Abstract
Background:
Mosquitoes are considered as the vectors of dirofilariasis and some vector borne disease in Iran. The objective of this study was to determine the susceptibility level of the vectors to various insecticides recommended by WHO for any control measures in an endemic area in northwestern Iran.
Methods:
Mosquito larval and adult collections were carried out using different methods provided by WHO including dipping and hand catch techniques. The susceptibility level was assessed to DDT 4%, malathion 5%, propoxur 0.1%, deltamethrin 0.05% and lambda-cyhalothrin 0.05%.
Results:
Totally, 749 adults and 5060 larvae of Culicidae mosquitoes were collected comprising seven species of adult and larvae, including: Anopheles claviger, An. maculipennis, An. sacharovi, Culex hortensis, Cx. pipiens, Cx. theileri and Culiseta longiaerolata. Frequency of larvae and adults of An. maculipennis was very low, so susceptibility tests on this species did not performed. Results showed that Cx. theileri, Cs. longiaerolata and Cx. pipiens were resistant to DDT 4%, lambda-cyhalothrin 0.05%, and propoxur 0.1% whereas found tolerant to deltamethrin 0.05% and malathion 5%. The LT50 and LT90 values for five insecticides were calculated.
Conclusion:
We suggest the same study in different parts of the world to obtain the data due to bionomic and susceptibility status of dirofilariasis vectors. This information will help the health authorities for monitoring and evaluation of control measures.
Keywords: Culicidae, Vector, Dirofilaria immitis, Susceptibility test, Iran
Introduction
Mosquitoes (Diptera: Culicidae) are the most important groups of arthropods in medical and veterinary. They act as vectors of several diseases such as malaria, yellow fever, dengue, filariasis, setariasis and encephalitis, causing serious health problems to humans (Service 2003, Almeida et al. 2008).
The Culicidae mosquitoes are vector of dogs heartworm parasites, Dirofilaria immitis, Di. repens, Wuchereria bancrofti, some arboviral diseases such as Japanese Encephalitis, Rift valley fever, Western equine encephalitis and Eastern equine encephalitis, Tahyna, Sagiyama, Trivitatus, Lymphocytic Choriomanangitis, West Nile virus, St Louis encephalitis, California encephalitis (Muller 2002, Mullen and Durden 2009).
Dirofilariasis is a metazoonotic disease that is transmitted by certain species of mosquitoes. Dirofilaria immitis could be found in carnivores especially dogs and reported from various countries including Turkey (Atas et al. 1997), Japan (Hatsushika et al. 1997), Brazil (Reifur et al. 2004), Canada (Slocombe and Villeneure 1993) and other countries (Chen and Liang 1993, Rosa et al. 2002).
Dirofilaria immitis is commonly causes canine heartworm disease in many countries (Genchi et al. 2005). Human dirofilariasis has been shown as pulmonary or subcutaneous infection, but in Korea one case of hepatic dirofilariasis has been reported by Di. immitis agent (Choi et al. 2002). Dirofilariasis produced by Di. immitis parasites have been reported from all tropical, subtropical and moderate areas and the highest prevalence is in North America, South America, Australia, Italy and Japan (Venco et al. 2004). Dirofilariasis is a worldwide distributed disease, but the most endemic areas are those with moderate, tropical and subtropical climates, where mosquito populations are high and stable. Other regions with cold climates or with hot summers and with rivers, lakes and widely irrigated lands are also suitable for the development of the disease. Endemic occurrence of Di. immitis has been reported in the USA, Canada, South America, Africa, Australia, Asia and Europe (Yildirim et al. 2006).
