Abstract
Helicobacter heilmannii-like organisms (HHLOs) are associated with mucosa-associated lymphoid tissue lymphoma and peptic ulcer. However, the sensitivity of diagnostic tests for HHLOs, such as rapid urease test (RUT), urea breath test (UBT) and blood antibody, is not high. Tightly coiled spiral microorganisms were found in the gastric mucosal biopsy specimen of a 48-year-old asymptomatic woman. Her findings were positive for RUT and UBT, but negative for blood antibody and stool antigen against H. pylori. A 7-day course of esomeprazole, amoxicillin and clarithromycin was administered, resulting in the successful eradication of the HHLOs. Analysis of the 16S rRNA and urease genes suggested a diagnosis of the HHLO H. suis. The sensitivity results of RUT, UBT, culture, blood antibody, immunohistochemistry and stool antigen were 40.0, 14.8, 0, 23.1, 40.0 and 0%, respectively. We report asymptomatic nodular gastritis due to an HHLO. Histological techniques, most likely with smears, are expected to be the most effective method for diagnosing infections by HHLOs, and genetic diagnosis by polymerase chain reaction can be very useful to identify the species of HHLOs.
Key Words: Helicobacter heilmannii-like organisms, Helicobacter suis, Urea breath test, Rapid urease test, Stool antigen test
Introduction
Helicobacter species are Gram-negative, spiral-shaped bacteria. Helicobacter species other than H. pylori, including H. heilmannii and H. felis, also referred to as H. heilmannii-like organisms (HHLOs), have been identified in gastric mucosa-associated lymphoid tissue lymphoma and peptic ulcer in humans. HHLOs are found in a small percentage of human subjects (0.1–6%) [1, 2]; however, HHLO infection is prevalent in gastric mucosa-associated lymphoid tissue lymphoma [3, 4]. HHLO infection is very common in dogs, cats, pigs and nonhuman primates [5, 6].
HHLO infection is most frequently diagnosed by histopathology to identify the organism's morphology. Culture of HHLOs by traditional H. pylori culture techniques is difficult, although one research group has been successful [7]. The 13C urea breath test (UBT) may be used to detect gastric HHLOs in animals [5, 8]. Some human cases have yielded positive results by the rapid urease test (RUT), the UBT and anti-H. pylori antibody, although the sensitivity of these tests is unclear. We report a case of nodular gastritis with HHLO infection diagnosed as H. suis by genetic sequencing. We also review the sensitivity of HHLO diagnostic methods.
Methods
DNA Extraction
Gastric biopsy samples were digested in SNET buffer (1% SDS, 400 mm NaCl, 5 mm EDTA, 20 mm Tris-HCl, pH 8.0) containing proteinase K (0.2 mg/ml) overnight at 50°C. After 10-min incubation at 95°C, the sample was serially diluted tenfold. The DNA was stored at −20°C until use.
Amplification of HHLO-Specific or H. pylori-Specific DNA
At least 1-, 10- or 100-fold diluted samples were used as DNA templates for polymerase chain reaction (PCR). PCR amplification involved Ampdirect® Plus (Shimadzu Corporation, Kyoto, Japan), BIOTAQTM HS DNA Polymerase (Shimadzu Corporation), 0.5 µm each of primers HeilF and HeilR (HHLO-specific) or VAC3624F and VAC4041R (H. pylori-specific) [9] using a CFX96 thermal cycler (Bio-Rad Laboratories Inc., Hercules, Calif., USA).
