Abstract
Melanopsin ganglion cells express the photopigment melanopsin and are the first functional photoreceptors to develop in the mammalian retina. They have been shown to play a variety of important roles in visual development and behavior in the early postnatal period (Johnson et al., 2010; Kirkby and Feller, 2013; Rao et al., 2013; Renna et al., 2011). Here, we probed the maturation of the dendritic arbors of melanopsin ganglion cells during this developmental period in mice. We found that some melanopsin ganglion cells (mainly the M1-subtype) transiently extend their dendrites not only into the inner plexiform layer (where they receive synaptic inputs from bipolar and amacrine cells) but also into the outer plexiform layer, where in mature retina, rod and cone photoreceptors are thought to contact only bipolar and horizontal cells. Thus, some immature melanopsin ganglion cells are biplexiform. This feature is much less common though still present in the mature retina. It reaches peak incidence 8–12 days after birth, before the eyes open and bipolar cells are sufficiently mature to link rods and cones to ganglion cells. At this age, some outer dendrites of melanopsin ganglion cells lie in close apposition to the axon terminals of cone photoreceptors and express a postsynaptic marker of glutamatergic transmission, postsynaptic density-95 protein (PSD-95). These findings raise the possibility of direct, monosynaptic connections between cones and melanopsin ganglion cells in the early postnatal retina. We provide a detailed description of the developmental profile of these processes and consider their possible functional and evolutionary significance.
Keywords: melanopsin, ipRGC, biplexiform, ganglion cell, retina
INTRODUCTION
In the mammalian retina, a small population of retinal ganglion cells has been shown to express the photopigment melanopsin and to be autonomously sensitive to light (Berson et al., 2002; Hattar et al., 2002). These intrinsically photosensitive ganglion cells (ipRGCs) signal directly to various brainstem and hypothalamic regions to regulate physiological processes such as circadian rhythms and the pupillary light reflex (Lucas et al., 2003; Panda et al., 2002). In the adult retina, melanopsin ganglion cells receive rod and cone signals from the outer retina by way of synaptic contacts from bipolar cells in the inner retina (Guler et al., 2007; Guler et al., 2008; Wong et al., 2007). In this sense, melanopsin ganglion cells resemble conventional retinal ganglion cells. However, they are highly unusual in at least two respects. First, as already noted, they are intrinsically photosensitive. Second, they send signals not only out of the retina, like conventional ganglion cells, but also back into the inner retina to modulate visual processing (Joo et al., 2013; Zhang et al., 2012; Zhang et al., 2008).
In early postnatal mice, rod and cone photoreceptors are functionally uncoupled from the inner retina until around postnatal day 10 (P10), when bipolar cells form functional connections with photoreceptors of the outer retina and amacrine and ganglion cells of the inner retina (Rich et al., 1997). Melanopsin ganglion cells are the first functional photoreceptors; they can detect light as early as the day of birth and transmit signals into the brain as early as P6 (Johnson et al., 2010; Schmidt et al., 2008; Sekaran et al., 2005; Tu et al., 2005). At this same developmental time point, waves of spontaneous activity sweep across the developing retina. These are central to the formation of functional neural circuits in many higher level visual centers of the brain (Ackman and Crair, 2014; Meister et al., 1991). Melanopsin ganglion cells modulate Stage 2 retinal waves but are also, in turn, affected by them; they spike in concert with other retinal ganglion cells during such waves (Renna et al., 2011). Stage 2 waves occur independent of light and are mediated by acetylcholine release from cholinergic (starburst) amacrine cells and activation of nicotinic cholinergic receptors (Feller et al., 1996; Ford et al., 2012; Zheng et al., 2006). In adulthood, there is little opportunity for direct contact between starburst amacrine cells and melanopsin ganglion cells because their dendrites occupy non-overlapping strata within the inner plexiform layer (IPL). However, the laminar relationships between these two cell types have not been previously described during the period of Stage 2 waves, so such direct contacts might be possible during this period. If present, might suggest as dendrodendritic mechanism for melanopsin ganglion cells to photically modulate the starburst network that drives wave activity.
In the course of addressing this question experimentally we discovered that some melanopsin ganglion cells extend dendrites into the outer plexiform layer, where rods and cones normally contact bipolar and horizontal cells. Melanopsin-immunopositive dendrites in the outer retina can be found in close association with cone axon terminals and express there a postsynaptic marker of glutamatergic transmission, postsynaptic density-95 protein (PSD-95), suggestive of direct synaptic contact. These findings suggest a novel anatomical circuit by which cone photoreceptors might influence the inner retina and brain far earlier than previously recognized. This arrangement is most fully developed at an age when retinal activity profoundly shapes the wiring of the visual centers of the brain, so this presumptive circuit may have important implications for visual system development. While biplexiform ganglion cells have been observed previously in the retinas of non-mammalian vertebrates, they are not thought to exist in mammals. Thus, our report provides the first anatomical evidence of biplexiform ganglion cells (and of direct photoreceptor-to-ganglion-cell contacts) in the mammalian retina.
METHODS
Animals
All experiments were performed in accordance with NIH guidelines under protocols approved by the Institutional Animal Care and Use Committees of Brown University and the University of Akron. Wild type C57BL/6 mice were used for all experiments. All animals were maintained on a 12h:12h light-dark cycle and sacrificed 10.5 – 11.5 hours after light-ON.
Immunohistochemistry
We used vertical retinal sections to compare the development of dendritic stratification of cholinergic starburst amacrine cells and of melanopsin ganglion cells (labeled by melanopsin immunostaining, enhanced by tyramide signal amplification; TSA: Invitrogen TSA kit# T-20925). Eyes were removed immediately after euthanasia, fixed (4% phosphate-buffered paraformaldehyde; 24 h), cryoprotected (30% phosphate-buffered sucrose; >24 hours), embedded (Optimum Cutting Temperature medium), cut at 25 µm on a cryostat (Leica CM3050s), and stored at −80° C. For immunolabeling, sections were equilibrated to room temperature for 1 hour and rinsed with phosphate-buffered saline (PBS; 3 times for 15 minutes each). Endogenous peroxidase activity was quenched by incubation in 1% H2O2, and 0.5 % Triton-X in PBS for 1 hour. The retinas were again washed (3 × 10 min in PBS) and incubated overnight in the blocking solution provided with the TSA kit. Sections were incubated for 36–48 hours in a mixture of primary antibodies in blocking solution: rabbit anti-melanopsin (UF006; kind gift of Ignacio Provencio; 1:10,000 dilution) and goat anti-ChAT (Millipore AB144P; 1:200). Tissue was then washed (6 × 10 min in PBS) and incubated overnight in the secondary antibody for the ChAT antibody (donkey anti-goat Alexa 594; Invitrogen #A-11058; 1:200). The next day, sections were washed (6 × 10 min in PBS) and immersed in goat normal serum (1:100 in PBS; 4° C; overnight). Finally, to reveal anti-melanopsin immunolabeling, sections were incubated in goat anti-rabbit antibody tagged with horseradish peroxidase supplied with the TSA kit; 3 hours at room temperature), rinsed (6 × 10 min in PBS) and immersed in a solution of tyramide-488 at 1:100 in Perkin-Elmer amplification buffer (FP1135) for 6 minutes. Sections were rinsed (3 × 15 min in PBS), and coverslipped with Aqua-mount.
