Abstract
Background:
Ferulago carduchorum Boiss and Hausskn belongs to the Apiaceae family. This plant grows in west part of Iran that local people added it to dairy and oil ghee to delay expiration date and give them a pleasant taste. The aim of this study was to investigate the antioxidant, antimicrobial, acetyl cholinesterase inhibition, cytotoxic, larvicidal activities and composition of essential oil of F. carduchorum.
Methods:
Acetyl cholinesterase (AChE) inhibitory, larvicidal activities and chemical composition of essential oil of F. carduchorum were investigated. Besides, antioxidant, antimicrobial and cytotoxic activities of essential oil were tested using DPPH, microdilution method and MTT assay, respectively.
Results:
The major components of essential oil were (z)-β-ocimene (43.3%), α-pinene (18.23%) and bornyl acetate (3.98%). Among 43 identified components, monoterpenes were the most compounds (84.63%). The essential oil had noticeable efficiency against Candida albicans (MIC= 2340 μg ml−1) and it was effective against Anopheles stephensi with LC50 and LC90 values of 12.78 and 47.43 ppm, respectively. The essential oil could inhibit AChE (IC50= 23.6 μl ml−1). The essential oil showed high cytotoxicity on T47D, HEP-G2 and HT-29 cell lines (IC50< 2 μg ml−1).
Conclusion:
The essential oil of F. carduchorum collected from west of Iran had anti-Candida, larvicidal and cytotoxicity effects and should be further investigated in others in vitro and in vivo experimental models.
Keywords: Ferulago carduchorum, Essential oil, Antimicrobial, Antiacetyl cholinesterase, Larvicidal activity
Introduction
Ferulago carduchorum Boiss and Hausskn (Apiaceae) known as an endemic plant of Iran, grows in west part of Iran (Mozaffarian 2007). In west of Iran, F. carduchorum has been traditionally added to dairy and oil ghee to increase hold time and give them a pleasant taste. In the past, this plant was used as natural preservative to delay expiration date of meat, too. Some species of Ferulago are benefit for remedy of digestive pains, hemorrhoid (Sodeifian et al. 2011), disease of spleen, headache, ulcers and snake bites (Demetzos et al. 2000).
Phytochemical studies on Ferulago species have led to identification of different coumarins (Ognyanov et al. 1969, Andrianova et al. 1975, Serkerov et al. 1976, Sklyar et al. 1982, De Pascual et al. 1979, Doganca et al. 1991, Doganca et al. 1992, Ruberto et al. 1994, Jimenez et al. 2000, Khalighi-Sigaroodi et al. 2006). There are also some reports about the acetyl cholinesterase inhibitory (Dall'Acqua et al. 2010), cytotoxic (Rosselli et al. 2009), antimicrobial and antioxidant (Basile et al. 2009) activities of coumarins of F. campestris Furthermore composition of essential oil of 6 different Ferulago species (Erdurak et al. 2006, Kilic et al. 2010) and antimicrobial activity of essential oil of F. bernardii (Khalighi-Sigaroodi et al. 2005) and F. campestris (Cecchini et al. 2010) have been reported later.
Alzheimer’s disease (AD) is the most common and important degenerative disease of brain among the elderly and is the fourth leading cause of death in western countries (Shen et al. 2005, Zhou et al. 2008). Reports suggest that AD is caused by reduced synthesis of acetylcholine (Weinstock et al. 1997), so the use of acetyl cholinesterase enzyme inhibitors (AChEIs) could help improve AD (Grutzendler et al. 2001). Traditional AD drugs exhibit side effects and many efforts have been made to achieve a variety of natural AChEIs with less adverse effects (Karimi et al. 2010). Furthermore, a lot of medications for cancer treatment are not effective enough so, researchers are trying to find the most effective medicinal plants to treat cancers (Rahimifard et al. 2009).
Mosquitoes are important vectors in transmission of some human diseases such as malaria, dengue fever, yellow fever and filariasis, which they are substantially among the greatest health problems all over the world (James et al. 1992). Different species of Anopheles transmit malaria, filariasis and certain arboviruses (Sedaghat et al. 2005). Malaria is one of the most important diseases in southern Iran (Vatandoost et al. 2011). In previous reports different plants showed toxic effects against public health pests (Hadjiakhoondi et al. 2005, Hadjiakhoondi et al. 2006, Hadjiakhoondi et al. 2008a, Hadjiakhoondi et al. 2008b, Vatandoost et al. 2008)
The in vitro investigation of essential oils is important to estimate their potential to use as an antibiotic or as a drug for AD, cancers, malaria and as a supplement to edible or pharmaceutical products.
The aim of this study was to investigate the antioxidant, antimicrobial, acetyl cholinesterase inhibition, cytotoxic, larvicidal activities and composition of essential oil of F. carduchorum.
Materials and Methods
Plant material
The aerial parts of Ferulago carduchorum (Apiaceae family) were collected from Manesht mountain of Illam Province in June 2011. The longitude and latitude of Manesht Mountain are 33°40′60″ N and 46°28′0″ E. This plant is an endemic plant of Iran, which grows in west part of Iran. The plant was identified and authenticated by Mr Yousef Ajani using flora iranica (Rechinger 1978). The voucher specimen is deposited in herbarium Institute of Medicinal Plants (ACECR), Karaj, Iran (Herbarium number: 1450).