Amplification and Sequencing of Urease Genes
Urease genes were amplified using primer pairs U430F and U1735R or U430F and U2235R (urease two, 1,752 bp) [10]. The products were sequenced commercially (Fasmac Co. Ltd., Atsugi, Japan) with the same primers and other primers (U850F, U1050R and U1350F) [10]. The sequences were compared with those in the NCBI GenBank by using the BLAST search tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Case Presentation
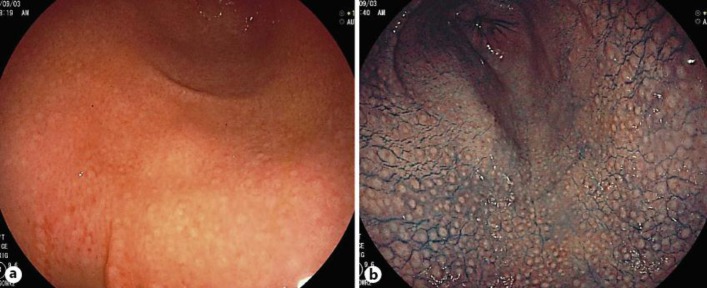
In June 2013, a 48-year-old woman was diagnosed with nodular gastritis by esophagogastroduodenal endoscopy at Marin clinic. Histology was positive for HHLO. Nothing abnormal except for gastritis was pointed out. The patient was referred to Aichi Medical University Hospital for treatment in August. She did not have any related medical history and had never come in contact with domestic animals, including cats and dogs. Esophagogastroduodenal endoscopy showed antral nodular gastritis without mucosal atrophy; the corpus was normal (fig. 1). The 13C UBT finding was positive with a 13C value of 3.3% (cutoff value 2.5%). The RUT (PYLORITEK®, Serim Research Corp., Elkhart, Ind., USA) finding was positive in 30 min, but the results of the stool antigen and serum anti-H. pylori IgG antibody tests were negative. However, HHLO of characteristic morphology was found in the antral mucosa and in a gastric pit only (fig. 2). Histopathology showed mild chronic gastritis in the stomach antrum and corpus mucosa and no atrophy or intestinal metaplasia. The patient was treated with a 7-day course of triple therapy consisting of esomeprazole, amoxicillin and clarithromycin. There were no adverse events. Two months after therapy, UBT findings were negative (0.8%).
Fig. 1.
a White light endoscopy of the antrum showed small, round, yellowish-white nodules, which were the features of H. pylori-infected mucosa. b The nodular pattern can be clearly observed with indigo carmine spreading.
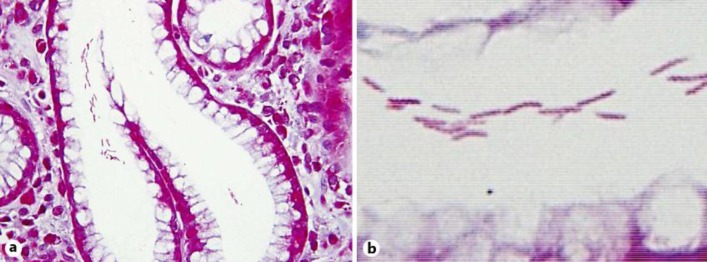
Fig. 2.
a Gimenez staining of the antral mucosa showed mild chronic gastritis and microbial colonies in a pit (×400). b Long, tightly spiraled microorganisms were observed (×1,000).
We obtained informed consent from patients and investigated the HHLO by PCR. An HHLO-specific amplicon was obtained from the sample. The sequences of the 16S rRNA gene (1,167 bp; fig. 3a) and the urease gene (916 bp, including a 4-bp undetermined sequence; fig. 3b) were determined. The 16S rRNA gene sequence of the sample showed 99% (1,162/1,167) and 96% (1,117/1,165) sequence identity with that of H. suis type strain HS1T (NR_044169) and H. heilmannii type strain ASB1T (HE984298), respectively. The urease gene sequence of the sample showed 96% (882/918) sequence identity with sequences from GenBank accession no. AB968248, AB968247 and AF508001 from H. suis SH8, SH10 and HU4, respectively. The urease gene had 95% (772/815) identity with the sequence of H. suis type strain HS1T (EF204592), whereas it had only 82% (659/801), 81% (727/894) and 80% (692/860) identity with corresponding sequences of H. bizzozeronii type strain StorkisT (AF508003), Candidatus H. heilmannii reference strain HU2R (AF508012) and H. heilmannii type strain ASB1T (HE984298), respectively. On the basis of both sequences, we conclude that the species of the strain is H. suis.
