate less steep discounting, i.e., a preference for larger-later over smaller-sooner options. (B) The proportion of trials that the larger-later option was chosen is shown for the AN and HC groups at each Session, separated into three bins indicating how much greater the larger-later choice was than then smaller-sooner choice in percentage terms. The AN group shows an overall decrease in the proportion of trials that they chose the delayed option, rather than for a specific subset of trials.
a Session 2 sample size (N=31 HC, 43 AN)
* p<0.05 (error bars are SEM).
As a secondary analysis, discount rates were compared between AN-R, AN-BP and HC in the mixed-effects model and showed a significant Session effect (F1,71.4=24.23, p<0.0001) and a significant Diagnosis by Session interaction (F1,71.3=10.82, p<0.0001). Compared with HC at Session 1, the AN-R group had lower discount rates (i.e. more patience) (t62=2.48, p=0.016), and no difference from HC at Session 2 (t51=0.096, p=0.92). Individuals with AN-BP did not differ significantly from HC or AN-R at Session 1 (HC: t66=1.37, p=0.175, AN-R: t52=−1.09, p=0.28) or Session 2 (HC: t50=−1.62, p= 0.11 and AN-R: t41=−1.56, p=0.13). Both AN-R (t17=−3.38p=0.004) and AN-BP (t18=−3.40,p=0.003) groups changed significantly across Sessions.
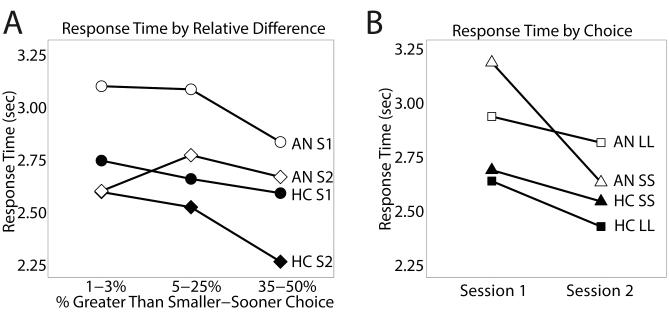
Overall response times quickened across sessions for both groups (mean difference=−311ms, SD=815ms, t68=3.17, p=0.002), with no diagnosis-by-session interaction (p=0.67). Response times for HC did not differ between SS and LL choices across sessions (difference=−50ms, SD=819ms, t27=−0.32, p=0.75). Individuals with AN showed a significant shift in response time between sessions: At Session 1, AN were slower for SS choices than for LL choices, and after treatment, responses were faster for SS choices than for LL choices (difference=−336ms, SD=797ms, t34=2.5, p=0.018)(Figure 3B). When absolute difference in subjective value of the two options was included in the analysis, to account for choice difficulty, the pattern was the same, but only at trend level (p=0.063)(i.e., slower response for SS choices in the underweight phase, Supplemental Methods).
Figure 3. Individuals with AN chose smaller-sooner options more slowly than larger-later options when underweight, and switched when weight restored.
(A) Response time by percent that the larger-later (LL) option is greater than the smaller-sooner (SS) option. This shows that both groups quickened their responses across session, and for some trial types more than the other. (B) Response time by session, split by SS and LL choice. The AN group shows a significant switch from being slower during SS than during LL choices when underweight, to being faster during SS than during LL choices once weight restored.
