Abstract
Contrast medium-induced acute kidney injury (CI-AKI) is a predominant cause of hospital-acquired renal insufficiency. With an increasing number of contrast medium-enhanced radiological procedures being performed in a rapidly increasing ageing population in the Western world, it is imperative that more attention is given to understand the aetiology of CI-AKI to devise novel diagnostic methods and to formulate effective prophylactic and therapeutic regimens to reduce its incidence and its associated morbidity and mortality. This article presents high-yield information on the above-mentioned aspects of CI-AKI, primarily based on results of randomised controlled trials, meta-analyses, systematic reviews and international consensus guidelines.
Key Words: Contrast medium, Acute kidney injury, Risk prediction, Serum creatinine, Neutrophil gelatinase-associated lipocalcin, N-acetylcysteine, Ascorbic acid, Statins, RenalGuard™ system
Introduction
Acute kidney injury (AKI) affects 13-18% of all patients admitted to hospital in developed countries [1]. Contrast medium (CM)-induced AKI (CI-AKI) from radiological procedures is a predominant cause. According to the National Health Service (NHS) Kidney Care, the cost of AKI to the NHS (excluding AKI in the community) has been estimated to be between GBP 434 and 620 million per year, which is more than expenditure on breast cancer or lung and skin cancer combined [2]. Up to 30% of AKI is preventable through simple interventions if symptoms are spotted early and prompt action is taken. With an increasing number of CM-enhanced imaging studies being performed at present, it is important that more attention is given to understand the aetiology of CI-AKI to devise novel diagnostic methods and to formulate effective prophylactic and therapeutic regimens to reduce its incidence and its associated morbidity and mortality. It has also been estimated that raising awareness about AKI in general and delivery of optimal care could save the NHS GBP 130-186 million each year [2].
Methodology
We searched MEDLINE and the Cochrane Database of Systematic Reviews with the search terms ‘contrast induced-acute kidney injury’ or ‘contrast induced nephropathy’. We focused on randomised controlled trials (RCTs), meta-analyses, systematic reviews and international consensus guidelines where possible. Newer studies were given priority over older ones.
Definition of CI-AKI
CI-AKI is commonly defined as a rise in serum creatinine (SCr) of 0.5 mg/dl or a 25% increase from the baseline value assessed at 48 h following CM administration [3].
SCr only rises out of normal range when more than 50% of the functioning kidney mass is lost. There is a significant subset of patients which incurs insult to less than 50% of the functioning nephrons with either no or marginal clinical change in SCr. These rather modest changes in SCr have a strong association with in-hospital mortality [4]. These observations have led to modification of consensus statements such as Kidney Disease Improving Global Outcomes (KDIGO) and a call to further refinement to include as minimal diagnostic criteria such as changes in SCr as low as 0.3 mg/dl, etc. to better correlate with worsening kidney function, need for renal replacement therapy (RRT) and death [5,6].
Incidence of CI-AKI
The lack of standardisation by which CI-AKI is defined has led to the lack of optimal estimation of disease burden. The estimated general incidence of CI-AKI is approximately 7% [7]. Its incidence may increase to >50% in the presence of risk factors such as chronic kidney disease (CKD), diabetes mellitus and nephrotoxic drugs.
Pathophysiology of CI-AKI
It is believed that CM exerts direct cytotoxic effects on renal tissue (epithelial cells and endothelial cells) by inducing renal cell apoptosis in a dose- and time-dependent manner. Generation of reactive oxygen species (ROS) drives these nephrotoxic effects of CM [8]. CM alters renal vascular function by inducing hypoperfusion, particularly in the medulla, by causing constriction of vasa recta [9]. The predominant effect in the medulla is due to its high absorptive activity, which increases its oxygen requirements. The presence of CM within renal tubules also increases the viscosity of tubular fluid, impeding its flow, leading to prolonged renal retention of CM and exposure to CM cytotoxic effects.
Risk Factors for Development of CI-AKI
The risk for development of CI-AKI can be determined prior to CM administration by consideration of the pharmacokinetics of CM and the unique function of the kidney. CM is cleared entirely by the kidney, with a half-life of a few hours. It follows that the dose of CM (volume or gram of iodine) and the half-life [increased with decreased glomerular function rate (GFR)] will determine the net exposure (volume/GFR or g of I/GFR) [10,11].