We immuno-stained retinal wholemounts to assess the origin and abundance of melanopsin-positive outer retina dendrites (ORDs), their origin in specific subtypes of melanopsin ganglion cells, and their relationship to cone pedicles (identified by immunolabeling for the vesicular glutamate transporter 1; vGluT1 and the postsynaptic marker PSD-95). These retinas were dissected from the eye immediately after enucleation, mounted ganglion-cell side up on a nitrocellulose membrane, fixed in 4% phosphate-buffered paraformaldehyde for one hour and immediately processed immunohistochemically. Following a wash (3 × 15 min in PBS), endogenous peroxidase activity was quenched and the overnight blocking step for TSA applied as already described for tissue sections. Retinas were then incubated for 5 days at 4° C in a mixture of primary antibodies (rabbit anti-melanopsin as described for retinal sections; guinea pig anti-VGlut1; Millipore #AB5905 at a dilution ratio of 1:1,000; and mouse anti-PSD-95; BD Biosciences #610496 at 1:500). The long incubation appeared to be necessary for optimal vGlut1 and PSD-95 immunolabeling in young animals. Retinas were then washed (3 × 1 hour in PBS) and incubated overnight in a mixture of secondary antibodies (HRP-tagged goat anti-rabbit at 1:200 for melanopsin; Alexa-488-tagged goat anti-guinea pig forVGlut1; Invitrogen #A-11073 at a dilution ratio of 1:200; and Alexa-647-tagged donkey anti-mouse for PSD-95; Millipore #A31571 at 1:200). Retinas were washed (3 × 1 hour in PBS) and the tyramide amplification step was performed using tyramide-594. Retinas were washed (3 × 15 min in PBS), incubated for 5 minutes in 4',6-diamidino-2-phenylindole (DAPI) a nuclear dye to reveal cell layers (Invitrogen #D-1306; dilution ratio of 1:100). Retinas were again washed (3 × 15 min in PBS), mounted and coverslipped on a glass slide using ProLong Gold anti-fade reagent.
We immunostained vertical sections of retina to examine the relationship between melanopsin ganglion cell dendrites and cone pedicles. Vertical sections of retina were prepared and processed as previously described. Melanopsin ganglion cells were labeled as previously described for vertical sections, and guinea pig anti-VGlut1 (1:1000) was co-incubated with the melanopsin primary antibody. Secondary antibodies (HRP-tagged goat anti-rabbit at 1:100 for melanopsin; and Alexa-488-tagged goat anti-guinea pig at forVGlut1; at 1:100) were co-applied for 3 hours at room temperature.
Image Acquisition and Quantification
Epifluorescence images were acquired on a Nikon E600 microscope equipped with a SPOT-RT Slider digital microscope camera. For wholemounts, we collected z-stacks of confocal images separated in depth by 0.5 or 1.0 µm on a Zeiss LSM510 Meta or Leica TCS SP5 confocal laser scanning microscope. Confocal images were analyzed using Zeiss LSM Image Browser software and further processed with Adobe Photoshop and ImageJ software.
To assess the density of outer retinal dendrites, we selected 3–5 regions of interest (680 µm × 450 µm) in wholemount retinas from each age studied (P4, P8, P12 and adult). In each of these regions, we counted the number of outer retinal dendrites (ORDs), defined as melanopsin immunopositive dendrites that extended outward into the inner plexiform layer. These dendrites were subdivided into simple and complex varieties based on whether, after entering the INL, they terminated within it (simple) or extended into and coursed within the OPL (complex).
Statistical Analysis
A single-factor ANOVA was used to assess the significance of any developmental differences in the densities of simple, complex and total outer retinal dendrites. A Bonferroni correction for multiple comparisons was applied to reduce the possibility of Type I error (significance was set at p<0.0083).
Electrophysiology
Whole-cell patch experiments were performed as described previously (Van Hook and Berson 2010). Briefly, patch pipettes were filled with a K-gluconate-based internal solution containing Alexa 488 hydrazide (120 K-gluconate, 5 NaCl, 4 KCl, 2 EGTA, 10 HEPES, 4 ATP-Mg, 7 phosphocreatine-Tris, and 0.3 GTP-Tris; osmolality was adjusted to 260–280 mOsm and pH to 7.3 with KOH). Extracellular Ames medium (Sigma) was supplemented with 10 mM D-glucose and 23mM NaHCO3 and bubbled with 5%CO2.
td-Tomato reporter mice (Jackson Laboratory; B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze) were crossed with ChAT-Cre (Jackson Laboratory; B6;129S6-Chattm1(cre)Lowl) mice to selectively label starburst amacrine cells. P4-P7 mice were dark adapted for 1 hour and then, under dim red light, given a lethal intraperitoneal dose of Beauthanasia. Retinas were isolated and mounted ganglion-cell side up. The tissue was continuously perfused with heated (33–37°C) and oxygenated Ames medium. Fluorescent starburst amacrine cells in the ganglion cell layer were targeted by epifluorescence and a loose-patch seal was obtained. Cells were dark adapted for 10–30 minutes thereafter. Spikes were recorded in current-clamp mode both in both darkness and light. Illumination was introduced from below through the microscope's condenser lens. We used unfiltered, broad-band light from a 100 W tungsten-halogen lamp. Stimulus irradiance (in) was 2.8×1014 photons·s−1·cm−2 measured at 480 nm. Starburst morphology of the recorded amacrine cell was visually confirmed at the conclusion of the recordings on the basis of Alexa 488 dye filling. Only cells that exhibited starburst amacrine cell morphology and wave-like bursts (Zheng et al. 2006) were analyzed further.
