Abstract
We report a case of hypokalemia resulting from colonic pseudo-obstruction or Ogilvie's syndrome. Colonic pseudo-obstruction is characterized by profuse watery diarrhea that has a low sodium and high potassium concentration. It is seen in a variety of medical and surgical conditions, but its exact cause remains unknown. It is thought to result from an imbalance of sympathetic and parasympathetic input in the distal colon. The diarrhea is secretory and driven by potassium secretion rather than the inhibition of sodium reabsorption or chloride secretion, which are the most common pathophysiologic mechanisms of secretory diarrhea. Affected patients often lose >100 mmol of potassium daily. Colonic pseudo-obstruction is associated with a dramatic upregulation of the maxiK or BK potassium channel. This channel plays a prominent role in flow-mediated potassium secretion in the connecting tubule and collecting duct and is also upregulated in the distal colon in patients with advanced chronic kidney disease and end-stage renal disease. In vitro studies show that the channel is regulated by catecholamine binding to the β receptor and cyclic AMP upregulation, somatostatin and aldosterone, insights that can be used to help guide pharmacologic therapy. Nephrologists should be aware of colonic pseudo-obstruction as a cause of extrarenal potassium loss.
Key Words: Hypokalemia, Colonic pseudo-obstruction, Ogilvie's syndrome
Case Presentation
The patient was a 68-year-old man with a history of type II diabetes mellitus, peripheral neuropathy, hypertension, chronic obstructive pulmonary disease on home oxygen, bipolar depression, and gastroesophageal reflux who presented with shortness of breath and cough for 2 weeks. He had noted a decrease in exercise tolerance and intermittent diarrhea over this time. In the emergency department, he was treated with vancomycin and piperacillin/tazobactam and transferred to the medical intensive care unit where dopamine was started for hypotension and presumed sepsis. His condition stabilized and he was transferred to a medical floor where the hospital course was complicated by a pulmonary embolus, colonic distension and profuse watery diarrhea. The patient was diagnosed with colonic pseudo-obstruction (Ogilvie's syndrome), and nasogastric and rectal tubes were placed. The renal service was consulted for hypokalemia that was difficult to control with potassium supplementation. Medications at the time of consultation included: aspirin 81 mg daily, atorvastatin 80 mg daily, budesonide/formoterol, levalbuterol, tiotroprium, insulin, pantoprazole 40 mg daily, piperacillin/tazobactam and a total of 100 mEq of potassium chloride daily. His blood pressure was 103/50 mm Hg, his pulse was 102 beats per minute, and the respiratory rate was 24 breaths per minute. On physical examination, the patient was tachypneic and was using accessory muscles. Rhonchi were present in the anterior lung fields. The abdomen was distended with very hypoactive bowel sounds. There was tenderness to palpation in the right upper quadrant and midepigastric area. Trace lower extremity edema was noted. A Foley catheter and rectal tube were in place. Laboratory evaluation revealed a serum sodium concentration of 146 mmol/l, chloride 118 mmol/l, potassium 2.7 mmol/l, and bicarbonate 19.9 mmol/l. Blood urea nitrogen and serum creatinine concentrations were 6.1 mmol/l and 110 μmol/l, respectively. Of note, the serum potassium concentration on admission was 4.1 mmol/l. Arterial blood gases showed a pH of 7.27, pCO2 of 36.9 mm Hg and bicarbonate of 17.1 mEq/l, compatible with a simple metabolic acidosis. Urine electrolytes: sodium 49 mmol/l, potassium 20 mmol/l, and chloride 90 mmol/l with a urine anion gap of minus 21. A 24-hour urine collection showed 9.1 mmol of potassium excreted. Stool electrolytes revealed a sodium concentration of <10 mmol/l and a potassium concentration of 139.7 mmol/l. Marked colonic distension (13 cm) was seen on a radiographic examination of the abdomen (fig. 1). A diagnosis of severe gastrointestinal potassium wasting as a result of colonic pseudo-obstruction (Ogilvie's syndrome) was made. The patient received large doses of potassium chloride (>100 mmol/day) with serum potassium concentrations maintained in the 3.5–4.0 mmol/l range. His respiratory status deteriorated. The family decided to withdraw care, and the patient expired.