Fig. 3.
The 16S rRNA gene (1,167 bp, a) and the urease gene (916 bp, b) were sequenced commercially (Fasmac Co. Ltd.). N = Undetermined gene.
Discussion
We reported an asymptomatic patient with H. suis-infected nodular gastritis demonstrated by biopsy specimens. Genetic diagnosis of this infection by PCR was very useful to identify the HHLO species as H. suis.
HHLO is a zoonotic agent transmitted from animals to humans [11], and most infected persons complain of dyspepsia, epigastric pain or acid reflux [12, 13]. However, the current patient had no contact with domestic animals, including cats and dogs. Moreover, she had no symptoms despite histologically mild chronic gastritis. Stolte and colleagues [3, 4, 14] reported that HHLO infection is more often focal, with fewer organisms, and usually restricted to the gastric antrum, with less severe gastritis than H. pylori infection, leading to the rarity of concurrent erosions and ulcers.
Gastric non-H. pylori helicobacters, which are morphologically long, spiral-shaped bacteria, were originally referred to as Gastrospirillum hominis and later as H. heilmannii.
H. heilmannii was further subdivided in two taxa, types 1 and 2. H. heilmannii type 2 is a group of species that colonize the gastric mucosa: H. felis, H. bizzozeronii, H. salomonis, H. cynogastricus, H. baculiformis and Candidatus H. heilmannii. On the other hand, H. suis has been accepted as a new gastric Helicobacter taxon corresponding to type 1 H. heilmannii [15]. The cells of this new species are tightly coiled spirals with up to six turns, which are approximately 2.3–6.7 µm long and 0.9–1.2 µm wide. They have bipolar tufts of 4–10 sheathed flagella that are blunt-ended or end in spherical knobs [15].
The characteristic morphology of HHLOs can be identified in biopsy specimens stained with hematoxylin and eosin, Giemsa or Warthin-Starry silver stains. The present case was also diagnosed by morphology on the basis of gastric biopsy examination, as in many reported cases. However, HHLOs are better diagnosed on smears (touch cytology) than biopsies [13, 16] because they are typically found in the mucus layer above the surface and foveolar epithelial cells and do not show the intimate adherence, pedestal formation or invasion in the intercellular spaces often seen with H. pylori.
Other diagnostic tests in the present case showed positive results for UBT and RUT and negative results for stool antigen and serum anti-H. pylori IgG antibody. The sensitivity of diagnostic tests for HHLOs is summarized in table 1. The findings were as follows: RUT 40.0%, UBT 14.8%, culture 0%, blood antibody 23.1%, immunohistochemistry 40.0% and stool antigen 0%. As expected, in vitro culture of HHLO failed in 55 patients, although culture was successful in the cat stomach [17]. The urease test seems useful for diagnosing HHLO infection, although it is difficult to distinguish HHLO from H. pylori infection. The rate of urease positivity appears to be lower than that for H. pylori. HHLOs probably have a lower amount of urease than H. pylori, and the test might be positive only when numerous organisms are present in the tested specimen. The UBT and RUT tests are highly sensitive and specific for HHLO in animals, and the discrepancy in these diagnostic tests in humans and animals is unclear [5, 7]. Serological and immunohistochemical tests with H. pylori antibody yielded lower positivity rates for HHLO than for H. pylori infection itself. The enzyme-linked immunosorbent assay of stool with H. pylori polyclonal antigens showed cross-reactivity with HHLO antigens. It is critical to develop HHLO-specific antibodies for serological, immunohistochemical and stool tests.
Table 1.