How the patient's kidney excretes this CM is the other important variable. CM will be concentrated as it traverses through the nephron due to the extraction of solute and water. The higher concentration within the nephron will favour direct tubule toxicity. CM is also concentrated in the vasa recti as they enter the medulla as a result of the osmotic movement of water out of the vessels into the hypertonic medullary interstitium. This will enhance the vasoconstriction within the vasa recti that carry oxygen to the medulla. Any factor (see below) that facilitates the concentration of CM within the nephron and/or the vasa recti may act to magnify the toxicity of the CM and promote CI-AKI.
A decrease in renal blood flow because of hypotension, volume depletion or redistribution, congestive heart failure, or drugs (such as non-steroidal anti-inflammatory drugs) will stimulate solute/water reabsorption along the nephron and water removal in the vasa recti, increasing the risk for CI-AKI. In CKD, there are less functioning nephrons. Since these nephrons are working maximally to maintain solute balance and have little vascular reserve, they are more sensitive to ischaemic injury. Vascular reserve is also compromised in conditions like diabetes mellitus and long-standing hypertension in which endothelial dysfunction limits the ability of the vasa recti to generate NO and vasodilate. Such dysfunction is also seen in the metabolic syndrome in pre-diabetes [12]. Finally, anaemia is associated with a higher risk of CI-AKI presumably because it further exacerbates the effects of CM-induced vasoconstriction of the vasa recti with resulting medullary ischaemia.
To What Extent Does Each Risk Factor Contribute to Developing CI-AKI?
The effect of risk factors is additive, i.e. the likelihood of CI-AKI rises with an increase in the number of risk factors [13], e.g. a 50% risk of CI-AKI has been reported in the presence of all 4 independent risk factors undergoing peripheral angiography [14]. A similar additive effect for development of CI-AKI requiring dialysis has been reported [15,16].
Effect of Type of CM on CI-AKI
High-osmolality CM are associated with a higher incidence of CI-AKI [17]. They have been replaced by low-osmolality CM (LOCM) and iso-osmolality CM (IOCM) in the Western world [6]. Whether IOCM are less nephrotoxic than LOCM remains contentious [18]. This probably stems from the fact that CM properties besides osmolality, such as viscosity, may play an important role in this variability [19].
Effect of Route of CM on CI-AKI
Intravenous and intra-arterial administration of CM is associated with CI-AKI as most intra-arterial injections of CM are intravenous relative to the kidneys [20]. CM volume is a key risk factor for CI-AKI and matters the most in high-risk patients [21]. Use of automated contrast injectors can help achieve this as it reduces the CM volume used [22].
Role of Risk Prediction for Development of CI-AKI
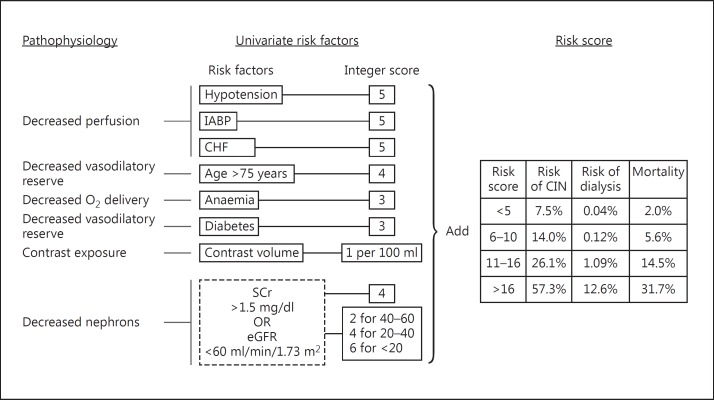
A number of risk scoring tools have been developed from analyses of large databases of patients exposed to intra-arterial CM during coronary angiography [15,16,23]. None has been validated in a prospective study. More importantly, none of these have been developed or validated in patients receiving intravenous CM. The most widely used risk assessment model reflects factors discussed above (fig. 1) and predicts the incidence of CI-AKI, need for RRT and death [15]. There is however no recommendation about the use of these risk assessment tools due to above limitations.
Fig. 1.
The pathophysiologic basis of kidney's vulnerability to CM exposure is reflected in the univariate risk factors for CI-AKI and creates the risk score predicting CI-AKI, need for dialysis and mortality. IABP = Intra-aortic balloon pump; CIN = contrast-induced nephropathy. Adapted from Mehran et al. [15].