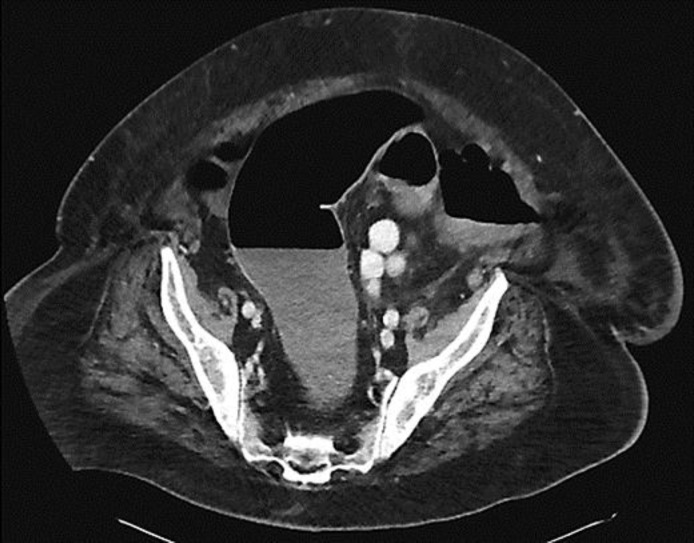
Fig. 1.
A CT scan of the abdomen. A representative image of the scan showing marked dilation of the colon. There was distention of the entire colon including the rectum, with the colon measuring up to 7 cm in diameter, and the cecum up to 9 cm in diameter.
Discussion
Hypokalemia may be the result of one or more basic processes including: inadequate oral intake, a shift of potassium from the extracellular fluid to the intracellular fluid, renal losses or gastrointestinal losses [1]. Inadequate intake alone is rarely a cause of hypokalemia given the kidney's ability to reduce potassium excretion to about 10 mmol/day. Renal and gastrointestinal losses are associated with a normal or low blood pressure. With renal losses, the urinary potassium excretion generally exceeds 20–30 mmol per day, whereas with gastrointestinal losses, there is renal potassium conservation, and urinary potassium excretion is generally less than 20 mmol/day. Gastrointestinal losses from the upper tract, such as with vomiting or nasogastric drainage, are associated with metabolic alkalosis, whereas metabolic acidosis is seen with diarrhea and laxative abuse. Our patient had hypokalemia, metabolic acidosis, and a low 24-hour urinary potassium excretion as a result of diarrheal losses. His potassium concentration on admission was normal. Stool electrolyte studies showed a very low sodium (<10 mmol/l) and very high potassium concentration (139.7 mmol/l). This pattern is typical in patients with colonic pseudo-obstruction also known as Ogilvie's syndrome.
Colonic pseudo-obstruction or Ogilvie's syndrome was first described in 1948 by Sir William Ogilvie [2]. As its name implies, it is characterized by a functional and not mechanical obstruction of the colon for reasons that are poorly understood. It has been speculated that the syndrome results from an imbalance in autonomic nervous system input in the colon with a stimulation of sympathetic and a suppression of parasympathetic activity. In the 2 patients originally reported by Ogilvie, there was an invasion of the celiac plexus by malignancy. Vanek and Al-Salti [3] analyzed 400 patients with colonic pseudo-obstruction. About half of the cases occurred in the postoperative period. Patients presented with symptoms typical of bowel obstruction including abdominal distension, nausea and vomiting. The majority had diarrhea, but constipation was described in a subset of patients. The diagnosis was most often made on plain film of the abdomen, which was most commonly suggestive of a distal colonic obstruction that ultimately was found to be functional in nature. The distal colon receives its parasympathetic innervation from spinal segments S2–S4, and the disruption of this input (which normally stimulates intestinal contraction) can result in a functional obstruction.
Binder's group carefully examined a 78-year-old woman that presented 1 week after surgery for a hip fracture with colonic pseudo-obstruction [4]. Severe diarrhea, hypernatremia, hypokalemia and metabolic acidosis were noted. Fecal potassium concentrations ranged from 130 to 170 mmol/l, and fecal sodium concentrations were between 4 and 15 mmol/l. They concluded that colonic pseudo-obstruction was a unique form of secretory diarrhea that is mediated by excessive potassium and not the sodium losses in stool. Up until this time, secretory diarrhea was recognized to result from either the inhibition of sodium reabsorption or active chloride secretion, followed by the passive movement of sodium to maintain electroneutrality. The accumulation of sodium salts in the intestinal lumen obligates the isotonic loss of water resulting in sodium and water losses in stool along with some potassium. In patients with Ogilvie's syndrome, it is the active secretion of potassium that drives the secretory diarrhea and stool potassium losses are high, while stool sodium losses are low. This is reflected in stool electrolytes that reveal a very high potassium concentration, >130 mmol/l, and a very low sodium concentration, <15 mmol/l. This same pattern of stool electrolytes was subsequently confirmed in a case series of 5 patients with colonic pseudo-obstruction [5]. Binder speculated that 3 mechanisms could be responsible for the stimulation of active colonic potassium secretion including hyperaldosteronism resulting from diarrhea-induced volume depletion, the activation of β-adrenergic receptors by catecholamines as well as colonic distension. An imbalance in autonomic stimulation was postulated to be responsible for the potassium secretion that occurs in the setting of catecholamine-stimulated sodium reabsorption. This results in hypokalemia as a result of fecal potassium losses and hypernatremia due to fecal water losses in the setting of low sodium losses.