Sensitivity of diagnostic tests for HHLOs
| Year | First author | RUT |
UBT |
Culture |
Blood Ab (H. pylori) |
IHC (H. pylori) |
Stool Ag (H. pylori) |
Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | + | − | + | − | |||
| 1989 | Dye | 2 | 0 | 0 | 1 | 19 | ||||||||
| 1990 | Morris | 2 | 0 | 0 | 2 | 0 | 2 | 20 | ||||||
| 1990 | Figura | 1 | 1 | 0 | 2 | 0 | 2 | 21 | ||||||
| 1990 | Fischer | 0 | 4 | 0 | 3 | 22 | ||||||||
| 1991 | Wegmann | 2 | 3 | 23 | ||||||||||
| 1991 | Borody | 0 | 2 | 0 | 2 | 24 | ||||||||
| 1993 | Mazzucchelli | 0 | 20 | 0 | 2 | 0 | 2 | 25 | ||||||
| 1994 | Lavelle | 1 | 0 | 26 | ||||||||||
| 1995 | Yang | 1 | 0 | 27 | ||||||||||
| 1995 | Hilzenrat | 0 | 3 | 12 | ||||||||||
| 1995 | Akin | 1 | 0 | 28 | ||||||||||
| 1995 | Koyanagi | 0 | 1 | 29 | ||||||||||
| 1997 | Goddard | 1 | 0 | 0 | 1 | 30 | ||||||||
| 1997 | Isomoto | 0 | 1 | 31 | ||||||||||
| 1998 | Chen | 14 | 6 | 3 | 17 | 0 | 20 | 32 | ||||||
| 1998 | Debongnie | 1 | 8 | 0 | 7 | 1 | 8 | 16 | ||||||
| 1999 | Jhala | 3 | 2 | 0 | 4 | 5 | 0 | 33 | ||||||
| 1999 | Yamamoto | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 34 | ||||
| 2000 | Kamoshida | 1 | 0 | 0 | 1 | 0 | 1 | 35 | ||||||
| 2002 | Yoshimura | 1 | 0 | 0 | 1 | 36 | ||||||||
| 2003 | Boyanova | 1 | 0 | 0 | 1 | 37 | ||||||||
| 2005 | Singhal | 1 | 0 | 38 | ||||||||||
| 2005 | Kato | 0 | 1 | 0 | 1 | 0 | 1 | 39 | ||||||
| 2006 | Oyauchi | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 40 | ||
| 2007 | Boyanova | 2 | 0 | 0 | 2 | 41 | ||||||||
| 2007 | Qualia | 1 | 1 | 42 | ||||||||||
| 2012 | Ohtaka | 0 | 1 | 0 | 1 | 43 | ||||||||
| 2014 | Matsumoto | 0 | 1 | 0 | 1 | 1 | 0 | 44 | ||||||
| 2015 | Goji | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | present case | ||||
| Total | 40 | 29 | 4 | 23 | 0 | 55 | 6 | 20 | 2 | 3 | 0 | 3 | ||
| Positive rate | 40.0% | 14.8% | 0% | 23.1% | 40.0% | 0% | ||||||||
Ab = Antibody; Ag = antigen; IHC = immunohistochemistry.
In the present case, PCR was used to identify the species of HHLO as H. suis. PCR-based studies have been used to identify HHLOs in humans [4] and animals [3, 4, 18]. Chisholm and Owen [9] performed PCR using gastric biopsies, showing an HHLO prevalence rate of 2.3% in Southeast England. However, this prevalence rate is not as high as that diagnosed by morphology. PCR sensitivity for HHLOs may be lower in clinical practice, and the typical morphological features on hematoxylin and eosin staining seem sufficient to diagnose HHLO infection with chronic gastritis.
Conclusion
We describe asymptomatic nodular gastritis infection associated with an HHLO. Histological techniques, most likely with smears, will be the most effective method for diagnosing HHLO infection. Furthermore, genetic diagnosis by PCR was shown to be very useful to identify the species of non-H. pylori helicobacters.
Disclosure Statement
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in this paper.