Short- and Long-Term Effects of Transient and Persistent CM-Related Renal Dysfunction
Both transient and persistent CM-related renal dysfunction is associated with poor prognosis for short- and long-term survival and in-hospital morbidities such as major adverse cardiac events, stroke, RRT and post-procedure length of stay [24,25]. Patients with either type of renal dysfunction have a 2- to 3-fold increased risk of mortality compared with patients with no pre-existing renal dysfunction [24,26]. CI-AKI in patients with CKD is also associated with increased long-term major adverse cardiac events compared with those without CKD [27]. The predictors of CI-AKI are similar in patients with and without CKD [28].
Diagnostic Testing for CI-AKI
SCr is the most commonly used biomarker, an increase in which is taken to represent a decrease in GFR. SCr has a non-linear association with GFR; the performance of this marker is associated with ascertainment bias and poor sensitivity. Since renal tubules are nearly always damaged during CI-AKI, changes in SCr and GFR do not indicate tubular (i.e. structural) damage but rather late functional damage when more than 50% of the functioning kidney mass is lost. Recent research has allowed early diagnosis of structural and functional kidney damage or a combination thereof [29,30] in the absence of classical signs that characterise CI-AKI based on SCr changes. This includes identification of biomarkers such as NGAL (neutrophil gelatinase-associated lipocalcin) [31] and KIM (kidney injury molecule)-1 [32]. In the absence of rigorous validation studies measuring the association between these novel biomarkers and clinically relevant outcomes, there are currently insufficient data to support their use for staging CI-AKI [33]. It can however be speculated that this modern approach may help identify the subset of patients with subclinical CI-AKI which display changes in other structural biomarkers but no changes in SCr and GFR [34].
Management of CI-AKI
In the absence of kidney function, and when therapeutic measures that promote the intracellular shift of potassium (such as correction of acidosis with bicarbonate, glucose and insulin infusion, and β2-agonists) are exhausted, an excess of potassium can only be eliminated with RRT [6]. No RCT exists on dialysis for life-threatening indications; however, there is general consensus that patients with severe hyperkalaemia, severe acidosis, pulmonary oedema and uraemic complications should be dialysed emergently. Tips for management of CI-AKI once it has developed are shown in table 1.
Table 1.
Tips for non-specialists [3]
| 1 | Identify patients with recognised risk factors for developing CI-AKI. |
| 2 | Assess pre-procedure renal function (eGFR and SCr) before the CM examination in patients with risk factors, >70 years of age, those due to undergo intra-arterial CM administration, and known eGFR <60 ml/min/1.73 m2. |
| 3 | Consider stopping nephrotoxic drugs such as NSAIDs and aminoglycosides. |
| 4 | Stop metformin based on eGFR: |
| eGFR ≥60 ml/min/1.73m2 – continue metformin. | |
| eGFR 30 – 59 ml/min/1.73m2 | |
| Intra-arterial CM administration – stop metformin 48 h before CM administration and start 48 h later if renal function not deteriorated. | |
| Intravenous CM administration – continue with metformin if eGFR ≥45 ml/min/1.73m2. If eGFR | |
| 30 – 44 ml/min/1.73 m2 – as above for intra-arterial route. | |
| eGFR <30 ml/min/1.73m2 – metformin is contraindicated. | |
| In emergency patients: stop metformin, monitor for signs of lactic acidosis and restart metformin after 48 h of CM if renal function unchanged from the pre-imaging level. | |
| 5 | Nephrotoxic role of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is controversial, and at present there is no strong evidence to stop these before CM administration. |
| 6 | Intravenous volume expansion with saline, with or without additional sodium bicarbonate supplementation is the only recommended prophylaxis against CI-AKI. |
| 7 | Determine eGFR and SCr 48 – 72 h after CM examination in high-risk patients. |
| 8 | Discuss with nephrologist if CI-AKI is identified. |
Prophylactic Measures against CI-AKI
Since our understanding of the pathophysiology of CI-AKI suggests a complex interplay of vascular and tubular effects, this makes it unlikely that a single intervention will always be successful.
Volume Replacement
To overcome the reduction in renal blood flow secondary to CM exposure, volume replacement is recommended in patients at high risk for developing CI-AKI [6] at an infusion rate of 1.0-1.5 ml/kg/h for at least 6 h before and after CM administration [3]. Normal saline (0.9%) appears to be more effective than half-normal saline (0.45%) [35]. This regimen however is impractical in the outpatient setting.