Isolation of the volatile oil
The essential oil was obtained by hydrodistillation using a Clevenger type apparatus (Advanced Technocracy Inc., India) for 4 h according to the European Pharmacopoeia (1975) (Maisonneune 1975). The aerial parts of Ferulago carduchorum (Apiaceae family) were dried under shade and powdered. The air-dried parts of F. carduchorum (150 g) and 1,000 ml distilled water placed in a round bottom flask (using a fire source from below) connected to a Clevenger-type apparatus. Hydrodistillation is often used to isolate non-water soluble compounds. Hydrodistillation is a method that the plant parts being boiled in water, using a heating source from below the vessel. In the presence of boiling water volatile compounds are volatilized at a temperature close to 100 °C, at atmospheric pressure. The volatile materials escape in vapor form through some tubes and then are cool. The essential oil is removed from the top of the hydrosol.
1 ml essential oil obtained duration 1 h hydrodistillation. The oil was dried over anhydrous sodium sulphate and kept at 4 °C in the sealed brown vial until required. 1.3 ml essential oil has been obtained from 100 gram dried plant. The oil yield of the plant was determined as 1.3% v/w. The isolation of the volatile oil has been carried out in laboratory of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
Gas chromatography mass spectroscopy
Analytical gas chromatography (GC) was carried out using a Shimadzu 15A gas chromatograph with capillary column HP-5ms (60m×0.25mm, ft 0.25mm), carrier gas, He, split ratio 1:25 and using a flame ionization detector (FID). The column temperature was programmed at 60 °C for 3 min and then it was heated to 260 °C at a rate of 5 °C min−1 and the temperature was then kept constant at 260 °C for 15 min. Gas chromatography mass spectroscopy (GC/MS) was carried out on a HP 68900 with a HP 5973 quadruple detector, on capillary column HP-5ms (5% phenyl methyl siloxane) (60m×0.25mm, ft 0.25mm), carrier gas, He, flow rate, 1 ml min−1. The column was held at 60 °C for 3 min and programmed up to 260 °C at the rate of 5 °C min−1, then kept constant at 260 °C for 15 min. The MS was operated at 70 eV ionization energy. Retention indices were calculated using the retention time of n-alkanes that were injected after the oil at the same chromatographic conditions. Quantitative data were obtained from the electronic integration of the FID peak areas. The components of the oils were identified by comparison of their mass spectra and retention indices with Wiley library and those published in the literature (Adams 1995).
DPPH radical scavenging activity
The 1, 1-diphenyl-2-picryl hydrazyl radical (DPPH) (Merck, Germany) has a maximum absorption at 517 nm which was used for the investigation of the free radical-scavenging activity of the essential oil (Yokozawa et al. 1998, Khanavi et al. 2009).
Antimicrobial activity
Antimicrobial activities of essential oil of aerial parts of F. carduchorum was determined against both Gram-positive (Staphylococcus aureus ATCC 6538), Gram-negative (Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027) bacteria and a fungal strain (Candida albicans ATCC 1023) by microdilution method. Negative control was prepared using dimethylsulphoxide (DMSO), which was solvent used to dissolve the essential oil. Gentamycin was used as positive control against S. aureus, E. coli, P. aeruginosa while, Nystatin was used as positive control against C. albicans. Their dilutions ranged from 10 to 0.009 μg/ml concentrations in microtitre plates. The antimicrobial activity of essential oil was assessed by the agar well diffusion method. The plates were incubated at 37 °C for 24 hours for bacteria and 20–25 °C for C. albicans. Inhibition was detected by measuring clear zones around the wells in millimeters. Minimum Inhibitory Concentration (MIC) of the essential oil was assessed by broth microdilution method with visible growth observed by using 96 U-shaped wells plates (NCCLS 2006). After 24 h of incubation at 35 °C (bacteria) and 20–25 °C (C. albicans), the microdilution plates were tested for the absence or presence of visible growth in comparison with that of the growth in drug-free control well. The endpoint of MIC is the lowest concentration of the compound at which the test strain does not demonstrate visible growth.
Acetyl cholinesterase (AChE) inhibition
The enzymatic activity was measured using by the method described by Aazza et al. (2011) with minor modifications. 50 μl of buffer 0.1 M (pH 8), 25 μl of essential oil dissolved in dimethyl sulphoxide (DMSO) with different concentrations and 25 μl of 0.22 U/ml of AChE enzyme were mixed. After 15 min incubation at 37 °C, 25 μl of 15 mM acetyl thiocholine iodide (AChI) and 125 μl of 3 mM 5,5′-dithiobis [2-nitrobenzoic acid] (DTNB) were added and the resulting mixture incubated for 30 min at room temperature. Absorbance of the mixture was measured at 405 nm by using a microplate reader (ELX808, BioTek, USA). The inhibitory effect of test compound was calculated by comparing to the negative control: %= [(A0–A1)/A0]* 100 where A0 was the absorbance of the blank sample and A1 was the absorbance of the sample.
The test was repeated three times. The inhibition of enzyme activity was expressed as IC50 (the concentration of the sample (μl ml−1), required to inhibit 50% of enzyme), calculated by a linear regression analysis.