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Ierardi E, Monno RA, Gentile A, Francavilla R, Burattini O, Marangi S, Pollice L, Francavilla A. Helicobacter heilmannii gastritis: a histological and immunohistochemical trait. J Clin Pathol. 2001;54:774–777. doi: 10.1136/jcp.54.10.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yakoob J, Abbas Z, Khan R, Naz S, Ahmad Z, Islam M, Awan S, Jafri F, Jafri W. Prevalence of non Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol. 2012;12:3. doi: 10.1186/1471-230X-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolte M, Bayerdorffer E, Morgner A, Alpen B, Wundisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut. 2002;50(suppl 3):III19–III24. doi: 10.1136/gut.50.suppl_3.iii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdorffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. doi: 10.1016/s0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 5.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthesy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Queiroz DM, Rocha GA, Mendes EN, De Moura SB, De Oliveira AM, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996;111:19–27. doi: 10.1053/gast.1996.v111.pm8698198. [DOI] [PubMed] [Google Scholar]
- 7.Trevena WB, Hooper RS, Wray C, Willshaw GA, Cheasty T, Domingue G. Vero cytotoxin-producing Escherichia coli O157 associated with companion animals. Vet Rec. 1996;138:400. [PubMed] [Google Scholar]
- 8.Kubota S, Ohno K, Tsukamoto A, Maeda S, Murata Y, Nakashima K, Fukushima K, Uchida K, Fujino Y, Tsujimoto H. Value of the 13C-urea breath test for detection of gastric Helicobacter spp. infection in dogs undergoing endoscopic examination. J Vet Med Sci. 2013;75:1049–1054. doi: 10.1292/jvms.12-0528. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm SA, Owen RJ. Development and application of a novel screening PCR assay for direct detection of ‘Helicobacter heilmannii’-like organisms in human gastric biopsies in Southeast England. Diagn Microbiol Infect Dis. 2003;46:1–7. doi: 10.1016/s0732-8893(02)00547-3. [DOI] [PubMed] [Google Scholar]
- 10.O'Rourke JL, Solnick JV, Neilan BA, Seidel K, Hayter R, Hansen LM, Lee A. Description of ‘Candidatus Helicobacter heilmannii’ based on DNA sequence analysis of 16S rRNA and urease genes. Int J Syst Evol Microbiol. 2004;54:2203–2211. doi: 10.1099/ijs.0.63117-0. [DOI] [PubMed] [Google Scholar]
- 11.Baele M, Pasmans F, Flahou B, Chiers K, Ducatelle R, Haesebrouck F. Non-Helicobacter pylori helicobacters detected in the stomach of humans comprise several naturally occurring Helicobacter species in animals. FEMS Immunol Med Microbiol. 2009;55:306–313. doi: 10.1111/j.1574-695X.2009.00535.x. [DOI] [PubMed] [Google Scholar]
- 12.Hilzenrat N, Lamoureux E, Weintrub I, Alpert E, Lichter M, Alpert L. Helicobacter heilmannii-like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995;119:1149–1153. [PubMed] [Google Scholar]
- 13.Debongnie JC, Donnay M, Mairesse J. Gastrospirillum hominis (‘Helicobacter heilmannii’): a cause of gastritis, sometimes transient, better diagnosed by touch cytology? Am J Gastroenterol. 1995;90:411–416. [PubMed] [Google Scholar]
- 14.Stolte M, Kroher G, Meining A, Morgner A, Bayerdorffer E, Bethke B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997;32:28–33. doi: 10.3109/00365529709025059. [DOI] [PubMed] [Google Scholar]