In an effort to overcome this limitation, the use of pre-procedure oral hydration followed by post-procedure intravenous hydration in patients admitted for catheterisation studies on the day of procedure has been reported [36]. Since adequately powered trials are awaited, oral hydration alone is yet not recommended [6].
Volume replacement with saline hydration seems to offer nephroprotection against CI-AKI by plasma volume expansion, reduction in renin activation, loss of nitric oxide, reduced production of ROS and through dilution of CM within the tubular lumen. The subsequent decrease in renin and vasopressin may increase urine flow rates that can reduce the CM concentration in tubular fluid.
Sodium bicarbonate-based saline hydration has been found superior to intravenous saline hydration alone [37] and is recommended [6]. This is based on the hypothesis that since CM administration increases the oxidative stress and increases generation of free radicals and ROS in the presence of acidic environment, alkalinising renal tubular fluid with bicarbonate would be a logical strategy to reduce renal injury. The most common protocol used in the literature is 3 ml/kg/h for 1 h before and 1 mg/kg/h for 6 h after procedure. This protocol is quicker than the intravenous saline hydration protocol and hence more practicable in an outpatient setting [3].
Intravenous volume expansion with saline, with or without additional sodium bicarbonate supplementation, is the only recommended prophylaxis against CI-AKI.
Diuretics
Theoretically, use of diuretics (furosemide and mannitol) as a prophylactic strategy against CI-AKI may decrease its severity by preventing renal tubule obstruction and reduction of oxygen consumption by inhibition of the Na-K-2Cl co-transporter. The administration of such agents has however been found to have either no or deleterious effects [38,39].
The RenalGuardTM System
The cornerstone of this strategy is that hydration (to allow for intravascular volume expansion) and forced diuresis (to ensure a high urine flow rate to ≥150 ml/h) would result in less CI-AKI [40]. The efficacy of this technique has been reported in patients with pre-existing renal impairment only in a few RCTs [41,42]. Large multicentre RCTs are warranted as it is unclear whether this strategy should be limited to high-risk patients or extended to all patient populations.
Antioxidants
Since ROS have been implicated in the aetiology of CI-AKI, use of antioxidants is therefore but logical. N-acetylcysteine remains the most widely investigated agent; however, its efficacy remains controversial at present [43]. Ascorbic acid has also been reported to have a nephroprotective role [44]. None of the above agents have been recommended [3].
Vasodilators
To improve blood flow to the renal medulla, vasodilators such as fenoldopam, which is a selective dopamine-1 agonist, have been used for prevention of CI-AKI, with variable efficacy [45,46]. This has been attributed to the vasodilator-induced systemic hypotension that might have offset its localised beneficial effects on the kidneys [47]. Targeted renal therapy with direct delivery of such agents to the kidneys via renal arteries during interventional radiological procedures has been reported to overcome such potential limitations [48]. Reduction in the incidence of CI-AKI has been reported with its use [49]. However, its efficacy and safety remains untested in a planned RCT and hence is not recommended [6].
Statins
Pleiotropic effects of statins have been explored to offer nephroprotection against CI-AKI and have shown promise with a high-dose short-term use of these agents [50]. At present however there are no recommendations on their use [6].
Renal Replacement Therapy
Haemodialysis has been observed to increase the risk of CI-AKI [51]. This may result from either RRT-related toxicity caused by activation of inflammatory reactions or release of vasoactive substances on blood-membrane contact that may induce acute hypotension and renal ischaemia. Also RRT may not be able to remove CM quickly enough to prevent renal injury. At present, prophylactic RRT is not recommended [6].
Development of and adherence to institutional protocols based upon the evidence presented above have been shown to reduce the incidence of CI-AKI [52]. However, even with national protocols, adherence requires continual monitoring and effort [53].
Questions for Future Research
• Routinely used definitions of CI-AKI have limitations as they fail to define it in terms of hard clinical outcome such as the need for RRT, rather than by the occurrence of a specific decline in the renal function. An evidence-based definition of CI-AKI in terms of clinical outcome is awaited.
• Prospective validation of risk assessment tools for CI-AKI is required in well-performed large-sample-size prospective studies in patients undergoing coronary and peripheral angiography and in those exposed to intravenous CM.