Bioassays and larval mortality
Fourth instar larvae of Anopheles stephensi Bandar-Abbas strain was exposed to test concentrations of 0.625, 1.25, 2.5, 5, 10, 20, 40 and 80 ppm of essential oil (solvent: Ethanol) for 24 hours according to standard method described by WHO (1981). In briefly 1 ml of appropriate dilution of essential oil with 224 ml of water and 25 larvae in 25 ml water mixed and total volume was 250 ml (Dharmagadda et al. 2005). For control, only 1 ml of ethanol with 224 ml of water and 25 larvae in 25 ml water mixed and total volume was 250 ml. The experiment was repeated four times on different days. The percentage of mortality was reported from the average for the four replicates after 24 hour exposure period. From the regression line between logarithmic dose and probit mortality, the LC50 was determined. The investigation of larvicidal activity has been carried out in the insectarium of Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
Cytotoxicity assay
The colon carcinoma (HT-29), breast carcinoma (T47D), hepatocellular carcinoma (HepG2) was obtained from Pasture Institute of Iran, Tehran, Iran. The colon carcinoma HT-29 and T47D (breast carcinoma) cell lines were mentioned as exponentially growing cultures in RPMI 1640 cell culture medium (PAA, Germany), supplemented with 10% fetal bovine serum (FBS: Gibco, USA), for HT-29 cells and 15% FBS for T47D cells. The hepatocellular carcinoma (HepG2) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, PAA, Germany) supplemented with 10% FBS. The Swiss mouse embryo fibroblast (NIH 3T3) cell line was kept in Dulbecco’s modified Eagle’s medium (DMEM, PAA, Germany) supplemented with 10% FBS. 100 IU/ml penicillin and 100 μg/ml streptomycin (Roche, Germany) were added to the media. All the cell lines were cultured at 37 °C in air /carbon dioxide (95:5) atmosphere.
Cytotoxic activity was measured using modified MTT assay (Newman DJ 2007). 1×104 cells/well were plated in 96-well plates (Nunc, Denmark) and incubated for 24 h before the addition of drugs. After 48 h of incubation in HT-29, NIH/3T3 and MCF-7 cells, 20 μl of MTT (Merck, Germany) reagent (5 mg/ml) in phosphate buffered saline (PBS) was added to each well. The plates were incubated at 37 °C for 4 h. The medium was discharged and the formazan blue, which had been formed in the cells, were dissolved with 100 μl dimethyl sulphoxide (DMSO). After the incubation at 37 °C for 10 min, absorbance at 570 nm at the dissolved solutions was detected using a micro plate reader (Anthos, Austria). The cell viability in MTT assay was calculated as the percentage of control value (Khanavi et al. 2012b). Methotrexate was used as the positive control. Cytotoxicity was expressed as the concentration of extract inhibiting cell growth with 50% (IC50±SD). All tests and analysis were run in triplicate.
Statistical analysis
In antioxidant and antiacetyl cholinesterase assays, analyses were carried out in triplicate and the data were expressed as mean ±SD. One-way ANOVA and Tukey post-hoc multicomparison tests were used for the analyses after data normality test. In cytotoxicity assay, IC50 (the median growth inhibitory concentration) values were calculated from the IC50 of dose-response curve in the sigma plot 11 software. Data representative of three independent experiments with similar results were presented as mean ± SD. For larvicidal activity was used from MicroProbit software (version 3.0). The percentages of mortality were corrected for the mortality in controls by using Abbott's correction. From the regression line between the logarithmic dose and probit mortality, all the parameters including LC50, LC90, confidence interval (CI) and slope values were determined (Abbott 1925).
Results
Chemical composition of the essential oil
The results of essential oil analysis of F. carduchorum were led to identification of 43 compounds (Table 1), represented 92.3% of the total oil. Gas chromatography-Mass diagram of F. carduchorum essential oil showed in figure 1. The oil yield of the plant was determined as 1.3% v/w. Major component of essential oil were identified as (Z)-β-ocimene (43.3%), α-pinene (18.23%), bornyl acetate (3.98%) and myrcene (3.15%).
Table 1.
Chemical composition of essential oil of Ferulago carduchorum
| No. | Component | RTa | RIb | Composition (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 10.133 | 937 | 18.23 |
| 2 | Camphene | 10.403 | 946 | 1.63 |
| 3 | Verbenene | 10.485 | 958 | Trace |
| 4 | β-Pinene | 11.443 | 971 | 1.81 |
