Abstract
Background:
The most part of Iran become malaria-free region and fall in prevention of re-introduction stage. These regions however are struggling with imported of malaria cases where malaria vectors exist. Therefore, understanding the situation of mosquito vectors is crucial. This study was carried out to find out the present situation of malaria vectors and malaria transmission potential in a malaria-free area.
Methods:
The study was conducted in a malaria free area, Izeh County, Khuzestan Province during 12 months in 2011–2012. Five villages, including 2 in highlands and 3 in plain area, were selected randomly. The mosquito sampling methods were conducted using spray sheet and hand catch collection methods from indoor/outdoors, window trap and larvae collections.
Results:
In total, 3352 female Anopheles were captured, 1826 mosquito from highland and 1526 from plain areas. Five species, An. stephensi, An. fluviatilis s.l., An. dthali, An. superpictus and An. pulcherrimus were identified. The seasonal activities were started from April to March. The abdominal conditions of collected mosquitoes from indoor/outdoor places pointed to exophilic propensity of An. fluviatilis.l. s.l. and endophilic behaviour for rest of the vectors. The results of window trap also confirmed these behaviors. The larval habitats of four species were widely dispersed and included spring, margin of rivers, irrigation channels, stagnant water and rice filed.
Conclusion:
Understanding the present situation of malaria vectors in free-malaria area is crucial particularly where is struggling with imported cases. The results of present study can be expanded to other area of northern Khuzestan for malaria vector control planning in reintroduction prevention stage.
Keywords: Malaria, Anopheles vector, Seasonal activity, Iran
Introduction
Malaria is very geographically specific and remains an important cause of mortality and morbidity in many parts of the world. In 2012, there were 99 countries and territories which highly struggling malaria transmission and 5 countries in the prevention of reintroduction phase (WHO 2013). Although this disease has been eradicated from many regions, there is more possible to reintroduce to an area particularly where Anopheles vectors are present circumstances are suitable for parasite transmission (Roll Back Malaria 2013). Therefore, to prevent malaria reintroduction is a big challenge (Hemami et al. 2013).
Malaria was native in the most parts of Iran but after six decade effort, the disease has been limited to south-eastern part of the country (Raeisi et al. 2013). Anti-malaria operation was started in Iran in 1945 and which cause malaria infection rate considerably decreased during 1948–1956 in the most endemic areas. Later malaria eradication programme started and since 1980 almost interrupted malaria transmission in the north and middle parts of the country. Then the programme shifted to malaria control programme which has been continuing up to present time (Azizi and Bahadori 2013). Seven Anopheles including An. maculipennis s.l., An. sacharovi, An. superpictus, An. fluviatilis s.l., An. stephensi, An. culicifacies and An. dthali are considered to be main malaria vectors in Iran (Zahar 1974, Hanafi-Bojd et al. 2011). In addition, An. pulcherrimus has been reported as suspected potential vector of malaria in the district of Ghassreghand, Baluchistan, Iran (Zaim et al. 1993). More recently four Anopheles species, An. stephenis, An. dthali, An. pulcherrimus and An. superpictus have been reported from Mahshar County located in south of Khuzestan Province, Iran (Farhadinejad et al. 2013).
At present, Iran fall to pre-elimination of malaria but the main challenges to sustaining elimination are addressing the potential re-introduction of cases, either via long border with malaria-endemic countries at east or from migrant populations scatter to whole country (Raeisi et al. 2013). However, malaria transmission is highly depend on community and Anopheles vectors behavior particularly in southern Iran where is threaded by imported cases (Shahandeh et al. 2010).
Khuzestan Province recently became free from malaria transmission but the area is threatened by imported malaria cases (Akbari et al. 2013). In this circumstance, vector control plays a main role to prevent malaria outbreak. Therefore, understanding the vectors' situation in advance is so essential for hampering of malaria occurrence. Izeh County is located at north of Khuzestan known as a malaria eliminated zone. Due to travel of job seeker labors or immigrants into the county, reintroduction of malaria particularly cases that lead to re-establishment of local transmission is the most important issue (personal communication with Izeh district Health Centre). Izeh has suitable environments for mosquito development but for more than five years no any malaria transmission has been reported, therefore, vector control programmes have been limited to larviciding operation and environmental management (County Health Centre of Izeh, personal communication).
The present study illustrates the current situation of malaria vectors and their activities in the Izeh County with a scope of role of the vector in reintroduction of malaria. The results of this study can be used for similar area which has imported malaria cases and potent vectors.