Dirofilaria immitis was first reported from a dog in Iran in 1969 (Sadighian 1969). Then infection to heartworm was reported from different areas of Iran including Ardebil (Bokai et al. 1998), Shiraz (Jafari et al. 1996), Tehran (Meshgi and Eslami 2001), Tabriz (Meshgi et al. 2002) Tonekabon (Ranjbar-Bahadori et al. 2005), and Mashhad (Razmi 1999). Iran reported one of the disease foci in the world (WHO/FAO/OIE 1984). Zoonotic dirofilariasis has been reported in 11 provinces of Iran (Azari-Hamidian et al. 2007). By now, Di. immitis has been isolated from peripheral blood in a cat in Tabriz (Ashrafi et al. 2001). Azari-hamidian et al. (2007) reported Di. immitis in dogs, jackals, foxes, wolves and cats, and Di. repens in dogs and jackals in different areas of the country. The natural infection has been reported about 26.7% on stray dogs in Tonekabon (former Shahsavar), Mazandaran Province (Sadighian 1969). In addition, contamination of 0.95 and 36.8 % has been reported in the East and West Azerbaijan, Ardebil and Tehran, Fars and Khuzestan, Mazandaran, Golestan and East Azerbaijan Provinces (Sadighian 1969, Abai 1990, Zarriffard 1993, Jafari et al. 1996, Jamali and Hashemzade Farhang 1996, Bokaie et al. 1998, Farahnak et al. 1998, Razm Arayi et al. 2000, Ariamanesh 2000, Mashgi and Eslami 2001, Sadjjadi et al. 2004, Fallah et al. 2005, Ranjbar-Bahadori and Eslami 2005). Infection of subcutaneous dirofilariasis has been reported in the Anzali Port and Lahijan, Karaj and Ahvaz County (Siavashi and Massoud 1995, Athari 2003, Maraghi et al. 2006, Radmanesh et al. 2006, (Khanmohammadi et al. 2011).
By now, human and animal dirofilariasis cases have been recorded in 11 of the 31 provinces of Iran. Azari–Hamidian et al. (2009) reported the fourteen human cases of infection, including eight subcutaneous and three ocular cases of Di. repens, a testicular hydrocele case of Di. immitis and two pulmonary cases probably attributable to Di. immitis.
Two species of Dirofilaria include Di. immitis (canine heartworm) and Di. repens have been reported in Iran. Microfilarial worms of Di. immitis as 258±7μm, and for infected larvae ranged 0.75–1.3mm (Anderson 2000). The length of adult calculated as 250–300×1 mm (Muller 2002). Female mosquitoes serve as an intermediate host by sucking blood from dog which circulating Di. immitis microfilaria (Montoya et al. 1998). The diagnosis of canine heartworm infection is based upon the detection of Di. immitis circulating in blood or upon the detection of serum antibodies by serologic methods (Peribanez et al. 2001). Wolbachia (Rickettsiales: Rickettsiaceae) found in filarial nematodes such as Dirofilaria spp., have an important role in the filarial infection pathogenesis (Bazzocchi et al. 2000). The immune response of dog against Wolbachiain, considered as a diagnostic method for canine heartworm diseases (Tiawsirisup et al. 2010).
Some of mosquito species are able to transmit infected of third-stage larvae of Dirofilaria parasite (Aranda et al. 1998). Many of Di. immitis vectors reported in the world (Grieve et al. 1983). The different species of the genera of Aedes, Ochlerotatus, Psorophora, Anopheles, and Culex found susceptible to Di. immitis in USA (Mullen et al. 2009). Anopheles maculipennis and Cx. theileri reported naturally infected with infective larvae of Di. immitis and Setaria labiatopapillosa in Iran (Azari-Hamidian et al. 2009).
In Iran, the insecticides residual spraying against malaria vectors was carried out during 1950–1968. Following the resistance of the vectors to DDT (1957), dieldrin (1960) and malathion (1976), carbamate and pyrethroid insecticides were used (Zaim 1987, Moosa-Kazemi et al. 2007). Some enzymes such as monooxygenases, esterases and glutathione S-transferases have been considered as a reason for resistance to Permethrin insecticides. The efficacy of these insecticides will be increased by using some synergists like piperonyl butoxide (PBO) that inhibits monooxygenases ortribufos (DEF) that inhibits esterases (Enayati et al. 2003).
Prior of this study, there are no reports on the susceptibility of mosquitoes to insecticides in Ahar County, East Azarbaijan Province. The aim of this study was to determine the susceptibility level of dirofialriasis vectors to insecticides in the area.
Materials and Methods
Study area
East Azarbaijan Province is located in the northwest of the country, bordering Armenia and the Republic of Azarbaijan. The investigation was carried out during the summer of 2011 in the rural district of Ahar County (38°45′N, 47°11′E). The population of Ahar County has been reported 147.781 and 34.067 families. Agriculture, horticulture, livestock and making hand crafts making carpet and needlework are introduced as the main jobs. Cold and moderate weather provides the suitable condition for agricultural and husbandry activities. Three fixed Afil, (38°37′N, 47°31′E), Noghdose (38°39′N, 47°36′E), Bohol (38°36′N, 47°35′E) and six variable villages selected randomly (Fig. 1).