Data analysis was performed offline in Clampfit 9 (Molecular Devices). The empirically determined liquid junction potential was subtracted from the measured voltage. For each cell, the resting membrane potential, the duration the cell was depolarized, the duration of the after-hyperpolarization, and the amplitude of the after-hyperpolarization were calculated for every burst and then averaged for each light condition. Statistical significance was calculated with a two tailed student-t test.
RESULTS
Development of cholinergic and melanopsin dendritic plexuses
We found that segregation of melanopsin-immunopositive dendrites into two distinct strata occurs as early as postnatal day four (P4; Fig. 1), when Stage 2 waves first emerge. At P4, these melanopsin bands are slightly more diffuse than in the adult, occupying roughly the inner and outer thirds of the IPL. At this age, the processes of starburst amacrine cells, as revealed by antibodies against choline acetyltransferase (ChAT) have yet to form two distinct bands. Even so, they exhibit little overlap in depth with melanopsin dendrites: they stratify diffusely in the middle of the IPL, sandwiched between the two bands of melanopsin processes. By P8, the inner and outer ChAT bands are clearly discernible and melanopsin dendrites have formed more crisply defined strata, a very narrow one at the boundary with the inner nuclear layer (INL) and a broader one in the inner fourth of the IPL. This pattern does not change appreciably at P12, and is essentially indistinguishable from that seen in adults. Thus, throughout the period of the Stage 2 cholinergic waves, starburst amacrine processes and melanopsin ganglion cell dendrites occupy distinct strata in the IPL suggesting that direct synaptic contacts between these cells must be rare, if they exist at all. Many melanopsin ganglion cells of the M1 type have cell bodies in the ganglion cell layer with dendrites that project to the outer IPL (passing through the middle of the IPL where starburst amacrine cell processes are located). Thus, it is theoretically possible that M1 melanopsin ganglion cells provide direct input to starburst amacrine cells through dendrodendritic contacts that could contribute to the photic modulation of Stage 2 retinal waves by melanopsin cells. However, whole-cell patch recordings of starburst amacrine cells in wholemounted P4 to P8 retinas revealed no obvious effects of light that might be expected from such a synaptic circuit.
Figure 1.
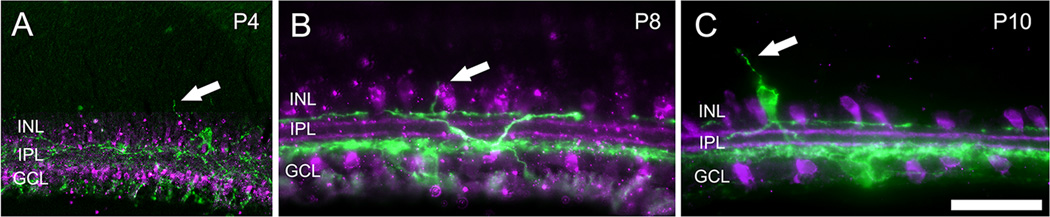
Development of dendritic stratification of melanopsin ganglion cells and starburst amacrine cells in the early postnatal period in mice. Epifluorescence photomicrographs of vertical retina sections in mice from three ages: P4 (A), P8 (B) and P10 (C). ChAT immunofluorescence (purple) marks starburst amacrine cells, and melanopsin immunofluorescence (green) reveals melanopsin ganglion cells types strongly expressing this photopigment (M1-M3 cells). Dendrites of melanopsin ganglion cells stratify in the innermost and outermost sublayers of the inner plexiform layer (IPL) as early as postnatal day 4 (P4) and lack any obvious overlap with starburst processes at any age. Simple outer retinal dendrites can be seen ascending from the S1 plexis into the INL in panels A, B, and from a displaced melanopsin ganglion cell in panel C, as indicated by the arrow. Scale bars: 50 µm.
In the dark, starburst amacrine cells exhibited typical compound bursts with calcium spikes and strong after-hyperpolarizations (AHP; Fig. 2A; (Zheng et al., 2006). Light had no measurable effect on key wave metrics in starburst amacrine cells (Fig. 2B), including the resting membrane potential, the wave burst duration, or the duration or peak amplitude of the AHP (Fig. 2C).
Figure 2.
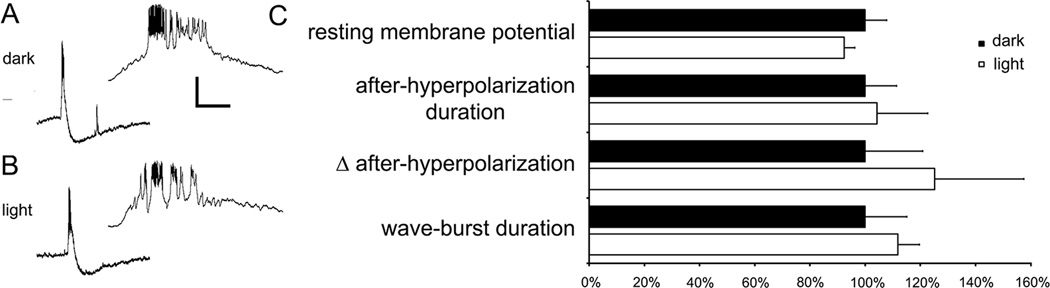
Melanopsin ganglion cells apparently do not modulate starburst amacrine cell wave bursts. A. Whole cell patch recordings of ON starburst amacrine cells located in the ganglion cell layer of isolated whole-mounted retinas from early postnatal mice (P4 – P8). The insert to the right shows the corresponding burst on an expanded time base. B. Light has no observable effect on starburst amacrine cell wave bursts. C. Normalized data of key wave burst metrics. In the dark, the resting membrane potential was 57.5 ± 3.8 mV, with a burst duration of 3.1 ± 0.5 seconds, peak AHP of 9.7 ± 2.0 mV, and AHP duration of 65.8 ±7.5 seconds (n=6 cells with 3–4 bursts per cells). Light had no observable effect on these key wave metrics as the resting membrane potential was 53.9 ± 1.9 mV, with a burst duration of 3.5 ± 0.2 seconds, peak AHP of 12.0 ± 3.0 mV, and AHP duration of 68.7 ± 12.1 seconds. Scale bars: 20 mV and 20 seconds (20 mV and 0.63 seconds for the insert). Error bars are SEM, p > 0.05).
Outer retinal dendrites of melanopsin ganglion cells
A striking and unexpected additional feature of melanopsin dendritic organization during this period was the abundance of melanopsin immunopositive dendrites extending outward beyond the IPL. These entered the inner nuclear layer and in some cases extended as far as the outer plexiform layer (OPL). An early study of melanopsin ganglion cells in adult rats similarly noted that melanopsin dendrites occasionally invade the INL (Hattar et al., 2002). However, these ‘outer retinal dendrites’ (ORDs) appeared to be far more abundant and elaborate in our material from the early postnatal mouse retina than in this earlier report.