• Currently, no ideal biomarker of renal impairment exists. An ideal biomarker would be diagnostic (i.e. specific) for CI-AKI, an ‘early’ indicator (i.e. sensitive) of CI-AKI, correlate with the degree of injury over time and be a simple, standardised and widely available assay that is cost effective with a rapid turnaround time.
• Strategies such as statins, targeted renal therapy with vasodilators and the RenalGuard™ system have shown promise but need well-performed multicentre RCTs before they can be recommended.
Conclusions
CI-AKI may occur in patients exposed to CM and has a general prevalence of approximately 7%. It is associated with clinically significant adverse outcomes including progressive loss of kidney function, need for dialysis, increased length of hospitalisation and death. The risk for developing CI-AKI can be determined prior to the index procedure by careful consideration of the risk factors. Intravenous volume expansion with saline, with or without additional sodium bicarbonate supplementation, is the only recommended prophylaxis against CI-AKI.
Disclosure Statement
R.J.S. is on the scientific advisory board for Ischemix Inc., USA, is a consultant for Bracco Diagnostics, Italy, has research funding for a trial by MDSci Inc., USA, and lectures for Guerbet Inc., USA. U.S., A.U., J.R.B. and P.D.H. have no competing interests to declare.
References
- 1.National Institute for Health and Care Excellence . Acute Kidney Injury – Prevention, Detection and Management up to the Point of Renal Replacement Therapy (Clinical Guideline 169) London: National Institute for Health and Care Excellence; 2013. [PubMed] [Google Scholar]
- 2.Calculating the cost. Interview with Marion Kerr. Health Service J. 2011;3(suppl 1) [PubMed] [Google Scholar]
- 3.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almen T, Aspelin P, Bellin MF, Clement O, Heinz-Peer G. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 4.Weisbord SD, Kip KE, Saul MI, Palevsky PM. Defining clinically significant radiocontrast nephropathy. J Am Soc Nephrol. 2003;14:280A–281A. [Google Scholar]
- 5.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lameire N, Kellum JA. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2) Crit Care. 2013;17:205. doi: 10.1186/cc11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Heyman SN, Rosen S, Khamaisi M, Idee JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45:188–195. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 9.Sendeski M, Patzak A, Persson PB. Constriction of the vasa recta, the vessels supplying the area at risk for acute kidney injury, by four different iodinated contrast media, evaluating ionic, nonionic, monomeric and dimeric agents. Invest Radiol. 2010;45:453–457. doi: 10.1097/RLI.0b013e3181d77eed. [DOI] [PubMed] [Google Scholar]
- 10.Laskey WK, Jenkins C, Selzer F, Marroquin OC, Wilensky RL, Glaser R, Cohen HA, Holmes DR, Jr, NHLBI Dynamic Registry Investigators Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:584–590. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Mager A, Vaknin Assa H, Lev EI, Bental T, Assali A, Kornowski R. The ratio of contrast volume to glomerular filtration rate predicts outcomes after percutaneous coronary intervention for ST-segment elevation acute myocardial infarction. Catheter Cardiovasc Interv. 2011;78:198–201. doi: 10.1002/ccd.22828. [DOI] [PubMed] [Google Scholar]
- 12.Toprak O, Cirit M, Yesil M, Bayata S, Tanrisev M, Varol U, Ersoy R, Esi E. Impact of diabetic and pre-diabetic state on development of contrast-induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22:819–826. doi: 10.1093/ndt/gfl636. [DOI] [PubMed] [Google Scholar]
- 13.McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, Tumlin J. Risk prediction of contrast-induced nephropathy. Am J Cardiol. 2006;98:27K–36K. doi: 10.1016/j.amjcard.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Gussenhoven MJ, Ravensbergen J, van Bockel JH, Feuth JD, Aarts JC. Renal dysfunction after angiography; a risk factor analysis in patients with peripheral vascular disease. J Cardiovasc Surg (Torino) 1991;32:81–86. [PubMed] [Google Scholar]