Materials and Methods
Study area
Izeh County is located at North of Khuzestan Province. The county is situated in slope of Zagrus Mountain chain at north and Khuzestan Plain in south (Fig. 1). It is geographically located on latitude 31°34′8″N, and longitude 49°34′0″E. Generally, the area comprises mountainous, hilly regions in the north, with plains regions in the south and west. The region has a mild climate and temperature reaches to 40 °C at maximum in summer and minimum near 10 °C in December and January. The water sources are permanent and temporary rivers, wells springs, ponds and pools. The annual rainfall ranged from 100 to 120 mm. The rainfall occasionally received during winter.
Fig. 1.
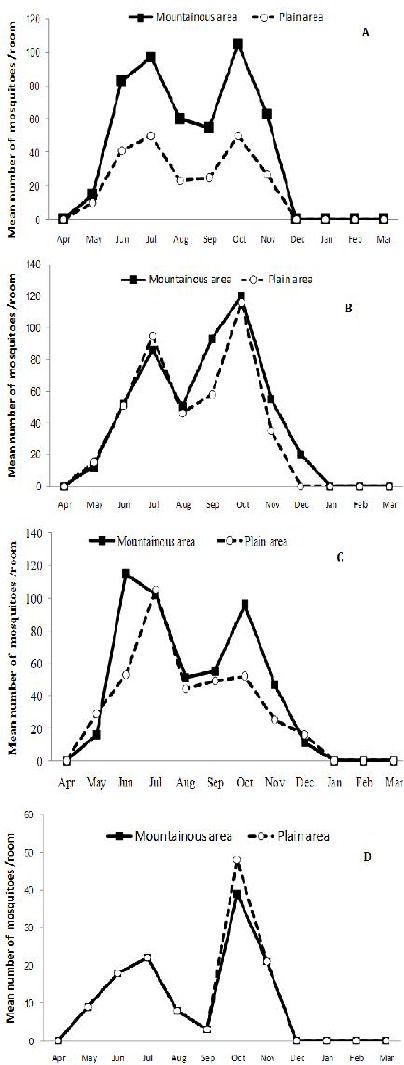
Seasonal prevalence of female mosquitoes: Anopheles stephensi (A), An. dthali (B), An. superpictus (C) and An. fluviatilis s.l. (D) collected from indoor places in both mountainous and plain areas of Izeh County (2011–2012). The population trends of mosquitoes were similar in both distinguishable areas
Izeh has Primary Health Care (PHC) since 1988 and all rural and urban areas have PHC services. By integrating malaria control activities into health system, the health workers involved in malaria cases finding as well as vector control programme.
Mosquito collection
Mosquito collections were standardized as fully described by WHO (WHO 2003). During the 12 months, mosquitoes were collected from 3 villages located in mountainous lands and 2 villages in plain lands. Collections were carried out from eight indoor and outdoor shelters in each village as follow.
Indoor collection
The mosquitoes were collected from human dwellings and sheep or cattle sheds by pyrethrum space spray method as fully described by Service and WHO (Service 1976, WHO 2003). Briefly, before spray, all the eves, windows, doors, and other exit points in each indoor shelter were closed and then white cloth sheets were spread on the floor. Pyrethrum extract (0.2% in kerosene) was sprayed in the entire space of the room and the room was closed for 15 minutes. After 15 minutes, all the knocked-down insects lying on the cloth sheet were collected carefully with the forceps and placed in petri dishes lined with moist filter paper and brought to the laboratory for further studies. The time of application in each village was early in the morning and during sunrise between 0530 and 0730 h.
Outdoor collection
Natural shelters inside and around the indicator villages were periodically searched during early morning and mosquitoes were collected using sucking tube and torch light. The mosquitoes were then captured using sucking tube and touch. All mosquitoes were identified based on species taxonomic keys of Shahgudian (Shahgudian 1960).
All details of the shelters including kind of habits, temperature, humidity, and date and time of collection were recorded on forms. In addition, collected female mosquitoes were graded to abdominal conditions in each sampling technique as described by (WHO 2003). Generally, endo/exophilic behavior of each mosquito species was categorized based on abdominal appearance of the collected mosquitoes as follows. The gravid (G) and/or semi-gravid (SG) appearance of the female abdomen demonstrate as resting stages of female mosquitoes, and the females with unfed (U) and freshly fed (F) guts are indicative of the seeking stages (seeking for blood meal or resting places). Therefore, ratio of the resting stages classified as tendency to rest in indoor or outdoor places (WHO 2003).