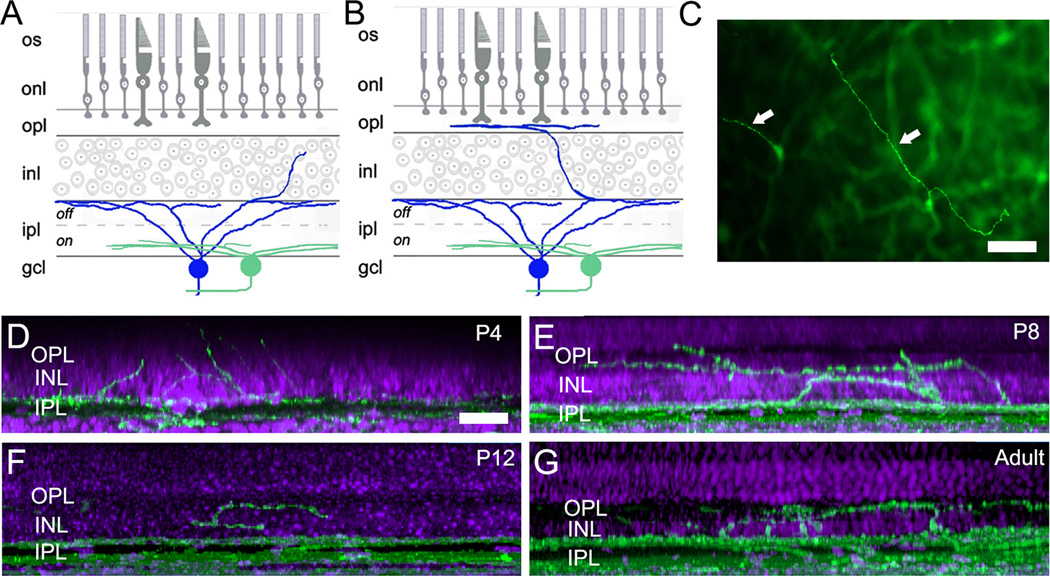
The appearance and abundance of ORDs was developmentally regulated. In the first postnatal week, most ORDs were relatively short and straight, consisting of a single dendritic process extending outward from the IPL to terminate within the INL or, rarely, at the level of the OPL. We term these “simple ORDs” (SORDs). By the second week, about half of the ORDs had taken on a more elaborate appearance. These “complex ORDs” (CORDs) were longer, sometimes branched, and, when they reached the outer margin of the INL, bent into the horizontal plane to run within the OPL for up to 100 µm or more (Fig. 3). Both types of ORDs were much less abundant in the adult retina, dropping to about a quarter of their peak spatial density, which occurred during the second postnatal week (Fig. 4). Nonetheless, both simple and complex ORDs were observed in adult retina.
Figure 3.
Morphology of outer retinal dendrites of melanopsin immunopositive melanopsin ganglion cells at various developmental stages. A, B: Schematic diagrams of simple (A) and complex (B) outer retinal dendrites ascending from the main dendritic arbor of an M1 melanopsin ganglion cell into the inner nuclear layer (INL) and as far as the outer plexiform layer (OPL) (B), where the dendrites form a sparse secondary dendritic plexus. C. Appearance of melanopsin-positive outer retinal dendrites as seen in a melanopsin immunolabeled retinal wholemount. The plane of focus lies in or near the OPL. The main plexus of melanopsin positive dendrites in the IPL is visible, especially on the right side of the image, but is out of focus. A bifurcating complex outer retinal dendrite is marked by the right arrow and a simple ORD by the left arrow. Scale bar: 20 µm. D – G. Outer retinal dendrites of melanopsin ganglion cells as observed at various developmental stages from P4 to adulthood. These images are maximum intensity projections of confocal z-stacks that have been compressed and rotated 90 degrees. Scale bar: 20 µm.
Figure 4.
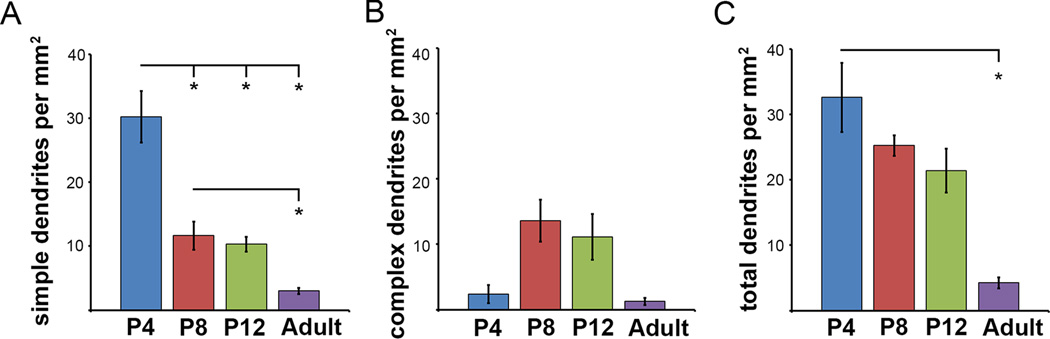
The density of outer retinal dendrites (ORDs) changes as the retina matures. A. The density of simple ORDs decreases from P4 to adult (30.2 ± 4.0 SORDs per mm2 at P4 [n=4]; 11.6 ± 2.3 per mm2 at P8 [n=5]; 10.3 ± 1.2 per mm2 at P12 [n=4]; and 3.0 ± 0.5 per mm2 in the adult [n=5]). B. The density of complex ORDs peaks at P8-P12, and then decreases (2.4 ± 1.4 CORDs per mm2 at P4; 13.6 ± 3.2 per mm2 at P8; 11.1 ± 3.5 per mm2 at P12; 2.6 ± 1.8 per mm2 in the mature retina). C. The total number of ORDs (sum of SORDs and CORDs) decreases throughout postnatal development leaving only 5.6 ± 2.0 ORDs per mm2 in the adult retina. D. The density of displaced melanopsin ganglion cells does not change significantly during postnatal development.