Imaging Results
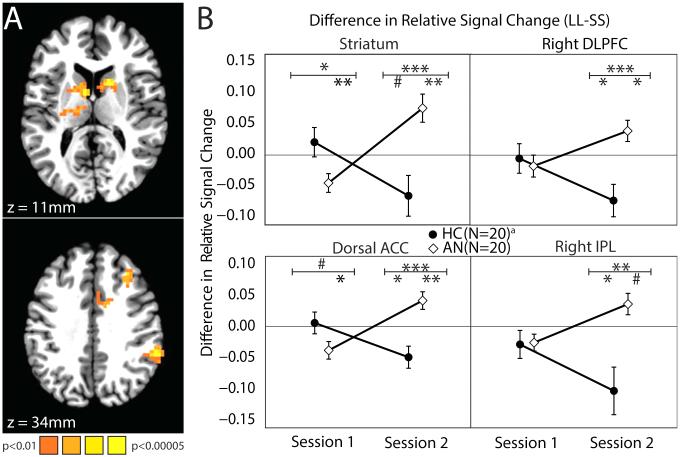
Imaging analyses probed the behavioral finding of differences in preference for delayed rewards among AN and HC. Analyses examined differences in neural activity between LL and SS choices. There was a significant Choice (SS/LL) by Diagnosis (AN/HC) by Session (S1/S2) interaction in multiple brain regions, including the striatum bilaterally, the dorsal anterior cingulate cortex (dACC), the right dlPFC (rdlPFC), and the right parietal lobule (rPar)(Figure 4; Table S9 in the Supplement). We compared the differences in Choice (LL minus SS) activity between diagnostic groups at each Session in these regions. At Session 1, HC showed no difference between LL and SS activity in any of these regions, whereas individuals with AN showed lower LL relative to SS activity in the striatum and dACC. At Session 2, HC showed lower LL relative to SS activity in the dACC, rdlPFC, and rPar, whereas individuals with AN showed greater LL relative to SS activity in the striatum, dACC, and rdlPFC. HCs showed a significant change across sessions: LL minus SS activity was smaller at Session 2 than Session 1 in the striatum, dACC, rdlPFC, and rPar, whereas AN showed the opposite change in these regions, with LL minus SS activity being greater at Session 2 than Session 1. These differences appear to be driven only by a decrease in LL activity in HC and by a concurrent increase in LL and decrease in SS activity in individuals with AN (Table S10 in the Supplement). When absolute difference in subjective value between SS and LL (to account for choice difficulty) was added as a regressor in the first level analysis, this pattern of activity remained in the striatum, but there were no longer any group and session differences in fronto-parietal activity (Supplemental Methods).
Figure 4. Individuals with AN have altered neural activity as compared with HC for larger-later (LL) versus smaller-sooner (SS) choices in cingulo-striatal and fronto-parietal circuitry.
(A) Areas with a significant interaction effect of Choice (LL or SS), Diagnosis (AN or HC), and Session (1 or 2) (whole-brain corrected p<0.01, individual voxel threshold p<0.01, spatial extent >= 41 voxels; see Table S9 in the Supplement). (B) Mean contrasts of LL minus SS choice neural activity between Diagnosis (AN or HC) and Session (1 or 2) in regions identified in the interaction effect. A positive value indicates greater neural activity when making LL choices than when making SS choices.
a Session 2 sample size (17AN, 14HC).
# p<0.10, * p<0.05, ** p<0.01, *** p<0.001 (error bars are SEM) Symbols below the horizontal bar indicate the test of the LL-SS contrast, those above indicate t-tests between diagnostic group of this contrast (Table S10 in the Supplement).
DISCUSSION
This study provides behavioral and neural data on monetary delay discounting from a large sample of acutely ill individuals with AN, tested before and after weight restoration, as well as a comparison with healthy peers. We replicated our previous result that in the underweight state, individuals with AN discount the value of a reward over time significantly less steeply than healthy peers(4). Specifically, individuals with AN selected a larger reward delivered after a delay more often than HC, a behavior commonly interpreted as indicating self-control. Additionally, underweight AN responded more slowly when choosing the smaller, earlier options than the larger, delayed options. Once weight-restored, individuals with AN showed normalized discount rates (i.e., less tendency to delay reward as their health improved), and quickened response times when choosing earlier options, suggesting a change in how the choices are perceived.