- 15.Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Mast J, Chiers K, Ducatelle R, Haesebrouck F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58:1350–1358. doi: 10.1099/ijs.0.65133-0. [DOI] [PubMed] [Google Scholar]
- 16.Debongnie JC, Donnay M, Mairesse J, Lamy V, Dekoninck X, Ramdani B. Gastric ulcers and Helicobacter heilmannii. Eur J Gastroenterol Hepatol. 1998;10:251–254. doi: 10.1097/00042737-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Lee A, Dent J, Hazell S, McNulty C. Origin of spiral organisms in human gastric antrum. Lancet. 1988;1:300–301. doi: 10.1016/s0140-6736(88)90382-0. [DOI] [PubMed] [Google Scholar]
- 18.Norris CR, Marks SL, Eaton KA, Torabian SZ, Munn RJ, Solnick JV. Healthy cats are commonly colonized with ‘Helicobacter heilmannii’ that is associated with minimal gastritis. J Clin Microbiol. 1999;37:189–194. doi: 10.1128/jcm.37.1.189-194.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dye KR, Marshall BJ, Frierson HF, Jr, Guerrant RL, McCallum RW. Ultrastructure of another spiral organism associated with human gastritis. Dig Dis Sci. 1989;34:1787–1791. doi: 10.1007/BF01540059. [DOI] [PubMed] [Google Scholar]
- 20.Morris A, Ali MR, Thomsen L, Hollis B. Tightly spiral shaped bacteria in the human stomach: another cause of active chronic gastritis? Gut. 1990;31:139–143. doi: 10.1136/gut.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figura N, Guglielmetti P, Quaranta S. Spiral shaped bacteria in gastric mucosa. J Clin Pathol. 1990;43:173. doi: 10.1136/jcp.43.2.173-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer R, Samisch W, Schwenke E. ‘Gastrospirillum hominis’: Another four cases. Lancet. 1990;335:59. doi: 10.1016/0140-6736(90)90195-b. [DOI] [PubMed] [Google Scholar]
- 23.Wegmann W, Aschwanden M, Schaub N, Aenishanslin W, Gyr K. Gastritis associated with Gastrospirillum hominis – a zoonosis? Schweiz Med Wochenschr. 1991;121:245–254. [PubMed] [Google Scholar]
- 24.Borody TJ, George LL, Brandl S, Andrews P, Ostapowicz N, Hyland L, Devine M. Helicobacter pylori-negative duodenal ulcer. Am J Gastroenterol. 1991;86:1154–1157. [PubMed] [Google Scholar]
- 25.Mazzucchelli L, Wilder-Smith CH, Ruchti C, Meyer-Wyss B, Merki HS. Gastrospirillum hominis in asymptomatic, healthy individuals. Dig Dis Sci. 1993;38:2087–2089. doi: 10.1007/BF01297089. [DOI] [PubMed] [Google Scholar]
- 26.Lavelle JP, Landas S, Mitros FA, Conklin JL. Acute gastritis associated with spiral organisms from cats. Dig Dis Sci. 1994;39:744–750. doi: 10.1007/BF02087417. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Li X, Xu Z, Zhou D. ‘Helicobacter heilmannii’ infection in a patient with gastric cancer. Dig Dis Sci. 1995;40:1013–1014. doi: 10.1007/BF02064190. [DOI] [PubMed] [Google Scholar]
- 28.Akin OY, Tsou VM, Werner AL. Gastrospirillum hominis-associated chronic active gastritis. Pediatr Pathol Lab Med. 1995;15:429–435. doi: 10.3109/15513819509026978. [DOI] [PubMed] [Google Scholar]
- 29.Koyanagi M, Tanaka M, Nishi T, Kawaguchi H, Kudo H. Acute gastric mucosal lesions accompanied by Gastrospirillum hominis, report of a case. Stomach Intestine. 1995;30:1079–1083. [Google Scholar]
- 30.Goddard AF, Logan RP, Atherton JC, Jenkins D, Spiller RC. Healing of duodenal ulcer after eradication of Helicobacter heilmannii. Lancet. 1997;349:1815–1816. doi: 10.1016/S0140-6736(05)61696-0. [DOI] [PubMed] [Google Scholar]