Window trap collection
Both entry and exit window traps (Length × width × height= 30× 30× 30 cm) were utilized to find moving behavior of mosquitoes in to the indoor shelters. Two entry and two exit traps were fixed on the windows of selected rooms in each villages from sun set until sun rise. All mosquitoes were collected alive with sucking tube and transferred to laboratory for identification.
Larval sampling and processing
Larval surveillance was conducted twice a month over a 12 month period in 2011. The larvae collection was carried using standard mosquito dipper from all potent breeding places out around the selected villages with a radius of about one kilometer. All collected larvae were counted and III-IV instars larvae transferred to lacto-phenol. The larvae were then identified 24 hours after conservation using morphological taxonomic key (Shahgudian 1960).
Results
In total, 3352 female Anopheles spp. were captured including 1826 mosquitoes from mountainous and 1526 from plain areas. The mosquito species were An. stephensi (ntotal= 837, nindoor= 701, noutdoor= 21, Window traps= 115), An. superpictus (ntotal= 1100, nindoor= 871, noutdoor= 48, Widow traps= 181), An. fluviatilis s.l. (ntotal= 326, nindoor= 129, noutdoor= 126, window traps= 71), An. dthali (ntotal= 1088, nindoor= 993, noutdoor= 47, Window traps= 137) and An. pulcherrimus (nindoor= 1).
As illustrated in Fig. 1, the seasonal population dynamics of the mosquitoes in indoor places were varied among four species either sampled from mountainous or plain areas. Totally, An. stephensi, An. superpictus and An. dthali population trend in mountainous followed the same pattern in plain areas (Fig. 1A, B, C) but An. fluviatilis s.l. had low indoor abundance in the plain areas comparing with mountainous areas (Fig. 1D). The seasonal activities of four species were started in April and the highest population of An. superpictus s.l. and An. dthali was observed in June while the maximum population of An. stephensi and An. fluviatilis s.l. occurred in October. An. superpictus exhibited more tendencies to be in indoor shelters (Fig. 1B) followed by An. stephensi and An. dthali populations (Fig. 1A). Generally, the population density of adults declined to minimum in winter.
Abundance of female mosquitoes collected from animal and human shelters was varied in either highland or low land areas (Table 1). Anopheles stephensi showed slightly more tendency to human shelters rather than animals while An. superpictus, An. dthali and An. fluviatilis s.l. were captured more in animal shelters comparing with human places. Totally, the pattern of using human/animal shelters as resting places by each species in highland areas was same as lowlands (Table 1).
Table 1.
Number of female mosquito captured from human and animal places in two different areas, Izeh County, Southwest of Iran, 2011–2012
| Species |
Mountainous areas |
Total |
Plain areas |
Total | ||
|---|---|---|---|---|---|---|
| Animal shelters (%) | Human shelters (%) | Animal shelters (%) | Human shelters (%) | |||
| An. stephensi | 230 (48.1) | 248 (51.8) | 478 | 103 (46.2) | 120 (53.8) | 223 |
| An. dthali | 302 (63.8) | 17 (35.2) | 473 | 299 (57.5) | 221 (42.5) | 520 |
| An. superpictus | 249 (50.1) | 244 (48.9) | 498 | 201 (53.8) | 172 (46.1) | 373 |
| An. fluviatilis s.l. | 46 (38.3) | 74 (61.6) | 120 | 9 (100) | 0 (0.0) | 9 |
| An. pulcherrimus | 0 | 0 | 0 | 1 (0.002) | 0 | 1 (0.0008) |
Four species also showed propensities for outdoor shelters (Fig. 2). The population dynamics of mosquitoes in outdoor shelters displaced similar pattern to indoor places. The mosquitos’ activities started from April to December or January. Anopheles fluviatilis s.l. showed more propensities for resting in outdoor shelters either in plain or mountain areas (Fig. 2D).
Fig. 2.