Subsequent reports further clarified the mechanism of the potassium secretory process in the colon. Simon et al. [6] described a patient with colonic pseudo-obstruction after hemorrhagic shock. Stool electrolytes showed the typical pattern of a low sodium concentration (11 mmol/l) and a high potassium concentration (143 mmol/l). The diarrhea did not resolve after ileostomy, and the patient eventually underwent a colectomy with the resolution of hypokalemia. Immunostaining showed a marked overexpression of the large conductance maxiK or BK channel in both surface and crypt colonocytes far beyond that seen in end-stage renal disease (ESRD). A cell model of a typical colonic epithelial cell is shown in figure 2. Potassium movement into the colonic epithelial cell across the basolateral membrane can occur on either the Na/K ATPase or the NCC1 isoform of the Na/K/2Cl cotransporter. Potassium then exits the cell via a large conductance potassium channel known as maxiK or BK. This is the same channel that is expressed in the connecting tubule and collecting ducts of the kidney that mediates flow-stimulated potassium secretion. It is regulated by cAMP and protein kinase A as is the chloride channel CFTR, and it is inhibited by somatostatin, an effect that is mediated via tyrosine phosphorylation [7]. Somatostatin was shown as early as 1984 in a case report to reduce the stool volume dramatically in a patient with colonic pseudo-obstruction [8]. BK is a heteromeric protein consisting of 4 α and 4 β subunits. There are multiple splice variants at the C-terminus of the α subunit. The ZERO splice variant, which is missing the 58 amino acid exon 18, is activated by cAMP [9]. In vitro studies show that adrenaline binding to the β receptor upregulates the ZERO splice variant, and this effect is blocked by propranolol. Our patient was also receiving inhaled β-2 agonists, and this may have contributed to the colonic potassium losses. The ZERO variant is also upregulated by a high potassium diet and aldosterone. Factors that stimulate and inhibit the channel are shown in table 1.
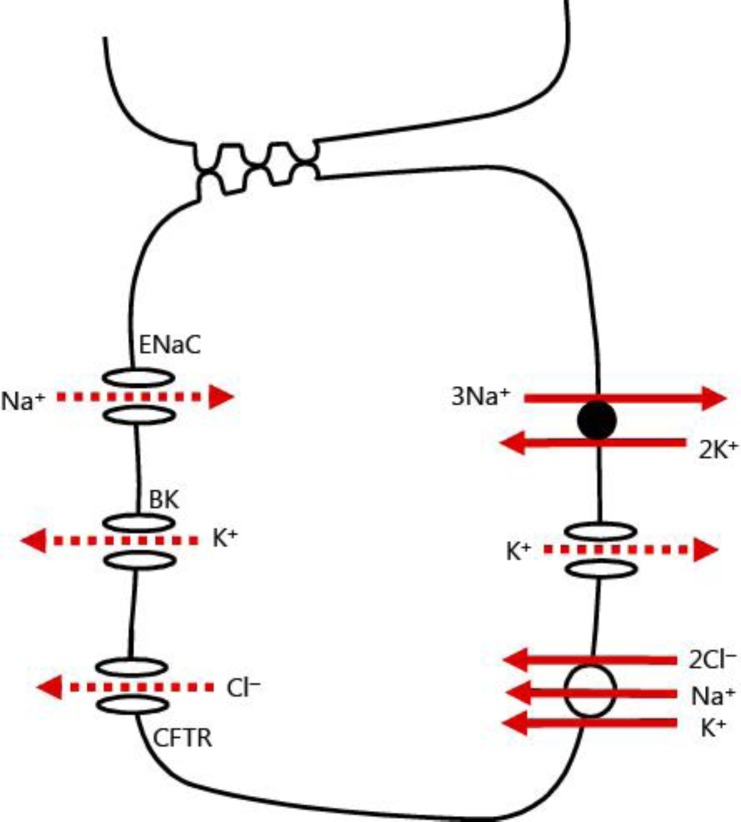
Fig. 2.