Neural activation patterns also differed from HC, though not in the expected ways. We predicted that individuals with AN might show increased neural activity compared to HC in regions associated with executive control (e.g. dlPFC), which has been shown to subserve the tendency to choose delayed rewards among HC(15). Instead, underweight AN showed relatively less activity than HC during delayed compared to earlier choices in the dACC and striatum, regions associated with multiple aspects of cognition and behavior. After behavior normalized with weight restoration, neural activity then differed between groups, specifically with differences in the cingulo-striatal and fronto-parietal systems to delayed versus earlier choices. However, when subjective value was included, capturing an aspect of choice difficulty for each individual, there were no observable group or session differences in this fronto-parietal circuit. In other words, results in these fronto-parietal regions vary by analytical approach (and may reflect changes in subjective difficulty that come with changes in discounting behavior); thus the most conservative interpretation would be that the main difference between groups is in striatal activity. These results suggest that phenotypic “excessive self-control” in AN might not result from executive-control circuit hyperactivity in the prefrontal cortex, but rather appears mainly associated with differences in striatal activity.
How, then, should we best understand increased “patience” in AN? The current study yields three sets of results. One, among AN in the underweight state, discount rate was abnormal, responses were slowed to the earlier choices, and cingulo-striatal activity was lower than HC during delayed choices relative to earlier choices. Two, with weight restoration, discount rate normalized and response times shifted to being faster for the earlier choices. Three, with weight restoration, neural activity in the cingulo-striatal and fronto-parietal circuits increased during delayed relative to earlier choices in the AN group, whereas activity decreased for the HC group. Taking these results together suggests a new hypothesis: the tendency to prefer larger, delayed rewards in the acutely ill state of AN may reflect a state-specific shift in decision-making. We can further speculate that acutely ill individuals with AN may be relying on choice strategies with reduced cognitive demands. While we cannot address this with the data in this study, perhaps choices amongst the underweight AN group are more habit driven(28), choosing to delay as a default response. Alternatively, the evaluation of delay and outcome-magnitude information might be changed(29–31) compared to healthy controls.
Faster response times can be an indication of a more automatic response(32; 33). For the AN group, response times were slower during earlier versus delayed choices when underweight, which reversed with treatment. This suggests that the delayed choice may be the default option and choosing earlier rewards required more deliberation. Considering the clinical phenomena, where delay of eating is likely rewarded initially, it may be that delay of gratification is incrementally reinforced and becomes a habitual choice(28), which may be amplified in the setting of starvation. This has yet to be tested in AN.
Although this interpretation is speculative, prior research suggests it is worthy of testing. Malnourishment is known to lead to many cognitive changes(34), and cognitive deficits have frequently been observed in AN(35). Furthermore, chronic starvation in animals has been shown to alter reward processing(36). The current data suggest that starvation may interact with the pathology of the illness to alter decision-making in ways that contribute to its entrenchment and create challenges in treatment. These data seem to differ from the reward-enhancing effects of acute hunger in food-related(37) and monetary paradigms(38), yet may relate to the literature that shows hunger does not lead to increased risk tolerance(39). Cingulo-striatal circuits have been suggested to play a role in modulation of basic reward signals(29). The hypoactivity in the dACC and striatum during delayed choices among underweight AN suggests a possible deficit in complex decision-making during delay discounting.
The absence of longitudinal imaging studies of delay discounting in HC makes interpreting the pattern of neural signal in HC across sessions difficult. One possibility is that neural activity decreases with task familiarity. Prior studies show variable neuroimaging results among HC, some with similarities to ours at either Session 1 or Session 2(12; 40), while others differ(41; 42)—likely related to differences in task design. Whereas HC showed less neural activity, individuals with AN showed increased activity during larger-later choices upon repeat administration of the task. Inpatient treatment may improve health such that, after treatment, individuals with AN are able to engage in more deliberate decision-making. These cognitive processes may be necessary in AN for making consistent healthy choices. Individuals with long-term remission of AN showed no behavioral difference from HC in a recent delay discounting study(43), suggesting that normalized discount rates persist once weight is restored.
The majority of research on delay discounting in psychiatry has suggested that discount rates are steeper than normal in behavioral disorders (e.g., substance abuse disorders)(44). AN thus appears unusual in being characterized by the opposite behavior. Additionally, one study has shown that less steep discount rates were associated with more lethal suicidal behavior(45), and another reported lower rates among individuals with obsessive compulsive personality disorder(46), a personality disorder often comorbid with AN. Our behavioral and neuroimaging results suggest that abnormally low discount rates in AN warrant further study. For example, does discount rate relate to maladaptive food choice, and does it predict response to treatment, as seen in disorders associated with high impulsivity and steeper discounting rates(47; 48)?