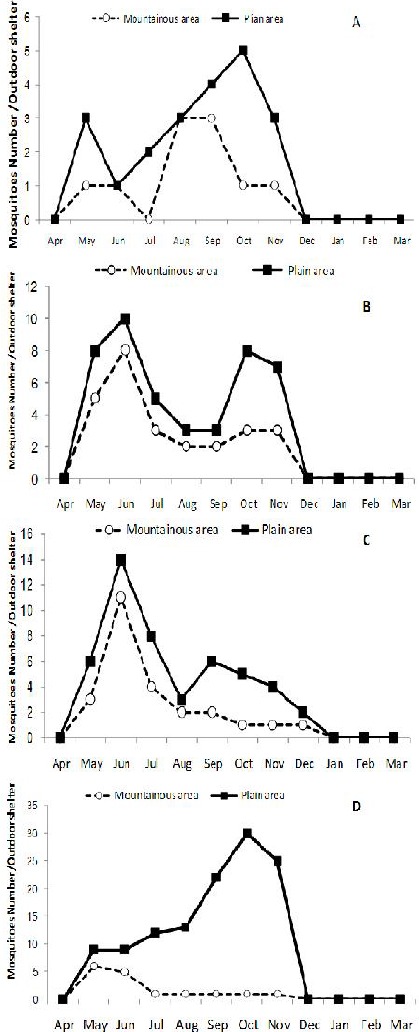
Seasonal density of female mosquitoes: An. stephensi (A), An. dthali (B), An. superpictus (C) and An. fluviatilis s.l. (D) collected from outdoor places in both mountainous and plain areas of Izeh County (2011–2012)
In addition, female mosquitoes were graded according to abdominal conditions collected indoors and outdoors. The gravid (G) and/or semi-gravid (SG) appearance of the abdomen demonstrate as resting stages, while the female mosquitoes with unfed guts (U) and/or freshly fed (F) are indicative of the seeking stages. The ratio of resting stages to seeking stages for An. stephensi showed that this species had a greater tendency to rest inside (G, SG/E, F= 0.75) rather than outdoors (G, SG/E, F= 0.50). Anopheles fluviatilis s.l. had a low proportion for endophilic behavior (G, SG/E, F= 0.17) and its preference for resting outside (G, SG/E, F= 0.56) was five times more than An. stephensi and nearly two times more than An. superpictus (Table 2). Anopheles superpictus showed slightly more exophilic behavior (G, SG/E, F= 0.71) than endophililc (G, SG/E, F= 0.73). Though the majority of An. dthali was collected in indoor shelters, resting tendency to indoor (G, SG/E, F= 0.88) was more than outdoors (G, SG/E, F= 0.51).
Table 2.
Abdominal condition of female mosquitoes based on collecting sites in Izeh County, southwest of Iran, 2011–2012
| Species |
Indoor collections |
G,SG/F, E (indoors) |
Outdoor collections |
G,SG/F, E (outdoors) | Outdoors/Indoors | ||
|---|---|---|---|---|---|---|---|
| F, E | G,SG | F, E | G, SG | ||||
| An. stephensi | 399 (57.1%) | 302 (42.9%) | 0.75 | 14 (72.2%) | 7 (27.8%) | 0.50 | 0.66 |
| An. superpictus | 504 (57.8%) | 367 (42.2%) | 0.73 | 28 (64.7%) | 20 (35.3%) | 0.71 | 0.97 |
| An. fluviatilis s.l. | 110 (85.3%) | 19 (14.7%) | 0.17 | 81 (70.0%) | 45 (30.0%) | 0.55 | 3.27 |
| An. dthali | 528 (53.21%) | 465 (46.8%) | 0.88 | 31 (68.1%) | 16 (31.9%) | 0.52 | 0.59 |
F: Fresh Fed female mosquito
E: Empty or Unfed female mosquito
G: Gravid female mosquito
SG: Semi-Gravid female mosquito
The number of mosquitoes caught by window traps is showed in Table 3. Generally, indoor tendency exhibited by four Anopheles species either in higher in lowland or highland. Entry behavior of An. stephensi, An. superpictus, An. dthali and An. fluviatilis s.l. was observed during whole activity seasons (Fig. 3). The dynamic of entry movement of four Anopheles species was alike to population dynamics of them sampled from indoors.
Table 3.
Frequency of female mosquito captured from exit andentry window trap in two different areas, Izeh County, southwest of Iran, 2011–2012
| Species |
Mountainous areas |
Total |
Plain areas |
Total | ||
|---|---|---|---|---|---|---|
| Exit (%) | Entry (%) | Exit (%) | Entry (%) | |||
| An. stephensi | 11 (34.3) | 21 (65.5) | 32 | 32 (38.5) | 51 (61.4) | 83 |
| An. dthali | 17 (36.1) | 30 (63.8) | 47 | 46 (51.1) | 44 (48.8) | 90 |
| An. superpictus | 40 (61.5) | 25 (38.4) | 65 | 62 (53.4) | 54 (46.5) | 116 |
| An. fluviatilis s.l. | 23 (62.1) | 14 (37.8) | 37 | 26 (76.4) | 8 (23.5) | 34 |
Fig. 3.