Colonic epithelial cell transport model. The Na-K ATPase is shown in the basolateral membrane. A basolateral potassium channel is present that plays a role in potassium recycling to support Na-K ATPase activity. Sodium and chloride enter the cell via a basolateral Na-K-2Cl cotransporter. The BK channel in the apical membrane mediates potassium secretion, while cystic fibrosis transmembrane conductance regulator (CFTR) mediates chloride secretion. Electrogenic sodium transport is carried out by epithelial sodium channel (ENaC).
Table 1.
Stimulators and inhibitors of BK channels in the colon
| Stimulators | Inhibitors |
|---|---|
| Protein kinase A | Somatostatin-G protein dependent |
| Aldosterone | |
| Epinephrine-β receptor | |
| High potassium diet | |
| ATP and UTP via P2Y2 and P2Y4 receptors |
The BK channel is the same channel that plays an important role in potassium homeostasis in later stages of chronic kidney disease and ESRD. Under normal circumstances, the majority of total body potassium excretion occurs via the kidney. However, as renal function declines, the colon plays an increasingly important role in potassium excretion. The distal colon becomes a potassium secretory organ, especially in patients with ESRD [10]. Under basal conditions, potassium secretion is about threefold greater in ESRD patients compared to those with normal renal function [11]. Immunostaining shows an increase in apical BK channels in surface colonocytes and crypt cells. This effect is lost in BK knockout mice, and the BK channel appears to be the sole exit pathway for potassium movement into the colonic lumen [12].
In summary, the nephrologist should be aware of colonic pseudo-obstruction because it can cause profound hypokalemia that requires large amounts of potassium supplementation to maintain potassium balance. The diagnosis is established by demonstrating marked colonic dilation in the absence of a mechanical obstruction on imaging studies associated with a high stool potassium concentration and a low stool sodium concentration.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Perazella MA, Rastegar M. Disorders of potassium homeostasis. In: Reilly RF, Perazella MA, editors. Nephrology in Thirty Days. ed 2. New York: McGraw Hill; 2013. pp. 71–88. chapter 6. [Google Scholar]
- 2.Ogilvie WH. Large intestine colic due to sympathetic deprivation: a new clinical syndrome. Br Med J. 1948;2:671–673. doi: 10.1136/bmj.2.4579.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie's syndrome): an analysis of 400 cases. Dis Colon Rectum. 1986;29:203–210. doi: 10.1007/BF02555027. [DOI] [PubMed] [Google Scholar]
- 4.van Dinter TG, Jr, Fuerst FC, Richardson CT, Santa Ana CA, Polter DE, Fordtran JS, Binder H. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology. 2005;129:1268–1273. doi: 10.1053/j.gastro.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Blondon H, Béchade D, Desramé J, Algayres JP. Secretory diarrhea with high fecal potassium concentrations: a new mechanism of diarrhea associated with colonic pseudo-obstruction? Report of 5 patients. Gastroenterol Clin Biol. 2008;32:401–404. doi: 10.1016/j.gcb.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Simon M, Duong J-P, Mallet V, Jian R, MacLennan KA, Sandle GI, Marteau P. Over-expression of colonic K+ channels associated with severe potassium secretory diarrhea after haemorrhagic shock. Nephrol Dial Transplant. 2008;23:3350–3352. doi: 10.1093/ndt/gfn411. [DOI] [PubMed] [Google Scholar]
- 7.Perry MD, Sandle GI. Regulation of colonic apical potassium (BK) channels by cAMP. Am J Physiol Gastrointest Liver Physiol. 2009;297:G159–G167. doi: 10.1152/ajpgi.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulvihill S, Passaro E, Jr, Debas H, Yamada T. Severe diarrhea after colonic pseudo-obstruction: treatment with somatostatin. N Engl J Med. 1984;310:467–468. doi: 10.1056/NEJM198402163100719. [DOI] [PubMed] [Google Scholar]
- 9.Sørensen MV, Sausbier M, Ruth P, Seidler U, Riederer B, Praetorius HA, Leipziger J. Adrenaline-induced colonic K+ secretion is mediated by KCa1.1 (BK) channels. J Physiol. 2010;588:1763–1777. doi: 10.1113/jphysiol.2009.181933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandle GI, Hunter M. Apical potassium (BK) channels and enhanced potassium secretion in human colon. QJM. 2010;103:85–89. doi: 10.1093/qjmed/hcp159. [DOI] [PubMed] [Google Scholar]
- 11.Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- 12.Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol. 2006;17:1275–1282. doi: 10.1681/ASN.2005101111. [DOI] [PubMed] [Google Scholar]