- 15.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O'Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. doi: 10.1016/j.amjcard.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–178. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 18.Biondi-Zoccai G, Lotrionte M, Thomsen HS, Romagnoli E, D'Ascenzo F, Giordano A, Frati G. Nephropathy after administration of iso-osmolar and low-osmolar contrast media: evidence from a network meta-analysis. Int J Cardiol. 2014;172:375–380. doi: 10.1016/j.ijcard.2014.01.075. [DOI] [PubMed] [Google Scholar]
- 19.Seeliger E, Lenhard DC, Persson PB. Contrast media viscosity versus osmolality in kidney injury: lessons from animal studies. Biomed Res Int. 2014;2014:358136. doi: 10.1155/2014/358136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyman U, Almen T, Jacobsson B, Aspelin P. Are intravenous injections of contrast media really less nephrotoxic than intra-arterial injections? Eur Radiol. 2012;22:1366–1371. doi: 10.1007/s00330-011-2371-4. [DOI] [PubMed] [Google Scholar]
- 21.Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O'Connor GT, Solomon RJ, Malenka DJ. Does safe dosing of iodinated contrast prevent contrast-induced acute kidney injury? Circ Cardiovasc Interv. 2010;3:346–350. doi: 10.1161/CIRCINTERVENTIONS.109.910638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minsinger KD, Kassis HM, Block CA, Sidhu M, Brown JR. Meta-analysis of the effect of automated contrast injection devices versus manual injection and contrast volume on risk of contrast-induced nephropathy. Am J Cardiol. 2014;113:49–53. doi: 10.1016/j.amjcard.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tziakas D, Chalikias G, Stakos D, Apostolakis S, Adina T, Kikas P, Alexoudis A, Passadakis P, Thodis E, Vargemezis V, Konstantinides S. Development of an easily applicable risk score model for contrast-induced nephropathy prediction after percutaneous coronary intervention: a novel approach tailored to current practice. Int J Cardiol. 2013;163:46–55. doi: 10.1016/j.ijcard.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 24.Brown JR, Malenka DJ, DeVries JT, Robb JF, Jayne JE, Friedman BJ, Hettleman BD, Niles NW, Kaplan AV, Schoolwerth AC, Thompson CA. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72:347–354. doi: 10.1002/ccd.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wi J, Ko YG, Kim JS, Kim BK, Choi D, Ha JW, Hong MK, Jang Y. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97:1753–1757. doi: 10.1136/hrt.2010.218677. [DOI] [PubMed] [Google Scholar]
- 26.Solomon RJ, Mehran R, Natarajan MK, Doucet S, Katholi RE, Staniloae CS, Sharma SK, Labinaz M, Gelormini JL, Barrett BJ. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–1169. doi: 10.2215/CJN.00550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appleby CE, Ivanov J, Lavi S, Mackie K, Horlick EM, Ing D, Overgaard CB, Seidelin PH, von Harsdorf R, Dzavik V. The adverse long-term impact of renal impairment in patients undergoing percutaneous coronary intervention in the drug-eluting stent era. Circ Cardiovasc Interv. 2009;2:309–316. doi: 10.1161/CIRCINTERVENTIONS.108.828954. [DOI] [PubMed] [Google Scholar]
- 28.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 29.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50:1505–1517. doi: 10.1515/cclm-2011-0814. [DOI] [PubMed] [Google Scholar]
- 32.Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, Ni Z, Mou S. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9:e84131. doi: 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCullough PA, Shaw AD, Haase M, Bouchard J, Waikar SS, Siew ED, Murray PT, Mehta RL, Ronco C. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 34.Ronco C, Stacul F, McCullough PA. Subclinical acute kidney injury (AKI) due to iodine-based contrast media. Eur Radiol. 2013;23:319–323. doi: 10.1007/s00330-012-2607-y. [DOI] [PubMed] [Google Scholar]
- 35.Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1,620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 36.Hiremath S, Akbari A, Shabana W, Fergusson DA, Knoll GA. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLoS One. 2013;8:e60009. doi: 10.1371/journal.pone.0060009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim TH, Urm SH, Kim DS, Seol SH, Kim DI, Cho KI, Kim BH, Park YH, Je HG, Ahn JM, Kim WJ, Lee JY, Lee SW. Sodium bicarbonate therapy for the prevention of contrast-induced acute kidney injury – a systematic review and meta-analysis. Circ J. 2012;76:2255–2265. doi: 10.1253/circj.cj-12-0096. [DOI] [PubMed] [Google Scholar]