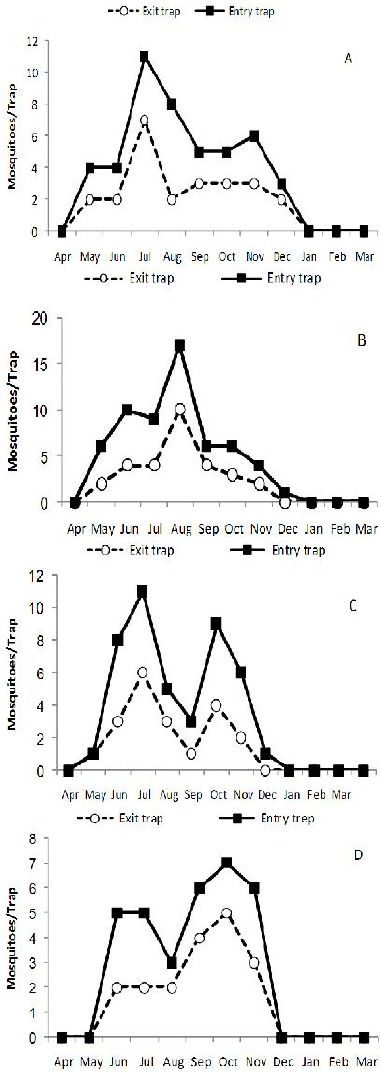
Mean number of female mosquitoes, Anopheles stephensi (A), An. dthali(B), An. superpictus (C) and An. fluviatilis s.l. (D) collected by exit and entry window traps in Izeh County (2011–2012). The population trends of mosquitoes were similar in both distinguishable areas
Totally, 931 larvae were collected from plain (418 larvae) and mountainous (513 larvae) areas. The collected larvae species in plain area were An. stephensi (117 larvae), An. superpictus (170 larvae), An. dthali (80 larvae) and An. fluviatilis s.l. (51 larvae) and in mountainous area An. stephensi (160 larvae), An. superpictus (105 larvae), An. dthali (222 larvae) and An. fluviatilis s.l. (26 larvae).
The larvae were generally found in spring, streams, margin of rivers, irrigation channels, stagnant water such as waste water, drainage, borrow pits as well as rice fields. The ratio of Anopheles species in each type of breeding places was highly varied. In addition high different between mountainous and plain areas was observed with respect to using different breeding places (Fig. 4, 5). Anopheles stephensi larvae were found mostly in stagnant waters or waters with low flow rate such as rice farm. Comparing to larvae combination in each type of breeding place, An. stephensi larvae ratio in the lowland area (plain) was mostly rice farm (Fig. 3C) while in the highland, it found mostly in springs. Anopheles dthali larvae collected in various breeding places with relatively high ratio comparing with other Anopheles mosquitoes (Fig. 4, 5) except in rivers margin of highland region (Fig. 4A). In contrary, high abundance of An. superpictus was collected in river margins particularly in highland (Fig. A). Anopheles fluviatilis s.l. was found at low ratio in stagnant waters and spring of highlands while more ratio of An. fluviatilis.l. s.l. larvae (19%) was collected in stagnant waters of in plain areas (Fig. 3B, Fig. 4B, C).
Fig. 4.
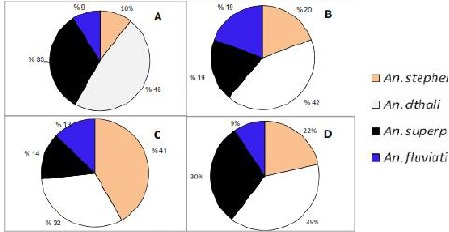
Larvae ratio of four Anopheles species, collected from different habitants in plain areas
A: River side
B: Stagnant water
C: Rice farm
D: Spring
Fig. 5.
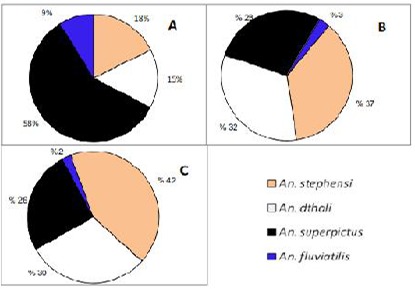
Larvae ratio of four Anopheles species collected from different habitants in mountainous area.
A: River side
B: Stagnant water
C: Spring
Discussion
Indeed, mosquito collections were carried out on probably occurrence of malaria outbreaks in an eliminated zone such as north of Khuzestan Province not based on a more comprehensive seasonal or temporal collection protocol may have resulted in a lower mosquito vectors activities overall. However, our results indicate that the activities and behavior of four Anopheles vectors in Izeh County indicates that the area may have potent for malaria transmission depend on mosquito vectors contact with any malaria cases.