| 5 | Myrcene | 12.306 | 989 | 3.15 |
| 6 | α-Phellandrene | 12.697 | 997 | 0.4 |
| 7 | p-Cymene | 13.654 | 1017 | 0.21 |
| 8 | o-Cymene | 13.500 | 1020 | 0.61 |
| 9 | δ-3-Carene | 13 | 1029 | 0.25 |
| 10 | β-Phelandrene | 13.819 | 1031 | 2.07 |
| 11 | (Z)-β-Ocimene | 14.969 | 1045 | 43.3 |
| 12 | (E)-β-Ocimene | 15.47 | 1055 | 2.71 |
| 13 | γ-Terpinene | 16.807 | 1062 | 1.82 |
| 14 | Terpinolene | 16.917 | 1088 | 0.16 |
| 15 | Linalool | 17.236 | 1100 | 0.16 |
| 16 | 1,3,8- para-Menthatriene | 18.155 | 1115 | Trace |
| 17 | allo-Ocimene | 18.744 | 1129 | 2.29 |
| 18 | cis-Verbenol | 18.904 | 1135 | 0.26 |
| 19 | neo-allo-Ocimene | 18.799 | 1150 | Trace |
| 20 | trans-Verbenol | 19.162 | 1156 | 1.01 |
| 21 | p-Mentha-1,5-dien-8-ol | 20.043 | 1165 | 0.39 |
| 22 | 4-Terpineol | 20.516 | 1170 | 0.13 |
| 23 | α –Terpineol | 21.159 | 1180 | 0.06 |
| 24 | Dodecane | 22.234 | 1200 | 1.02 |
| 25 | Bornyl acetate | 25.446 | 1277 | 3.98 |
| 26 | α - Cubebene | 33.892 | 1366 | 2.65 |
| 27 | α -Ylangene | 31.025 | 1370 | 0.16 |
| 28 | α -Copaene | 29.649 | 1378 | 0.36 |
| 29 | β–Bourbonene | 29.952 | 1382 | 0.43 |
| 30 | β -Cubebene | 30.156 | 1389 | 0.14 |
| 31 | β -Elemene | 30.271 | 1399 | 0.14 |
| 32 | α -Gurjunene | 36.654 | 1421 | 0.32 |
| 33 | γ -Elemene | 31.944 | 1428 | 0.1 |
| 34 | Aromadendrene | 31.306 | 1433 | 0.31 |
| 35 | α -Humulene | 32.659 | 1446 | Trace |
| 36 | trans-β-Farnesene | 33.017 | 1452 | Trace |
| 37 | Germacrene-D | 33.198 | 1475 | Trace |
| 38 | Bicyclogermacrene | 34.431 | 1498 | 0.68 |
| 39 | β - Bisabolene | 35.047 | 1500 | 0.15 |
| 40 | δ-Cadinene | 35.482 | 1513 | 0.5 |
| 41 | Spathulenol | 37.424 | 1568 | 0.71 |
| 42 | Salvial-4(14)-en-1-one | 37.754 | 1589 | Trace |
| 43 | α-Cadinol | 39.862 | 1650 | Trace |
| Total | 92.3 | |||
| Monoterpenes hydrocarbons | 78.64 | |||
| Monoterpenes oxygenated | 5.99 | |||
| Sesquiterpenes hydrocarbons | 5.94 | |||
| Sesquiterpenes oxygenated | 0.71 | |||
| Nonterpenoids | 1.02 |
Notes
Retention time,
RI Retention Index on HP-6890 with reference to nalkanes injected after the oil at the same chromatographic conditions.
Fig. 1.
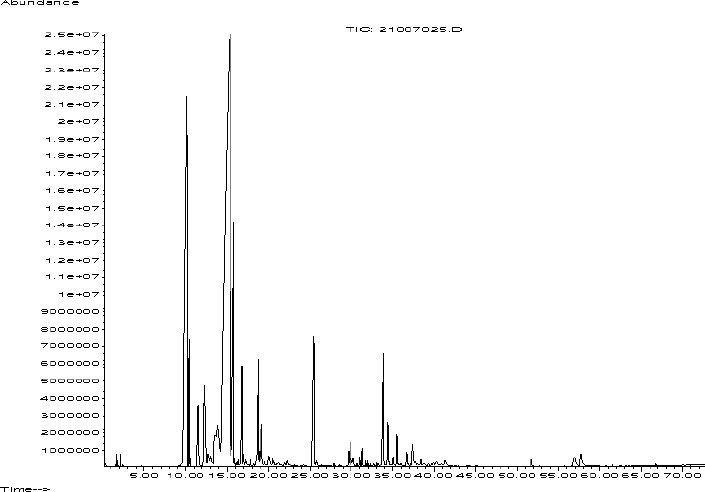
Gas chromatography-Mass diagram of Ferulago carduchorum essential oil
Antioxidant and antimicrobial activities
In this research radical scavenging activity of essential oil of F. carduchorum was determined and IC50 value was calculated as 29.61 μl. Vitamin E also used as a reference and positive control compound.
In our antimicrobial investigation, the results of Minimum Inhibitory Concentration (MIC) are shown in Table 2. The results of gentamycin and nystatin were used as positive control against bacterial and fungal strain, respectively. The results of positive (gentamycin and nystatin) and negative (DMSO) controls are shown in Table 2.
Table 2.
Minimum inhibitory concentration (MIC) of Ferulago carduchorum essential oil against selected bacteria and Candida albicans
| Microorganism | MICa | Positive control | Positive control | Negative control |
|---|---|---|---|---|
| Essential oil | Gentamycin | Nystatin | DMSO | |
| Staphylococcus aureus | 9000 | 0.62 | - | - |
| Escherichia coli | 23000 | 0.009 | - | - |
| Pseudomonas aeruginosa | 18000 | 2.48 | - | - |
| Candida albicans | 2340 | - | 0.02 | - |
Note
MIC was determined by broth micro dilution method and expressed in μg ml−1. - No effect.
Acetyl cholinesterase Inhibition Activity
Acetyl cholinesterase inhibition activity of F. carduchorum essential oil was studied for the first time. The results showed that the IC50 of essential oil was 23.6 μl ml−1.
Larval mortality
The larvicidal activity of our essential oil and methanol against Anopheles stephensi larvae under laboratory conditions are presented in Table 3. Essential oil of F. carduchorum was effective against An. stephensi with LC50 and LC90 values of 12.78 and 47.43 ppm, respectively.
Table 3.
Probit regression line of Anopheles stephensi exposed to different interval concentrations of essential oil of Ferulago carduchorum
| Intercept | Slope±SE* | LC50 (ppm) | 95% CI | LC90 (ppm) | 95% CI | χ2 | χ2table (df) | p-value |
|---|---|---|---|---|---|---|---|---|
| −2.4906 | 1.9303± | 12.7818 | 19.5101– | 47.4356 | 89.9890– | 35.78 | 22.458 (6) | 0.001 |
| 0.323 | 33.4434 | 357.8734 | * |
No heterogeneity; SE: Standard Error, LC50: Lethal Concentration to cause 50% mortality in population; LC90: Lethal Concentration to cause 90% mortality in population, CI: Confidence Interval, χ2 (df) = heterogeneity about the regression line (Degrees Of Freedom).