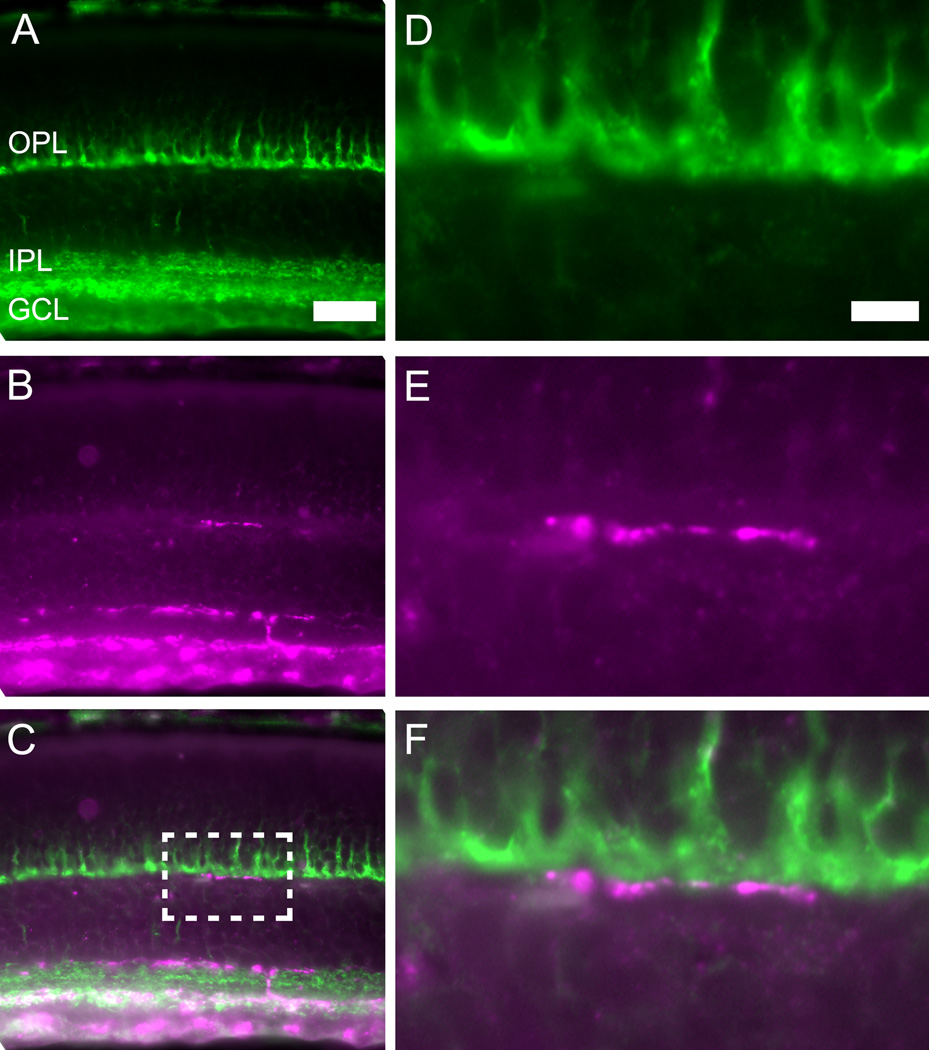
To visualize the relationship between complex ORDs and photoreceptor terminals, we double-labeled vertical retinal sections at P12 with antibodies against melanopsin to label ORDs and the vesicular glutamate transporter vGluT1, which labels cone pedicles and rod spherules. We observed numerous instances in which melanopsin-immunoreactive ORDs were in close apposition to the plate-like cone pedicles (Fig. 5). Other ORDs traveled more proximally within the OPL, so that a clear gap separated them from the cone pedicles. At this age, rod spherules were not clearly marked by vGluT1 immunostaining, but we never observed ORDs traveling higher in the OPL than the cone pedicles, where rod spherules would be expected to lie.
Figure 5.
Example of a close apposition between a complex outer retinal dendrite of a melanopsin ganglion cells and a cone axon terminal at P12. Epifluorescence photomicrographs of a vertical retina section. A. VGlut1 immunohistochemistry (green) labels photoreceptor terminals (mainly cone pedicles) in the outer plexiform layer (OPL) and bipolar cell terminals in the inner plexiform layer (IPL). B. Melanopsin immunohistochemistry (magenta) labels melanopsin ganglion cells including their dendrites. Arrow indicates a complex outer retinal dendrite (CORD) stratifying in the OPL. C. The merged image from panels A and B. D–F. Same as A–C but magnified to show the region indicated by the dashed white rectangle in C centered on the OPL. Note the close apposition of the CORD to the base of the cone pedicles. Scale bar: 20 µm (left); Scale bar: 5 µm (right).
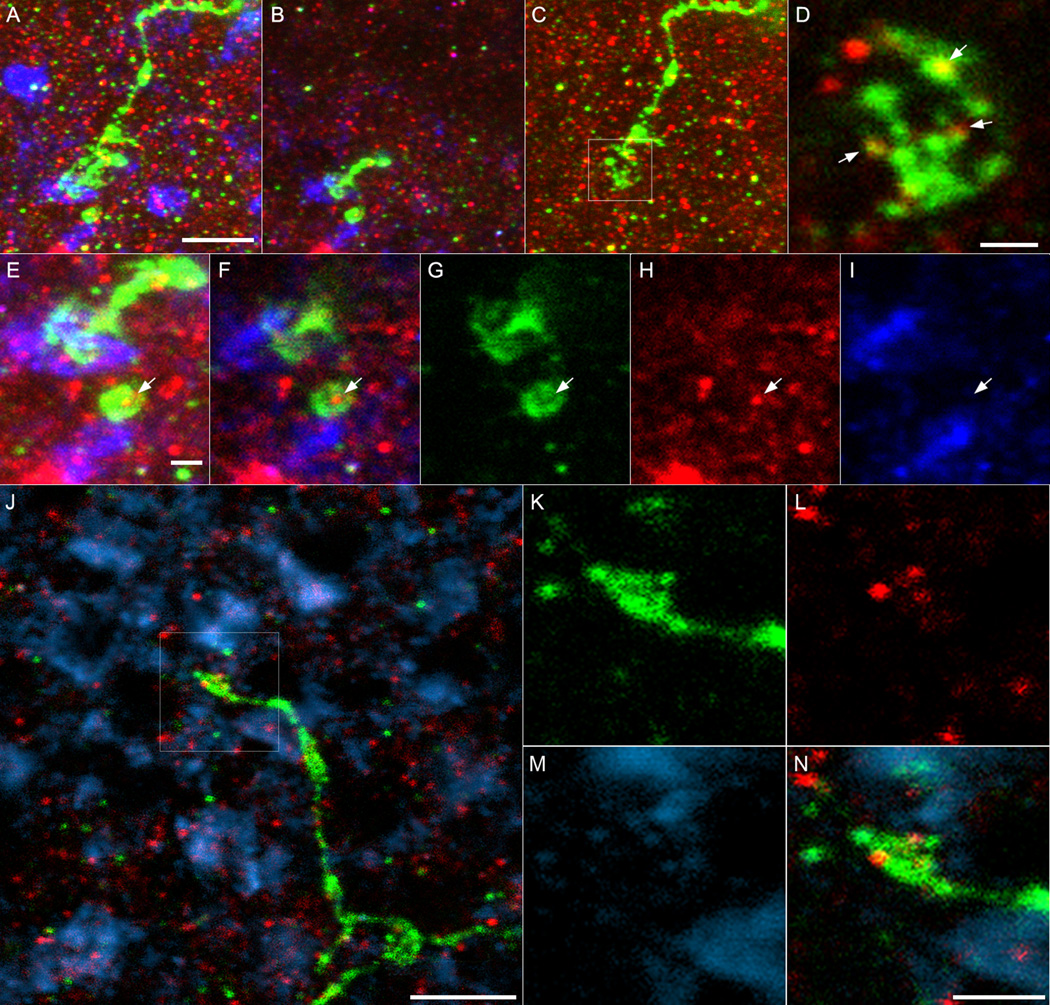
Cones use glutamate as their transmitter. As a test for possible synaptic machinery permitting direct glutamatergic transmission between cones and melanopsin dendrites (i.e., CORDs), we augmented the earlier immunohistochemical experiment by adding a third antibody against PSD-95, a postsynaptic marker at glutamatergic synapses. We routinely observed PSD-95 immunopositive puncta within the CORDs at both P8 (Fig. 6) and P12. These often appeared along the dendrite as it traversed the inner nuclear layer (Fig. 6A, 6C–D, 6J), sometimes in association with specialized appendages (Fig. 6C–D). Significantly, they could also be found in CORDs closely in close proximity to cone pedicles (Fig.6E–N).
Figure 6.
Complex outer retinal dendrites (CORDs) of melanopsin ganglion cells in relation to cone pedicles as seen in retinal wholemounts from P8 mice. All images reconstructed from z-stacks of deconvolved confocal sections. Panels A–I show one such dendrite, J–N another. In all panels, green pseudocolor shows melanopsin immunofluorescence; blue shows cone pedicles revealed by anti-VGluT1 immunostaining; red shows punctuate immunofluorescence for PSD-95, a postsynaptic marker for glutamatergic synapses. A: Maximum intensity projection (48 optical sections) showing the complex form of the first CORD (green). In the upper part of the panel, the dendrite is coursing through the inner nuclear layer (INL); it terminates in the outer plexiform layer at the bottom left. For clarity, the signal in the VGluT1 channel (blue) was omitted from planes above or below the level of the cone pedicles. Scale bar in A is 5 µm and applies to panels A–C. B: The dendrite bifurcates at its terminus and forms two swellings, each lying in close apposition to a cone pedicle (blue). Maximum intensity projection of 11 optical sections. C: Proximal portion of the outer retinal dendrite which includes a complex appendage (boxed region) which lies in the middle of the INL. Maximum intensity projection of 29 optical sections. D: single optical section showing enlarged view of the boxed complex appendage in C and the apparent presence of several