Totally, the population trend of three mosquito species in mountainous area followed the same pattern in plain (Fig. 1A, B, C). In contrary, An. fluviatilis s.l. had low indoor abundance in the plain area rather than mountainous. These results may indicate that the environmental condition in both areas is similar for endophilic species.
In the present study, An. superpictus and An. dthali were the most abundant and prevalent species showing preference to be indoor places while An. fluviatilis s.l. at low prevalent noticeably to outdoor places. The abundant of An. stephensi was also high in both indoor and outdoor shelters with more intendancy to indoors in Izeh areas. Anopheles fluviatilis s.l. was collected at very low frequency in indoor places particularly in plain areas indicating that this mosquito is more exophlic and this has been confirmed by the results of collection using window trap methods. Similar behavior of An. fluviatilis s.l. was declared by several authors (Viswanathan et al. 1945, Eshghi et al. 1976, Nanda et al. 1996, Sahu et al. 2009, Sahu et al. 2011).
It was illustrated that An. fluviatilis s.l. as a wild mosquito had relatively high tendency to human blood (Manouchehri et al. 1976a, Basseri et al. 2010). Totally, it seems host seeking behavior of four Anopheles adults may slightly different when they exhibited varied movement and resting behaviour in Izeh County.
We also found that larval habitats of An. fluviatilis s.l. may be small, widely dispersed, and transient, and this may be reason we could not find the larvae at high abundance in Izeh County. Overall, control of An. fluviatilis s.l. is big challenge due to the behavior of this mosquito vector in Izeh County. However, this mosquito considered as one of the most important malaria vector for stable malaria in Middle East and south of Asia countries (Eshghi et al. 1976, Manouchehri et al. 1976a, Gunasekaran 1994, Dev et al. 2003).
Anopheles stephensi was recognized as a domestic mosquito (Sharma 1995, Kar et al. 1996, Chakraborty et al. 1998). It was responsible for malaria epidemic in some part of southern Iran (Manouchehri et al. 1976b). In addition, this species exhibits a strong preference for human blood in south and south eastern Iran (Basseri et al. 2005, 2010). In the present study, An. stephensi showed endophilic behavior as well as using natural shelters for rest. Therefore, at outbreak situation of malaria, using indoor residual spraying may not be effective method for control of this vector, particularly this anopheles has become resistant to several insecticides in Iran (Enayati et al. 2003, Vatandoost et al. 2006).
Anopheles dthali was identified as secondary malaria vector in Iran (Manouchehri et al. 1972). As in previous study stated this mosquito use human dwelling as well as animal shelter in south of Iran (Manouchehri and Rohani 1975), we also found An. dthali in the same places at relatively high abundance. Anopheles dthali is still susceptible to all insecticide which used for indoor residual spraying (Vatandoost et al. 2007). However, it was stated that An. dthali can be incriminated for transmission malaria at high density where we comparatively found in Izeh County.
In the present study, Anopheles superpictus was found relatively at high density in both mountainous and plain areas of Izeh County. This mosquito was also captured by window traps more than other mosquito vectors. Anopheles superpictus is one of the most widespread malaria vectors in Iran (Hanafi-Bojd et al. 2011). Overall, the results obtained from window traps collection method (Table 3) and density of the female mosquito per room (Fig. 1) as well as abdominal condition ratio (Table 2) together indicating that this species has more propensity to indoor places than outdoors. Therefore, in any malaria outbreak situation, the behavior of An. superpictus should be considered as a potent vector in north of Khuzestan Province. This species had been responsible for maintaining malaria transmission due to its refractory behavior in southern slopes of the Zagros chain (Zahar AR 1990).
The study area is very fertile with different water source for breeding of Anopheles larvae. The agricultural activities start from beginning of spring and provide many jobs for immigrant labors. Furthermore, the environment of Izeh provides suitable condition for activity of four malaria vectors. According to report of Izeh Health Centre, the area is struggling with imported malaria cases annually. Therefore, occurrence of malaria transmission should be expected.
Conclusion
Although malaria has been eliminated from north of Khuzestan, but due to population movement form malaria endemic area, understanding the present situation of malaria vector is essential. The results of present study can be expanded to other area of northern Khuzestan for malaria vector control planning in reintroduction prevention stage.