The patients in this study were all receiving inpatient treatment, raising the question as to whether AN and HC differ because of context, such that monetary rewards are less valuable during inpatient treatment. However, patients showed similar behavior between cash and gift card trials, and their discount rates showed no correlation with time to discharge, which mitigates this concern. Nevertheless, we cannot rule out the possibility that discounting behavior was influenced by the prospect of leaving the inpatient setting. Our main interest was to compare AN and HC and accordingly, our study was not powered to make strong empirical conclusions regarding the AN subtypes, particularly in the fMRI sample. It may be that self-control differs between these groups, a possibility that deserves attention in future research. Discounting preferences have been shown to differ across development; as such we age-matched our groups, and there was no change to the results when age and IQ were included as covariates in the analysis.
In conclusion, these novel behavioral and brain imaging results illustrate how delay discounting differs among individuals with AN, pre- and post-treatment, compared with healthy peers. Our results suggest that self-control, as measured by a delay discounting task, is selectively altered in the acutely ill, underweight state rather than a trait-like abnormality of AN, and that this alteration is not due to heightened dorsolateral prefrontal cortex activity, as one might have expected based on previous work in healthy individuals(15). Thus, the “iron determination”(2) manifested by individuals with AN is perhaps not the result of persistent executive control, a cognitively demanding approach that may be too challenging for an undernourished brain. Rather, these findings may indicate a maladaptive rule-based or automatic tendency to select the larger, delayed option when undernourished. Treatment and weight restoration may facilitate the switch to cognitively more demanding strategies. This aberrant decision-making warrants exploration specifically as related to choices about eating and suggests new directions for understanding the basic mechanisms of AN.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank BJ Casey, B. Timothy Walsh, and Elke Weber for their mentorship on this project; Kris Caudle and Cate Hartley for their contributions to the work, and the patients and staff of the Eating Disorders Research Unit at the New York State Psychiatric Institute.
This work was supported by NIMH K23MH076195 (PI: Steinglass) and the Klarman Family Foundation Award in Eating Disorders.
Bernd Figner was supported by a grant from the U.S. NSF (NSF SES – 0922743) and a grant from the Swiss NSF (PA001 – 115327).
Johannes Decker was supported by an MSTP grant from the National Institute of General Medical Sciences of the NIH under award number: T32GM007739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work has been presented as a poster at the Cognitive Neuroscience conference in April 2014, and as part of a mini-symposium at Biological Psychiatry in May 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
Dr. Steinglass receives funding from the Global Foundation for Eating Disorders and the National Eating Disorder Association.
Dr. Figner and Mr. Decker report no biomedical financial interests or potential conflicts of interest.
References
- 1.Arcelus J, Mitchell A. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 2.Bruch H. The golden cage: The enigma of anorexia nervosa. Vintage Books; New York: 1978. [Google Scholar]
- 3.Green L, Fry AF, Myerson J. Discounting of Delayed Rewards: A Life-Span Comparison. Psychol Sci. 1994;5:33–36. [Google Scholar]
- 4.Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc. 2012;18:773–80. doi: 10.1017/S1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp C, Monterosso J, Montague PR. Neuroeconomics: a bridge for translational research. Biol Psychiatry. 2012;72:87–92. doi: 10.1016/j.biopsych.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chabris CF, Laibson D, Morris CL, Schuldt JP, Taubinsky D. Individual laboratory-measured discount rates predict field behavior. J Risk Uncertain. 2008;37:237–269. doi: 10.1007/s11166-008-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–8. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 8.Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: Strategic self-regulation for coping with rejection sensitivity. J Pers Soc Psychol. 2000;79:776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- 9.Frank GKW, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O’Reilly RC. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37:2031–46. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating C, Tilbrook AJ, Rossell SL, Enticott PG, Fitzgerald PB. Reward processing in anorexia nervosa. Neuropsychologia. 2012;50:567–75. doi: 10.1016/j.neuropsychologia.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 11.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 12.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter RM, Meyer JR, Huettel S a. Functional neuroimaging of intertemporal choice models: A review. J Neurosci Psychol Econ. 2010;3:27–45. [Google Scholar]