PSD-95 puncta (arrows) within it. Scale bar = 1 µm. E–I: higher-magnification view of the terminal dendritic region shown in B. E: maximum intensity projection of 23 optical sections to show the proximity of dendritic endings to cone pedicles. F–H: Single optical section showing a PSD-95 immunoreactive punctum within one of the terminal bulbs of the dendrite; a second adjacent optical section was added to the VGluT1 channel (blue) to improve the visibility of the cone pedicles. F: Merged image; G: melanopsin-immunoreactive dendrite; H: PSD-95 immunolabel; I: anti-VGluT1 labeling of cone pedicles. Scale bar in D is 1 µm and applies to E–I. J–N: Images of a second CORD suggesting possible glutamatergic synaptic contacts between photoreceptor axon terminals and outer dendritic processes of melanopsin ganglion cells. J: montage of single deconvolved confocal sections showing a melanopsin-immunopositive dendrite (green) coursing through the INL and terminating in the OPL at the level of cone pedicles, which appear as blue patches (anti-VGluT1 immunofluorescence). Several puncta immunopositive for PSD95 (red), a postsynaptic marker for glutamatergic synapses, are apparent within the distal terminus of the dendrite. K–N: Higher-power view of the dendritic terminus boxed in J, all from a single optical plane. K: melanopsin dendrite; L: PSD-95 puncta; M: VGluT1 immunopositive cone pedicles; N: merge of B–D. To assemble Panel J, each plane of a z-stack of confocal sections was regionally masked so as to display only the part of the image passing near the plane of the cone pedicles and/or through the melanopsin dendrite. For most locations, these planes were the same, but diverged toward the bottom right of the image, which includes the ascending portion of the CORD. Immunofluorescence shown at any x-y position in the image is derived from only a single confocal plane. Images in K–N are all from a single optic plane through the tip of the melanopsin dendrite. Calibrations: 5 µm in J; 2 µm in N (applies to K–N).
Which melanopsin ganglion cells subtypes give rise to melanopsin dendrites in the outer retina?
Outer retinal dendrites were easily traced back to the IPL. Most branched off the coarse melanopsin-immunoreactive processes in the outermost IPL (i.e., sublamina S1). The great majority of melanopsin dendrites in that plexus derived from melanopsin ganglion cells of the M1 type, though a small fraction came from M3 cells. In the first two postnatal weeks, many ORDs could be unambiguously traced back to M1 cells with cell bodies in the inner nuclear layer (“displaced M1 cells”), and others to conventionally placed M1 cells, in the ganglion cell layer. The origin of many ORDs was less uncertain because, though they could be linked to processes in the outer melanopsin plexus, these parent dendrites could not be traced with confidence through this highly overlapping plexus of melanopsin dendrites to their cellular origin. We suspect however, that they arose mainly from M1 cells (and perhaps M3 cells) in the ganglion cell layer, which are the main sources of this dendritic plexus. We saw few if any examples of ORDs that could be traced directly from the outer retina into the inner IPL without links to the outer retinal plexus. We therefore conclude that few if any ORDs derive from melanopsin ganglion cells of the M2 type, which deploys it dendritic arbor exclusively in the inner IPL. Other melanopsin ganglion cells types (M4 and M5) lack significant melanopsin immunofluorescence in their dendrites and can be discounted as a source of the ORDs. Thus, in the early postnatal period, it appears that the great majority of ORDs derive from M1 melanopsin cells and prominently from displaced M1 cells, though contributions from M2 and M3 cells cannot be excluded.