Acknowledgements
We thank the personnel of the Izeh Health Centers for kind assistances. The authors would like to thank Dr Ahmad Raeisi, the Head of Malaria Control Office, Ministry of Public Health, for his great technical supports. We appreciate the kind cooperation of Khuzestan Province Health Centre and special thank to Mrs Shahla Bigdeli for her kind official supports. This work received financial support from Tehran University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- 1. Akbari H, Majdzadeh R, Rahimi Foroushani A, Raeisi A. ( 2013) Timeliness of malaria surveillance system in iran. Iran J Publ Health. 42: 39– 47. [PMC free article] [PubMed] [Google Scholar]
- 2. Azizi MH, Bahadori M. ( 2013) Brief historical perspectives of malaria in Iran. Arch Iran Med. 16: 131– 135. [PubMed] [Google Scholar]
- 3. Basseri H, Raeisi A, Ranjbar Khakha M, Pakarai A, Abdolghafar H. ( 2010) Seasonal abundance and host-feeding patterns of anopheline vectors in malaria endemic area of Iran. J Parasitol Res. 20( 10): Article ID 671291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basseri H, Moosakazemi S, Yosafi M, Mohebali M, Hajaran H. ( 2005) Anthropophily of malaria vectors in Kahnouj d, south of Kerman, Iran. Iran J Publ Health. 34: 27– 37. [Google Scholar]
- 5. Chakraborty S, Ray S, Tandon N. ( 1998) Seasonal prevalence of Anopheles stephensi larvae and existence of two forms of the species in an urban garden in Calcutta City. Indian J Malariol. 35: 8– 14. [PubMed] [Google Scholar]
- 6. Dev V, Bhattacharyya PC, Talukdar R. ( 2003) Transmission of malaria and its control in the northeastern region of India. J Assoc Physicians India. 51: 1073– 1076. [PubMed] [Google Scholar]
- 7. Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. ( 2003) Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 17: 138– 144. [DOI] [PubMed] [Google Scholar]
- 8. Eshghi N, Motabar M, Javadian E, Manoucheri AV. ( 1976) Biological features of Anopheles fluviatilis.l. and its role in the transmission of malaria in Iran. Trop Geogr Med. 28: 41– 44. [PubMed] [Google Scholar]
- 9. Farhadinejad R, Mousavi M, Amraee K. ( 2013) Fauna and monthly activity of Anopheles mosquitoes in Mahshar County, Khuzestan Province in 2012. Iran J Infect Dis Trop Med. 62: 43– 47 (in Persian). [Google Scholar]
- 10. Gunasekaran K. ( 1994) Age composition, natural survival and population growth of Anopheles fluviatilis s.l. James, 1902, the major malaria vector in the endemic belt of Koraput County, Orissa, India. Southeast Asian J Trop Med Public Health. 25: 196– 200. [PubMed] [Google Scholar]
- 11. Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Charrahy Z. ( 2011) Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 4: 498– 504. [DOI] [PubMed] [Google Scholar]
- 12. Hemami MR, Sari AA, Raeisi A, Vatandoost H, Majdzadeh R. ( 2013) Malaria elimination in iran, importance and challenges. Int J Prev Med. 4: 88– 94. [PMC free article] [PubMed] [Google Scholar]
- 13. Kar I, Eapen A, Ravindran KJ. ( 1996) Domestic breeding sources and their contribution in Anopheles stephensi breeding in Dindigul, Tamil Nadu. Indian J Malariol. 33: 191– 199. [PubMed] [Google Scholar]
- 14. Manouchehri AV, Djanbakhsh B, Eshghi N. ( 1976a) The biting cycle of Anopheles dthali, A. fluviatilis and A. stephensi in southern Iran. Trop Geogr Med. 28: 224– 227. [PubMed] [Google Scholar]
- 15. Manouchehri AV, Javadian E, Eshighy N, Motabar M. ( 1976b) Ecology of Anopheles stephensi Liston in southern Iran. Trop Geogr Med. 28: 228– 232. [PubMed] [Google Scholar]
- 16. Manouchehri AV, Rohani F. ( 1975) Notes on the ecology of Anopheles dthali Patton in southern Iran. Ann Trop Med Parasitol. 69: 393– 397. [DOI] [PubMed] [Google Scholar]