Cytotoxicity effect
The effects of F. carduchorum essential oil on the proliferative response of the HT-29, HepG2 and T47D cell lines have been analyzed by treating the cells with different concentrations of the essential oil and significant decrease in cell lines proliferation were observed. The results of cytotoxic tests have been shown in Table 4.
Table 4.
Cytotoxic activity of essential oil of Ferulago carduchorum
| Cell Linesa (MTT assay) | Essential oil | Methotrexate | Doxorubicin |
|---|---|---|---|
| NIH-3T3 | 0.64±0.02 | 0.24 ± 0.013 | 0.21 ±0.03 |
| HT-29 | 1.74±0.14 | 0.23 ± 0.02 | - |
| T47D | 0.17±0.01 | 0.16 ± 0.09 | - |
| HEP-G2 | 0.35±0.02 | - | 1.04 ±0.07 |
Results are expressed as IC50 values (μg ml−1), Key to cell Lines employed: HT-29 (colon carcinoma), T47D (breast carcinoma), HEP-G2 (hepatocellular carcinoma), NIH 3T3 (Swiss embryo fibroblast).
Discussion
Among 43 identified components, monoterpenes were the most identified compounds (84.63%) that only 5.99% of them were oxygenated whereas, sesquiterpenes were totally detected about 6.65% with 0.71% oxygenated sesquiterpenes. (Z)-β-ocimene and myrcene as acyclic monoterpene hydrocarbons and α-pinene as a cyclic monoterpene hydrocarbon were identified as main compounds. Moreover, the major oxygen containing monoterpene and sesquiterpene are bornyl acetate (3.98 %) and spathulenol (0.71 %), respectively. Composition of the oil obtained from air-dried aerial parts of F. carduchorum from Kerman Province, was found to contain (z)-β-ocimene as the major component (Samiee et al. 2006), which agrees with our research. The amount of (z)-β-ocimene in our investigation (43.3 %) was more than previous study (21.2 %). More ever F. carduchorum from Illam and Kerman Provinces had shown the constituent of α-pinene 18.23 % and 4.8 % respectively. Moreover, other reports showed that in F. humillis, F. trachycarpa and F. angulata (z)-β-ocimene is the main component (Baser et al. 2002, Khanahmadi et al. 2006). The important compound in three species (F. aucheri and F. mughlae and F. sandrasica) was α-pinene. The major components in essential oil of F. macroseiadia, F. sylvatica and F. bernardii were methyl carvacrol, p-cymene and 2, 4, 5-trimethyl benzaldehyde, respectively (Baser et al. 2002, Khalighi-Sigaroodi et al. 2005).
Evaluation of MIC of the essential oil showed that sample had antimicrobial effect against both Gram-positive (Staphylococcus aureus), Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria and a fungal strain (Candida albicans). The essential oil indicated noticeable efficiency against C. albicans (MIC= 2340 μg ml−1).
The antioxidant assessment of fruits and roots of F. campestris were demonstrated that their IC50 were less than IC50 of essential oil of F. carduchorum (Cecchini et al. 2010). Moreover, antimicrobial effect of F. campestris was better than F. carduchorum, that MIC of roots and fruits essential oils were less than MIC of F. carduchorum essential oil (Cecchini et al. 2010). The results of antimicrobial activity of F. bernardii essential oil were shown MIC of essential oil againt Gram-positive, Gram-negative bacteria and fungal strain were <1000 μg ml−1 (Khalighi-Sigaroodi et al. 2005). So, antimicrobial activity of F. bernardii is better than F. carduchorum. Anti-candida effect of Ferulago capillaris essential oil was investigated and the results were demonstrated that MIC is less than F. carduchorum (Pinto et al. 2013). There have been reports on the AchE inhibitory activity of some bicyclic monoterpenoides including α-pinene and 3-carene (Miyazawa et al. 2005). Hence in our study, AchE inhibitory activity of essential oil could be attributed to its α-pinene.
In a previous study, larvicidal activity of Eucalyptus camaldulensis essential oil against An. stephensi was investigated and the LC50 and LC90 values were 89.85 and 215.26 ppm, respectively (Sedaghat et al. 2010). In one study, the larvicidal activities of methanolic extracts of some Iranian plants (Lawsonia inermis, Thymus kotschyanus, Cedrus deodara and eight species from Stachys) against malaria vector, An. stephensi were investigated (Khanavi et al. 2013). These results indicated that essential oil of F. carduchorum was more potent than these plants against An. stephensi. Compared to another studies on larvicidal activity of essential oils of Heracleum persicum, Foeniculum vulgare, Coriandrum sativum (Sedaghat et al. 2011), Cymbopogon olivieri (Hadjiakhoondi et al. 2003) and Nepeta menthoides (Khanavi et al. 2012a) it was found that F. carduchorum was most effective against An. stephensi (Khanavi et al. 2011). Therefore, the essential oil of F. carduchorum can be used in insect control as an alternative for chemical compounds on the environment.