DISCUSSION
Our data indicate that the dendritic arbors of melanopsin ganglion cells have already formed two distinct strata as early as P4, and that these are already segregated from the dendrites of cholinergic starburst amacrine cells at this age. Thus, the activation of melanopsin ganglion cells during Stage II (cholinergic) retinal waves is unlikely to occur through conventional synaptic contacts between starburst amacrine cells and melanopsin ganglion cells. This suggests that acetylcholine released during a wave may diffuse far enough from the starburst dendrites that release it to activate nicotinic receptors on melanopsin ganglion cells dendrites in entirely different strata of the IPL. This is consistent with data that has been published in other reports that strongly suggests volume transmission as the primary mechanism of cholinergic wave neurotransmission, rather than through direct synaptic contact with individual cells in the inner plexiform layer (Ford and Feller, 2012).
Light modulates retinal waves through the activation of melanopsin ganglion cells, though the mechanism has not yet been established (Renna et al., 2011). Data presented here suggests that melanopsin’s modulatory effects are not exerted at the level of the starburst amacrine cells because their wave-associated bursting was not modulated by light. This suggests melanopsin ganglion cells modulate wave activity through an alternative circuit, such as that proposed by Kirkby et al. 2013, in which melanopsin ganglion cell signals modulate dopamine amacrine cells which, in turn, exert paracrine effects throughout the IPL.
During this early postnatal period, surprisingly large numbers of melanopsin-positive dendrites extend beyond the IPL into the outer retina. These dendrites vary in appearance and abundance during development, and are most elaborate just before eye opening, when photoreceptors can first signal to RGCs through newly competent bipolar cells. At P4, ORDs are simple vertical processes. They appear to be undergoing a process of active outgrowth at this time, because a few days later (P8), many of them have reached the OPL and made a right-angle turn to run within this synaptic layer, where some closely approach and may contact the cone pedicles. Between P12 and adulthood, ORDs become far less common, presumably through dendritic pruning, attrition by programmed cell death, or both.
Parallels with dopaminergic interplexiform cells
Biplexiform melanopsin ganglion cells exhibit striking structural parallels with interplexiform cells, which are a variant of dopaminergic amacrine cells. Both have bistratified dendritic arbors in S1 and the OPL. All DA cells and many biplexiform melanopsin ganglion cells have somas in the inner nuclear layer. Furthermore, both types express remarkably similar stratification defects in mice with disruption of specific semaphorin-plexin interactions (Matsuoka et al., 2011). These structural and developmental similarities are matched by tight functional links between dopaminergic amacrine cells and melanopsin ganglion cells. For example, melanopsin ganglion cells provide excitatory drive to some DA cells, perhaps through intraretinal axon collaterals (Joo et al., 2013; Zhang et al., 2012; Zhang et al., 2008). In return, dopaminergic cells act on D1 receptors of melanopsin ganglion cells to suppress their phototransduction (Van Hook et al., 2012). Both cell types receive input from ectopic ON bipolar cell synapses in the OFF sublayer (Dumitrescu et al., 2009).
Synaptic circuits involving outer retinal dendrites of melanopsin ganglion cells
Do biplexiform melanopsin ganglion cells have synaptic partners in the OPL? If they do, it seems likely that ORDs are postsynaptic rather than presynaptic at such synapses, because they are dendritic rather than axonal in form and appear to express the postsynaptic marker-95 protein; to our knowledge, there are no precedents for presynaptic ganglion-cell dendrites in mammals. If ORDs are postsynaptic, their most likely presynaptic partner would be the cones. Cone pedicles were found closely apposing melanopsin dendrites (Fig. 5 and Fig. 6), whereas rod spherules (which terminate higher in the OPL) do not appear to be in a position to do so. Cones mature earlier than rods and may well be capable of light-driven synaptic signaling during the early postnatal period during which ORDs are most abundant. Many of the prerequisites for light-driven signaling by from cones seem to be in place by P4, including opsin expression (Arango-Gonzalez et al., 2010) and appropriate synaptic machinery, such as synaptic ribbons, synaptic vesicle proteins and vesicular glutamate transporter vGluT1 (Chen et al., 2012; Regus-Leidig et al., 2009; Rich et al., 1997).
In adulthood, cones drive ON responses in melanopsin ganglion cells, but it is unclear whether the same response polarity would apply if cones made direct synaptic contacts onto melanopsin ganglion cells during the early postnatal period. Light hyperpolarizes the cones and reduces the release of glutamate from their axon terminals. ON responses in adult ganglion cells, including melanopsin ganglion cells, reflect the sign-inverting metabotropic glutamatergic (mGluR) synapse between photoreceptors and ON bipolar cells, and the sign-conserving ionotropic glutamate receptor (iGluR) synapse between ON bipolar cells and ganglion cells.. Direct glutamatergic signaling from cones to melanopsin ganglion cells through sign-conserving iGluRs would thus be expected to generate hyperpolarizing light responses in the melanopsin ganglion cells. Alternatively, the sign of cone effects on melanopsin ganglion cells could be inverted, for example through a sign-inverting mGluR mechanism in immature melanopsin ganglion cells that resembles that in ON bipolar cells or though the involvement of horizontal cells in a less direct synaptic circuit. The present data should motivate a reexamination of possible cone-to-melanopsin ganglion cell contacts at the ultrastructural level as well as functional studies probing for possible functional indications of direct cone input to melanopsin ganglion cells, including possible light-driven effects.
The possibility that the outer arbor of biplexiform melanopsin ganglion cells might serve an output function should not be entirely dismissed. In adult retina, melanopsin ganglion cells are tracer-coupled to other ganglion cells and amacrine cells, ostensibly through gap junctions on their dendrites (Muller et al., 2010). Melanopsin ganglion cells shape the retinal waves during the period of maximal abundance of ORDs. Thus, if an output function of these dendrites could be established, it would raise the possibility that retinal waves, traditionally linked to inner retinal and brain developmental mechanisms, may also play some role in outer retinal development.