- 17. Manouchehri A, Ghiasseddin M, Shahgudian ER. ( 1972) Anopheles dthali Patton, 1905, a new secondary vector in southern Iran. Ann Trop Med Parasitol. 66: 537– 538. [DOI] [PubMed] [Google Scholar]
- 18. Nanda N, Joshi H, Subbarao SK, Yadav RS, Shukla RP, Dua VK, Sharma VP. ( 1996) Anopheles fluviatilis s.l. complex: host feeding patterns of species S, T, and U. J Am Mosq Control Assoc. 12: 147– 149. [PubMed] [Google Scholar]
- 19. Raeisi A, Gouya MM, Nadim A, Ranjbar M, Hasanzehi A, Fallahnezhad M, Sakeni M, Safari R, Saffari M, Mashyekhi M, Ahmadi Kahnali A, Mirkhani V, Almasian E, Faraji L, Paktinat Jalali B, Nikpour F. ( 2013) Determination of malaria epidemiological status in Iran's malarious areas as baseline information for implementation of malaria elimination program in Iran. Iran J Public Health. 42: 326– 333. [PMC free article] [PubMed] [Google Scholar]
- 20. Roll Back Malaria ( 2013) Global Malaria Action Plan for a Malaria-free World. Roll Back Malaria Partnership, WHO; Avaiable at: http://www.rollbackmalaria.org/gmap/2-3.html.) [Google Scholar]
- 21. Sahu SS, Gunasekaran K, Vanamail P, Jambulingam P. ( 2011) Seasonal prevalence and resting behaviour of Anopheles minimus Theobald and An. fluviatilis James (Diptera: Culicidae) in east-central India. Indian J Med Res. 133: 655– 661. [PMC free article] [PubMed] [Google Scholar]
- 22. Sahu SS, Gunasekaran K, Jambulingam P. ( 2009) Bionomics of Anopheles minimus and An. fluviatilis s.l. (Diptera: Culicidae) in east-central India, endemic for falciparum malaria: human landing rates, host feeding, and parity. J Med Entomol. 46: 1045– 1051. [DOI] [PubMed] [Google Scholar]
- 23. Service MW. ( 1976) Mosquito Ecology: Field Sampling Methods. John Wiley and Sons, New York. [Google Scholar]
- 24. Shahandeh K, Basseri H, Pakari A, Riazi A. ( 2010) Mosquito vector biting and community protection in a malarious area, Siahoo County, Hormozgan, Iran. Iran J Arthropod Borne Dis. 4: 35– 41. [PMC free article] [PubMed] [Google Scholar]
- 25. Shahgudian ER. ( 1960) A key to the Anopheles of Iran. Acta Med Iran. 3: 38– 48. [PubMed] [Google Scholar]
- 26. Sharma RS. ( 1995) Urban malaria and its vectors Anopheles stephensi and Anopheles culicifacies (Diptera: Culicidae) in Gurgaon, India. Southeast Asian J Trop Med Public Health. 26: 172– 176. [PubMed] [Google Scholar]
- 27. Vatandoost H, Shahi M, Hanafi-Bojd AA, Abai MR, Oshaghi MA, Rafii F. ( 2007) Ecology of Anopheles dthali Patton in Bandar Abbas County, Hormozgan Province, Southern Iran. Iran J Arthropod-Borne Dis. 1: 21– 27. [Google Scholar]
- 28. Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaaghoobi F, Baghaii M, Hanafi-Bojd AA, Zamani G, Townson H. ( 2006) Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, southern Iran, 2002. Acta Trop. 97: 196– 203. [DOI] [PubMed] [Google Scholar]
- 29. Viswanathan DK, Rao TR, Rao TS. ( 1945) The behaviour of Anopheles fluviatilis, the behaviour of gravid females. J Malar Inst India. 6: 243– 245. [PubMed] [Google Scholar]
- 30. WHO ( 2013) Malaria. Avaiable at: www.who.int/mediacentre/factsheets/fs094/en/.
- 31. WHO ( 2003) Malaria Entomology and Vector Control. WHO/CDS/CPE/SMT/2002.18 Rev.1.Part I. Geneva. [Google Scholar]
- 32. Zahar AR. ( 1990) Vector bionomics in the epidemiology and control of malaria The WHO African Region and the Southern WHO Eastern Mediterranean Region VBC/90.2, MAL/90.2. Geneva. [Google Scholar]
- 33. Zahar AR. ( 1974) Review of the ecology of malaria vectors in the WHO Eastern Mediterranean Region. Bull World Health Organ. 50: 427– 440. [PMC free article] [PubMed] [Google Scholar]
- 34. Zaim M, Subbarao SK, Manouchehri AV, Cochrane AH. ( 1993) Role of Anopheles culicifacies s.l. and An. pulcherrimus in malaria transmission in Ghassreghand (Baluchistan), Iran. J Am Mosq Control Assoc. 9( 1): 23– 26. [PubMed] [Google Scholar]