The essential oil of F. carduchorum showed a high cytotoxicity on T47D, HEPG2 and HT-29 cell lines (IC50< 2 μg ml−1). The effect of essential oil on T47D cell line was much stronger than HT-29 and HEP-G2. It indicated that the essential oil had potential cytotoxic selectivity on T47D cell line similar to Methotrexate (positive control), whereas, the essential oil had lower cytotoxic effect than Methotrexate on NIH 3T3 (Swiss embryo fibroblast). So, the oil could be safer than this drug. Another study on Vinca minor revealed that its cytotoxicity activity on T47D (IC50= 1.34±0.29) and HT-29 (IC50= 3.63±1.24) was less than F. carduchorum (Khanavi et al. 2010). In results, this plant has active cytotoxic components.
Conclusion
The essential oil of F. carduchorum collected from west of Iran give good biological activity including anti-Candida, AchE inhibitory, larvicidal and high cytotoxic effects and should be further investigated in others in vitro and in vivo experimental models. The determined biological activities of F. carduchorum essential oil are important information for its future application in treatment. Furthermore, the determination of antioxidant and antibacterial activity of the essential oil have great importance in preservation of food products and pharmaceutical industries.
Acknowledgements
This research has been supported by Tehran University of Medical Sciences (Grant No: 90-02-33-13855). The authors declare that there is no conflict of interests.
References
- 1. Aazza S, Lyoussi B, Miguel MG. ( 2011) Antioxidant and antiacetyl cholinesterase activities of some commercial essential oils and their major compounds. Molecules. 6: 7672– 7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbott WS. ( 1925) A method of computing the effectiveness of an insecticide. J Econ Entomol. 18: 265– 267. [Google Scholar]
- 3. Adams RP. ( 1995) Identification of Essential oil Components by Gas Chromatography/Mass Spectroscopy. Carol Stream, Illinois: Allured Publishing Corporation. ISBN-10: 1932633219. [Google Scholar]
- 4. Andrianova VB, Sklyar YuE, Pimenov MG. ( 1975) Coumarins of Ferulago turcomanica roots. Khim Prir Soedin. 11: 514. [Google Scholar]
- 5. Baser KHC, Demirci B, Ozek T, Akalin E, Ozhatay N. ( 2002) Micro Distilled Volatile Compounds from Ferulago Species Growing in Western Turkey. Pharm Boil. 40: 466– 471. [Google Scholar]
- 6. Basile A, Sorbo S, Spadaro V, Bruno M, Maggio A, Faraone N, Rosselli S. ( 2009) Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules. 14: 939– 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cecchini CM., Coman M, Cresci A, Tirillini B, Cristalli G, Papa F, Sagratini G, Vittoric S, Maggid F. ( 2010) Essential oil from fruits and roots of Ferulago campestris (Besser) Grecescu (Apiaceae): composition and antioxidant and anti-Candida activity. Flavour Frag. J. 25: 493– 502 [Google Scholar]
- 8. Dall'Acqua S, Maggi F, Minesso P, Salvagno M, Papa F, Vittori S, Innocenti G. ( 2010) Identification of nonalkaloid acetyl cholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae). Fitoterapia. 81: 1208– 1212. [DOI] [PubMed] [Google Scholar]
- 9. Demetzos C, Perdetzoglou D, Gazouli M, Tan K, Economakis C. ( 2000) Chemical analysis and antimicrobial studies on three species of Ferulago from Greece. Planta Med. 66( 6): 560– 563. [DOI] [PubMed] [Google Scholar]
- 10. De Pascual TJ, Jimenez B, Corrales B, Grande M. ( 1979) Coumarins from Ferulago granatensis group:isovaleryl marmesin. An Quim. 75: 175– 176. [Google Scholar]
- 11. Dharmagadda VSS, Naik SN, Mittal PK, Vasudevan P. ( 2005) Larvicidal activity of Tagetes patula essential oil against three mosquito species. Biores Techno. 96: 1235– 1240. [DOI] [PubMed] [Google Scholar]
- 12. Doganca S, Tuzlaci E, Ulubelen A. ( 1992) Constituents of Ferulago asperigifolia. Fitoterapia. 63( 6): 552. [Google Scholar]
- 13. Doganca S, Ulubelen A, Tuzlaci E. ( 1991) 1-Acetylhydroquinone 4-galactoside from Ferulago aucheri. Phytochemistry. 30: 2803– 2805. [Google Scholar]
- 14. Erdurak CS, Coskun M, Demirci B, Baser KHC. ( 2006) Composition of the essential oil of fruits and roots of Ferulago isaurica Pesmen and F. syriaca Boiss. (Umbelliferae) from Turkey. Flavour Frag J. 21: 118– 121. [Google Scholar]
- 15. Grutzendler J, Morris JC. ( 2001) Cholinesterase inhibitors for alzheimer's disease. Drugs. 61: 41– 52. [DOI] [PubMed] [Google Scholar]