Comparative and evolutionary considerations
Biplexiform ganglion cells have been observed previously in vertebrate retina. The only prior description of a biplexiform ganglion cell in mammals was based on observations in macaques (Mariani, 1982; Zrenner et al., 1983), but others have provided compelling evidence for reinterpreting that type as horizontal cells that have been displaced to the inner retina, perhaps through errors in retinal development (Wassle et al., 2000). In teleosts, a distinctive biplexiform ganglion cell has been described, with confirmed axon in the optic nerve, and dual dendritic arbors, one in the outermost layer of the IPL and a second in the OPL (Cook et al., 1996; Pushchin and Kalachev, 2010), a pattern strikingly similar to that of immature melanopsin ganglion cells with ORDs. Direct contacts between photoreceptors and ganglion-cell dendrites are the rule rather than the exception in the hagfish, a jawless vertebrate whose primitive retinas apparently lack horizontal, bipolar and amacrine cells (Holmberg, 1970; Lamb et al., 2007). The RGCs in this species further resemble mammalian M1 melanopsin ganglion cells in that their major projections are to the hypothalamus rather than the thalamus or midbrain (Kusunoki and Amemiya, 1983). RGCs have been suggested to be homologous to the rhabdomeric photoreceptors of invertebrates (Arendt et al., 2004), and this is most clearly reflected in the intrinsic photosensitivity, R-opsin expression and rhabdomeric phototransduction cascade of melanopsin ganglion cells. Lamb and colleagues have hypothesized a primordial synaptic circuit in vertebrate eye evolution in which ciliary photoreceptors directly contact rhabdomeric photoreceptors which, in turn, project to the brain (Lamb et al., 2007). In this context, the output cells of the hagfish retina, the biplexiform ganglion cells of teleosts and the immature M1 melanopsin ganglion cells of the vertebrate retina may all hark back to this primitive retinal arrangement.
Acknowledgments
We would like to thank Dianne Boghossian and Kimberly Boghossian from Brown University and Olivier Brun from Leica Microsystems, Inc., for technical assistance.
Funding Source
This project was supported by grants from the National Eye Institute of the National Institutes of Health to David Berson (R01 EY12793 and R01 EY17137), Jordan Renna (F32 EY20108), Maureen Stabio (F32 EY021994), and the University of Akron Faculty Research Fellowship to Jordan Renna (#1791).
REFERENCES
- 1.Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Current opinion in neurobiology. 2014;24:166–175. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arango-Gonzalez B, Szabo A, Pinzon-Duarte G, Lukats A, Guenther E, Kohler K. In vivo and in vitro development of S- and M-cones in rat retina. Investigative ophthalmology & visual science. 2010;51:5320–5327. doi: 10.1167/iovs.09-4741. [DOI] [PubMed] [Google Scholar]
- 3.Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- 4.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Wang K, Lin B. Development and degeneration of cone bipolar cells are independent of cone photoreceptors in a mouse model of retinitis pigmentosa. PloS one. 2012;7:e44036. doi: 10.1371/journal.pone.0044036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JE, Kondrashev SL, Podugolnikova TA. Biplexiform ganglion cells, characterized by dendrites in both outer and inner plexiform layers, are regular, mosaic-forming elements of teleost fish retinae. Visual neuroscience. 1996;13:517–528. doi: 10.1017/s0952523800008191. [DOI] [PubMed] [Google Scholar]
- 7.Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. The Journal of comparative neurology. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- 9.Ford KJ, Felix AL, Feller MB. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:850–863. doi: 10.1523/JNEUROSCI.5309-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Visual neuroscience. 2012;29:61–71. doi: 10.1017/S0952523811000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guler AD, Altimus CM, Ecker JL, Hattar S. Multiple photoreceptors contribute to nonimage-forming visual functions predominantly through melanopsin-containing retinal ganglion cells. Cold Spring Harb Sym. 2007;72:509–515. doi: 10.1101/sqb.2007.72.074. [DOI] [PubMed] [Google Scholar]
- 12.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmberg K. The hagfish retina: fine structure of retinal cells in Myxine glutinosa, L., with special reference to receptor and epithelial cells. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1970;111:519–538. doi: 10.1007/BF00330929. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, Van Gelder RN, Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joo HR, Peterson BB, Dacey DM, Hattar S, Chen SK. Recurrent axon collaterals of intrinsically photosensitive retinal ganglion cells. Visual neuroscience. 2013;30:175–182. doi: 10.1017/S0952523813000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkby LA, Feller MB. Intrinsically photosensitive ganglion cells contribute to plasticity in retinal wave circuits. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12090–12095. doi: 10.1073/pnas.1222150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusunoki T, Amemiya F. Retinal projections in the hagfish, Eptatretus burgeri. Brain Res. 1983;262:295–298. doi: 10.1016/0006-8993(83)91021-1. [DOI] [PubMed] [Google Scholar]
- 19.Lamb TD, Collin SP, Pugh EN., Jr Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nature reviews. Neuroscience. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 21.Mariani AP. Biplexiform cells - ganglion-cells of the primate retina that contact photoreceptors. Science. 1982;216:1134–1136. doi: 10.1126/science.6177044. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 24.Muller LP, Do MT, Yau KW, He S, Baldridge WH. Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. The Journal of comparative neurology. 2010;518:4813–4824. doi: 10.1002/cne.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 26.Pushchin I, Kalachev A. Biplexiform ganglion cells contact photoreceptors in the retina of the greenling Hexagrammos octogrammus. Synapse. 2010;64:937–940. doi: 10.1002/syn.20832. [DOI] [PubMed] [Google Scholar]
- 27.Rao S, Chun C, Fan JQ, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstatter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. The Journal of comparative neurology. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- 29.Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nature neuroscience. 2011;14:827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. The Journal of comparative neurology. 1997;388:47–63. [PubMed] [Google Scholar]
- 31.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. Journal of neurophysiology. 2008;100:371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Current biology : CB. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. The European journal of neuroscience. 2012;35:507–518. doi: 10.1111/j.1460-9568.2011.07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wassle H, Dacey DM, Haun T, Haverkamp S, Grunert U, Boycott BB. The mosaic of horizontal cells in the macaque monkey retina: with a comment on biplexiform ganglion cells. Visual neuroscience. 2000;17:591–608. doi: 10.1017/s0952523800174097. [DOI] [PubMed] [Google Scholar]
- 36.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. The Journal of physiology. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PloS one. 2012;7:e42647. doi: 10.1371/journal.pone.0042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nature neuroscience. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- 40.Zrenner E, Nelson R, Mariani A. Intracellular-Recordings from a Biplexiform Ganglion-Cell in Macaque Retina, Stained with Horseradish-Peroxidase. Brain Res. 1983;262:181–185. doi: 10.1016/0006-8993(83)91007-7. [DOI] [PubMed] [Google Scholar]