- 15.Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–9. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 16.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 17.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 18.Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–12. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff-Grethe A, McCurdy D, Grenesko-Stevens E, Irvine LEZ, Wagner A, Yau W-YW, et al. Altered brain response to reward and punishment in adolescents with Anorexia nervosa. Psychiatry Res. 2013;214:331–40. doi: 10.1016/j.pscychresns.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner A, Aizenstein H, Venkatraman V, Fudge J, May JC, Mazurkewicz L, et al. Altered Reward Processing in Women Recovered From From Anorexia Nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 21.Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attia E, Haiman C, Walsh BT, Flater SR. Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry. 1998;155:548–51. doi: 10.1176/ajp.155.4.548. [DOI] [PubMed] [Google Scholar]
- 23.Cooper Z, Fairburn C. The eating disorder examination: A semi structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;8:1–8. [Google Scholar]
- 24.Wechsler D. Weschler Test of Adult Reading. TX Psychol Corp; San Antonio: 2001. [Google Scholar]
- 25.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 26.R-Core-Team . R: A language and environment for statistical computing. R. F. for S. Computing Vol. 172. Vienna, Austria: 2012. pp. 167–172. [Google Scholar]
- 27.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh B. The Enigmatic Persistence of Anorexia Nervosa. Am J Psychiatry. 2013;170:477–484. doi: 10.1176/appi.ajp.2012.12081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters J, Büchel C. The neural mechanisms of inter-temporal decision- making: understanding variability. Trends Cogn Sci. 2011;15:227–39. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Kurth-Nelson Z, Bickel W, Redish AD. A theoretical account of cognitive effects in delay discounting. Eur J Neurosci. 2012;35:1052–64. doi: 10.1111/j.1460-9568.2012.08058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider W, Shiffrin R. Controlled and Automatic Human Information Processing: I. Detection, Search, and Attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- 33.Keramati M, Dezfouli A, Piray P. Speed/accuracy trade-off between the habitual and the goal-directed processes. PLoS Comput Biol. 2011;7:e1002055. doi: 10.1371/journal.pcbi.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation. Vol. 2. Univ. of Minnesota Press; 1950. [Google Scholar]
- 35.Steinglass JE, Glasofer DR. Eating Disorders and the Brain. NeuropsychologyWiley Online Library. 2011:106–121. [Google Scholar]
- 36.Carr K. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 37.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang XT, Dvorak RD. Sweet future: fluctuating blood glucose levels affect future discounting. Psychol Sci. 2010;21:183–8. doi: 10.1177/0956797609358096. [DOI] [PubMed] [Google Scholar]
- 39.Levy DJ, Thavikulwat AC, Glimcher PW. State dependent valuation: the effect of deprivation on risk preferences. PLoS One. 2013;8:e53978. doi: 10.1371/journal.pone.0053978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo S, Ainslie G, Giragosian L, Monterosso JR. Behavioral and Neural Evidence of Incentive Bias for Immediate Rewards Relative to Preference-Matched Delayed Rewards. J Neurosci. 2009;29:14820–14827. doi: 10.1523/JNEUROSCI.4261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–53. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- 42.Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54:1344–54. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 43.Wierenga C, Bischoff-Grethe A, Melrose B, Irvine Z, Torres B, Bailer U, et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.09.024. doi:10.101: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99:32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dombrovski AY, Szanto K, Siegle GJ, Wallace ML, Forman SD, Sahakian B, et al. Lethal forethought: delayed reward discounting differentiates high- and low- lethality suicide attempts in old age. Biol Psychiatry. 2011;70:138–44. doi: 10.1016/j.biopsych.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto A, Steinglass JE, Greene AL, Weber EU, Simpson HB. Capacity to Delay Reward Differentiates Obsessive-Compulsive Disorder and Obsessive Compulsive Personality Disorder. Biol Psychiatry. 2013;75:1–7. doi: 10.1016/j.biopsych.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15:176–86. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- 48.MacKillop J, Kahler C. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.