- 16. Hadjiakhoondi A, Aghel N, Zamanizadeh N, Vatandoost H. ( 2008a) Chemical and biological study of Mentha spicata L. essential oil from Iran. Daru. 8: 19– 21. [Google Scholar]
- 17. Hadjiakhoondi A, Vatandoost H, Abousaber M, Khanavi M, Abdi L. ( 2008b) Chemical composition of the essential oil of Tagetes minuta L. and its effects on Anopheles stephensi larvae in Iran. J Med Plant. 7( 26): 33– 100. [Google Scholar]
- 18. Hadjiakhoondi A, Sadeghipour-Roodsari HR, Vatandoost H, Khanavi M, Abaee MR, Vosoughi M. ( 2006) Fatty acid composition and toxicity of Melia azedarach L. fruits against malaria vector Anopheles stephensi. Iranian J Pharm Sci. 2( 2): 97– 102. [Google Scholar]
- 19. Hadjiakhoondi A, Vatandoost H, Khanavi M, Abaee MR. ( 2005) Biochemical investigation of different extracts and larvicidal activity of Tagetes minuta L on Anopheles stephensi larvae. Iran J Pharm Sci. 1: 81– 84. [Google Scholar]
- 20. Hadjiakhoondi A, Vatandoost H, Jamshidi A, Bagherj Amiri E. ( 2003) Chemical constituents and efficacy of Cymbopogon olivieri (Boiss) bar essential oil against malaria vector, Anopheles stephensi. Daru. 11( 3): 125– 128. [Google Scholar]
- 21. James AA. ( 1992) Mosquito molecular genetics: the hands that feed bite back. Science. 257: 37– 38. [DOI] [PubMed] [Google Scholar]
- 22. Jimenez B, Grande MC, Anaya J, Torres P, Grande M. ( 2000) Coumarins from Ferulago capillaris and F. brachyloba. Phytochemistry. 53: 1025– 1031. [DOI] [PubMed] [Google Scholar]
- 23. Karimi GH, Iranshahi M, Hosseinalizadeh F, Riahi B, Sahebkar A. ( 2010) Screening of acetyl cholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula. Pharmacologyonline. 1: 566– 574. [Google Scholar]
- 24. Khalighi-Sigaroodi F, Hadjiakhoondi A, Shafiee A, Mozaffarian VA, Shahverdi AR, Alavi SHR. ( 2006) Phytochemical analysis of Ferulogo Bernardii Tomk and M Pimen. Daru. 14: 214– 221. [Google Scholar]
- 25. Khalighi-Sigaroodi F, Hadjiakhoondi A, Shahverdi AR, Mozaffarian V, Shafiee A. ( 2005) Chemical composition and antimicrobial activity of the essential oil of Ferulago Bernardii Tomk and M Pimen. Daru. 13: 100– 104. [Google Scholar]
- 26. Khanahmadi M, Janfeshan K. ( 2006) Study on antioxidant property of Ferulago angulata plant. Asian J Plant Sci. 5: 521– 526. [Google Scholar]
- 27. Khanavi M, Vatandoost H, Khosravi Dehaghi N, Sanei Dehkordi A, Sedaghat MM, Hadjiakhoondi A, Hadjiakhoondi F. ( 2013) Larvicidal Activities of Some Iranian Native Plants against the Main Malaria Vector, Anopheles stephensi. Acta Med Iran. 51: 141– 147. [PubMed] [Google Scholar]
- 28. Khanavi M, Fallah A, Vatandoost H, Sedaghat M, Abai MR, Hadjiakhoondi A. ( 2012a) Larvicidal activity of essential oil and methanol extract of Nepeta menthoides against malaria vector Anopheles stephensi. Asian Pac J Trop Med. 5( 12): 962– 965. [DOI] [PubMed] [Google Scholar]
- 29. Khanavi M, Manayi A, Lotfi M, Abbasi R, Majdzadeh M, Ostad N. ( 2012b) Investigation of Cytotoxic Activity in Four Stachys Species from Iran. Iran J Pharm Res. 11: 589– 593. [PMC free article] [PubMed] [Google Scholar]
- 30. Khanavi M, Rajabi A, Behzad M, Hadjiakhoondi A, Vatandoost H, Abaee MR. ( 2011) Larvicidal Activity of Centaurea bruguierana ssp. belangerana Against Anopheles stephensi Larvae. Iran J Pharm Res. 10( 4): 829– 833. [PMC free article] [PubMed] [Google Scholar]
- 31. Khanavi M, Pourmoslemi Sh, Farahanikia B, Hadjiakhoondi A, Ostad N. ( 2010) Cytotoxicity of Vinca minor. Pharm Biol. 48( 1): 96– 100. [DOI] [PubMed] [Google Scholar]
- 32. Khanavi M, Saghari Z, Mohammadirad A, Khademi R, Hadjiakhoondi A, Abdollahi M. ( 2009) Comparison of antioxidant activity and total phenols of some date varieties. Daru. 17: 104– 108. [Google Scholar]
- 33. Kilic CS, Ozkan AMG, Demirci B, Coskun M, Baser KHC. ( 2010) Essential oil composition of four endemic Ferulago species growing in Turkey. Nat Prod Commun. 5: 1951– 1954. [PubMed] [Google Scholar]
- 34. Maisonneune SA. ( 1975) European Pharmacopoeia. Vol. 3 Saint-Ruffine; France. [Google Scholar]
- 35. Miyazawa M, Yamafuji C. ( 2005) Inhibition of acetyl cholinesterase activity by bicyclic monoterpenoids. J Agric Food Chem. 53: 1765– 1768. [DOI] [PubMed] [Google Scholar]
- 36. Mozaffarian V. ( 2007) A Dictionary of Iranian Plant Names. Farhange Moaser, Tehran. [Google Scholar]
- 37. NCCLS ( 2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A7; PA, Wayne. [Google Scholar]