- 31.Isomoto H, Matsunaga K, Shikuwa S, Ofukuji M, Mizuta Y, Makiyama K, Kohno S. A case of Gastrospirillum hominis-associated corpus gastritis. Gastroenterol Endosc. 1997;39:68–72. [Google Scholar]
- 32.Chen Y, Zhou D, Wang J. Biological diagnostic and therapeutic study on the infection of Helicobacter heilmannii. Zhonghua Yi Xue Za Zhi. 1998;78:490–493. [PubMed] [Google Scholar]
- 33.Jhala D, Jhala N, Lechago J, Haber M. Helicobacter heilmannii gastritis: association with acid peptic diseases and comparison with Helicobacter pylori gastritis. Mod Pathol. 1999;12:534–538. [PubMed] [Google Scholar]
- 34.Yamamoto T, Matsumoto J, Shiota K, Kitajima S, Goto M, Imaizumi M, Arima T. Helicobacter heilmannii associated erosive gastritis. Intern Med. 1999;38:240–243. doi: 10.2169/internalmedicine.38.240. [DOI] [PubMed] [Google Scholar]
- 35.Kamoshida T, Hotta S, Hirai S, Oka Y. A case of Helicobacter heilmannii-associated antral gastritis diagnosed by McMullen modified staining. Gastroenterol Endosc. 2000;42:974–979. [Google Scholar]
- 36.Yoshimura M, Isomoto H, Shikuwa S, Osabe M, Matsunaga K, Omagari K, Mizuta Y, Murase K, Murata I, Kohno S. A case of acute gastric mucosal lesions associated with Helicobacter heilmannii infection. Helicobacter. 2002;7:322–326. doi: 10.1046/j.1523-5378.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyanova L, Koumanova R, Lazarova E, Jelev C. Helicobacter pylori and Helicobacter heilmannii in children. A Bulgarian study. Diagn Microbiol Infect Dis. 2003;46:249–252. doi: 10.1016/s0732-8893(03)00085-3. [DOI] [PubMed] [Google Scholar]
- 38.Singhal AV, Sepulveda AR. Helicobacter heilmannii gastritis: a case study with review of literature. Am J Surg Pathol. 2005;29:1537–1539. doi: 10.1097/01.pas.0000169499.96658.6e. [DOI] [PubMed] [Google Scholar]
- 39.Kato S, Ozawa K, Sekine H, Ohyauchi M, Shimosegawa T, Minoura T, Iinuma K. Helicobacter heilmannii infection in a child after successful eradication of Helicobacter pylori: case report and review of literature. J Gastroenterol. 2005;40:94–97. doi: 10.1007/s00535-004-1499-2. [DOI] [PubMed] [Google Scholar]
- 40.Oyauchi M, Ohara S, Sekine H, Shimosegawa T. Barrett's adenocarcinoma with Helicobacter heilmannii infection, report of a case. Stomach Intestine. 2006;41:1089–1093. [Google Scholar]
- 41.Boyanova L, Lazarova E, Jelev C, Gergova G, Mitov I. Helicobacter pylori and Helicobacter heilmannii in untreated Bulgarian children over a period of 10 years. J Med Microbiol. 2007;56:1081–1085. doi: 10.1099/jmm.0.47181-0. [DOI] [PubMed] [Google Scholar]
- 42.Qualia CM, Katzman PJ, Brown MR, Kooros K. A report of two children with Helicobacter heilmannii gastritis and review of the literature. Pediatr Dev Pathol. 2007;10:391–394. doi: 10.2350/06-09-0159.1. [DOI] [PubMed] [Google Scholar]
- 43.Ohtaka M, Tatsumi A, Fukasawa M, Yamaguchi T, Uetake T, Ohtska H, Sato T, Enomoto N, Watanabe H, Mitani K. Complete remission of gastric plasmacytoma following eradication of ‘Candidatus Helicobacter heilmannii’. Clin J Gastroenterol. 2012;5:158–163. doi: 10.1007/s12328-012-0287-4. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto T, Kawakubo M, Akamatsu T, Koide N, Ogiwara N, Kubota S, Sugano M, Kawakami Y, Katsuyama T, Ota H. Helicobacter heilmannii sensu stricto-related gastric ulcers: a case report. World J Gastroenterol. 2014;20:3376–3382. doi: 10.3748/wjg.v20.i12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]