- 38.Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 39.Majumdar SR, Kjellstrand CM, Tymchak WJ, Hervas-Malo M, Taylor DA, Teo KK. Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:602–609. doi: 10.1053/j.ajkd.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Stevens MA, McCullough PA, Tobin KJ, Speck JP, Westveer DC, Guido-Allen DA, Timmis GC, O'Neill WW. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999;33:403–411. doi: 10.1016/s0735-1097(98)00574-9. [DOI] [PubMed] [Google Scholar]
- 41.Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, Golia B, Ricciardelli B, Condorelli G. Renal Insufficiency after Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124:1260–1269. doi: 10.1161/CIRCULATIONAHA.111.030759. [DOI] [PubMed] [Google Scholar]
- 42.Dorval JF, Dixon SR, Zelman RB, Davidson CJ, Rudko R, Resnic FS. Feasibility study of the RenalGuard balanced hydration system: a novel strategy for the prevention of contrast-induced nephropathy in high risk patients. Int J Cardiol. 2013;166:482–486. doi: 10.1016/j.ijcard.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 43.Gonzales DA, Norsworthy KJ, Kern SJ, Banks S, Sieving PC, Star RA, Natanson C, Danner RL. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007;5:32. doi: 10.1186/1741-7015-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadat U, Usman A, Gillard JH, Boyle JR. Does ascorbic acid protect against contrast-induced acute kidney injury in patients undergoing coronary angiography: a systematic review with meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;62:2167–2175. doi: 10.1016/j.jacc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 45.Stone GW, McCullough PA, Tumlin JA, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O'Neill WW. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003;290:2284–2291. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- 46.Ng TM, Shurmur SW, Silver M, Nissen LR, O'Leary EL, Rigmaiden RS, Cieciorka M, Porter LL, Ineck BA, Kline ME, Puumala SE. Comparison of N-acetylcysteine and fenoldopam for preventing contrast-induced nephropathy (CAFCIN) Int J Cardiol. 2006;109:322–328. doi: 10.1016/j.ijcard.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 47.Tumlin JA. Impaired blood flow in acute kidney injury: pathophysiology and potential efficacy of intrarenal vasodilator therapy. Curr Opin Crit Care. 2009;15:514–519. doi: 10.1097/MCC.0b013e328332f6f9. [DOI] [PubMed] [Google Scholar]
- 48.Teirstein PS, Price MJ, Mathur VS, Madyoon H, Sawhney N, Baim DS. Differential effects between intravenous and targeted renal delivery of fenoldopam on renal function and blood pressure in patients undergoing cardiac catheterization. Am J Cardiol. 2006;97:1076–1081. doi: 10.1016/j.amjcard.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Weisz G, Filby SJ, Cohen MG, Allie DE, Weinstock BS, Kyriazis D, Walker CM, Moses JW, Danna P, Fearon WF, Sachdev N, Wiechmann BN, Vora K, Findeiss L, Price MJ, Mehran R, Leon MB, Teirstein PS. Safety and performance of targeted renal therapy: the Be-RITe! Registry. J Endovasc Ther. 2009;16:1–12. doi: 10.1583/08-2515.1. [DOI] [PubMed] [Google Scholar]
- 50.Gandhi S, Mosleh W, Abdel-Qadir H, Farkouh ME. Statins and contrast-induced acute kidney injury with coronary angiography: systematic review and meta-analysis. Am J Med. 2014;127:987–1000. doi: 10.1016/j.amjmed.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Cruz DN, Goh CY, Marenzi G, Corradi V, Ronco C, Perazella MA. Renal replacement therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Med. 2012;125:66–78. doi: 10.1016/j.amjmed.2011.06.029. e63. [DOI] [PubMed] [Google Scholar]
- 52.Brown JR, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Robb JF, Boss R, Goldberg DJ, Fedele FA, Kellett MA, Phillips WJ, Ver Lee PN, Nelson EC, MacKenzie TA, O'Connor GT, Sarnak MJ, Malenka DJ. How do centres begin the process to prevent contrast-induced acute kidney injury: a report from a new regional collaborative. BMJ Qual Saf. 2012;21:54–62. doi: 10.1136/bmjqs-2011-000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schilp J, de Blok C, Langelaan M, Spreeuwenberg P, Wagner C. Guideline adherence for identification and hydration of high-risk hospital patients for contrast-induced nephropathy. BMC Nephrol. 2014;15:2. doi: 10.1186/1471-2369-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]