- 38. Newman DJ, Cragg GM. ( 2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod. 70: 461– 477. [DOI] [PubMed] [Google Scholar]
- 39. Ognyanov I, Bocheva D. ( 1969) Natural coumarins. II: Coumarins in Ferulago meoides. Planta Med. 17: 65– 70. [Google Scholar]
- 40. Pinto E, Hrimpeng K, Lopes G, Vaz S, Gonçalves MJ, Cavaleiro C, Salgueiro L. ( 2013) Antifungal activity of Ferulago capillaris essential oil against Candida, Cryptococcus, Aspergillus and dermatophyte species. Eur J Clin Microbiol Infect Dis. 32( 10): 1311– 1120. [DOI] [PubMed] [Google Scholar]
- 41. Rahimifard N, Pakzad SR, Shoeibi Sh, Hedayati MH, Hajimehdipour H, Motaharinia V, Mehrafshan L, Javadi A, Pirali Hamedani V, Mehrafshan L, Javadi A, Pirali Hamedani M. ( 2009) Effects of essential oil and extract of Thymus vulgaris, Zataria multiflora and Eugenia carryophilata on Vero, Hela, Hep2 cell lines by MTT assay. J Med Plants. 8: 152– 156. [Google Scholar]
- 42. Rechinger KH. ( 1978) Flora Iranica (Umbelliferae). Vol. 62 Graz: Akademische Druck-u. Verlagsanstalt; Germany. [Google Scholar]
- 43. Rosselli S, Maggio AM, Faraone N, Spadaro V, Morris-Natschke SL, Bastow KF, Lee KH, Bruno M. ( 2009) The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelino. Nat Prod Commun. 4( 12): 1701– 1706. [PubMed] [Google Scholar]
- 44. Ruberto G, Cannizzo S, Amico V, Bizzini M, Piattelli M. ( 1994) Chemical constituents of Ferulago nodosa. J Nat Prod. 57: 1731– 1733. [Google Scholar]
- 45. Samiee K, Akhgar MR, Rustaiyan A, Masoudi SH. ( 2006) Composition of the volatiles of Ferulago carduchorum Boiss and Hausskn and Levisticum officinale Koch obtained by hydrodistillation and extraction. J Essent Oil Res. 18: 19– 22. [Google Scholar]
- 46. Sedaghat MM, Sanei Dehkordi A, Abai MR, Khanavi M, Mohtarami F, Salim Abadi Y, Rafi F, Vatandoost H. ( 2011) Larvicidal activity of essential oils of Apiaceae plants against malaria vector, Anopheles stephensi. Iran J Arthropod Borne Dis. 5: 51– 59. [PMC free article] [PubMed] [Google Scholar]
- 47. Sedaghat MM, Sanei Dehkordi AR, Khnavi M, Abai MR, Hadjiakhoondi A, Mohtarami F, Vatandoost H. ( 2010) Phytochemistry and larvicidal activity of Eucalyptus camaldulensis against malaria vector, Anopheles stephensi. Asian Pac J Trop Med. 10: 841– 845. [Google Scholar]
- 48. Sedaghat MM, Harbach RE. ( 2005) An annotated checklist of the Anopheles mosquitoes (Diptera: Culicidae) in Iran. J Vect Ecol. 30: 272– 276. [PubMed] [Google Scholar]
- 49. Serkerov SV, Kagramanov AA, Abbasov RM. ( 1976) Coumarins of Ferulago turcomanica. Khim Prir Soedin. 1: 94. [Google Scholar]
- 50. Shen Q, Peng Q, Shao J, Liu X, Huang Z, Pu X, Ma L, Li YM, Chan AS, Gu L. ( 2005) Synthesis and biological evaluation of functionalized coumarins as acetyl cholinesterase inhibitors. Eur J Med Chem. 40: 1307– 1315. [DOI] [PubMed] [Google Scholar]
- 51. Sklyar YE, Andrianova VB, Pimenov MG. ( 1982) Coumarins of the roots of Ferulago sylvatica. Chem Nat Comp. 18: 488– 489. [Google Scholar]
- 52. Sodeifian GH, Ansari K, Bamoniri A. ( 2011) Study of chemical composition of the essential oil of Ferulago angulata (schelcht) Boiss from Iran using supercritical fluid extraction and nano scale injection. J Nanomaterials and Biostructures. 161– 168. [Google Scholar]
- 53. Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, Mahmoodi M, Akbarzadeh K, Sartipi M. ( 2011) Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan Province, southeast Islamic Republic of Iran. East Mediterr Health J. 17: 439– 445. [PubMed] [Google Scholar]
- 54. Vatandoost H, Khazani A, KebriainZadeh A, Rafinejad J, Vatandoost H, Khazani A, KebriainZadeh A, Rafinejad J, Khoobdel M, Abai MR. ( 2008) Comparative efficacy of Neem and dimethyl phthalate (DMP) against malaria vector, Anopheles stephensi (Diptera: Culicidae). Asian Pacific J Trop Med. 1( 3): 1– 6. [Google Scholar]
- 55. Weinstock M. ( 1997) Possible role of the cholinergic system and disease models. J Neural Transm. 49: 93– 102. [DOI] [PubMed] [Google Scholar]
- 56. Yokozawa T, Chen CP, Dong E, Tanka-Nonaka I. ( 1998) Study on the inhibitory effect on tannins and flavonoids against the DPPH radical. Biochem Pharmacol. 50: 213– 222. [DOI] [PubMed] [Google Scholar]
- 57. Zhou X, Wang XB, Wang T, Kong LY. ( 2008) Design, synthesis, and acetyl cholinesterase inhibitory activity of novel coumarin analogues. Bioorgan Med Chem. 16: 8011– 8021. [DOI] [PubMed] [Google Scholar]