Fig. 1.
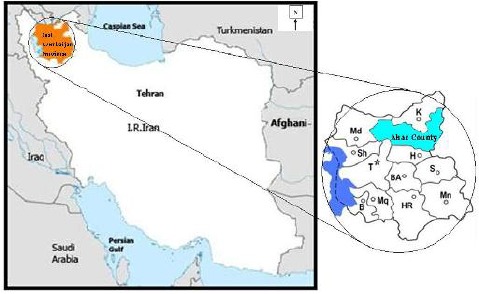
Map of the study area, East Azerbaijan Province, northwestern Iran
Mosquito collection
Hand collection:
In each fixed and variable places, mosquitoes were collected from indoor places by the using suction tubes. Adult mosquitoes were collected from the villages, 05.00 to 08.00 AM by using suction tube. Then the adult mosquitoes released in the cage and covered with wet towel and mosquitoes feed by sucrose 5 % solution (Silver 2008).
Larval collection:
In each fixed station the mosquitoes were collected from June to August 2011. The related data such as water temperature, larval type, larval number and the date of sampling were recorded. The larvae were collected from the villages, 08.00 to 11.00 AM by using standard dipper (350 ml) and eye dropper. The larvae were transferred into a closed container, sent to the laboratory and placed within a few cups into cages to obtain F1 generation. The samples were reared (Silver 2008).
Adult susceptibility test
The mosquitoes were transferred into the exposure tubes at different logarithmic exposure time, and mortality was then calculated after a 24 h recovery period (25 °C, 75%RH). The mortalities between 5.0 to 20.0 % were corrected by Abbott’s correction formula. All the tests were accepted when the mortality was less than 5% and ignored when the mortality was more than 20% in the control group (Abbott 1925, Silver 2008, WHO 1998). The susceptibility level of the species was considered in three classes as susceptible, tolerant and resistant based on WHO criteria (WHO 2002). The mortality more than 98 % was considered as susceptible, less than 80 % noted as resistant and between 97 to 98 % defined as tolerant.
Insecticides impregnated papers
Impregnated papers, DDT 4 %, malathion 5 %, deltamethrin 0.05 %, propoxur 0.1 % and lamda-cyhalothrin 0.05 % were supplied by WHO.
Statistical analysis
The LT50 was considered as lethal time causing 50 % mortality and its 95 % confidence interval and LT90 mentioned as lethal time causing 90 % mortality and its 9 5% confidence interval. The LT50 and LT90 values of Cx. theileri, Cx. pipiens and Cs. longiaerolata and probit regression line parameters were calculated to Finney’s test (Finney 1971). Plotting the regression line was calculated through the χ2 test using Microsoft Excel ver. 2007.
Identification of mosquito using morphological characteristics
The samples were mounted and identified by systematic keys (Shahgudian 1960, Zaim and Cranston 1986, Azari-Hamidian and Harbach 2009).
Results
Totally, 749 adults and 5060 larvae of Culicidae mosquitoes were collected including seven species, An. maculipennis sl, An. sacharovi, An. claviger, Cx. pipiens, Cx. hortensis, Cx. theileri and Cs. longiaerolata. Culex theileri was found to be dominant species as 42.8 %, 33.6 % allocated in adult and larval stages, respectively. Cx. pipiens was followed by 29.9 %, 25.3 % in adult and larvae respectively. An. maculipennis sl was found 6.9 % in larvae collection. An. claviger was collected as low density (4.7%), An. sacharovi, Cx. hortensis and Cs. longiaerolata were also collected in larval habitats. The maximum and minimum temperature in larval habitats was 29 °C and 22 °C, respectively.
An. maculipennis sl was collected very low, so susceptibility tests on this vector did not perform. The LT50 and LT90values for five insecticides are shown in Table 1, Figs. 2–6. The mortality rates of field collected Cs. longiareolata, Cx. theileri and Cx. pipiens to DDT 4 % at diagnostic exposure time were 18 %, 15 %, 23 %, respectively.
Table 1.
Probit regression line parameters of insecticides against mosquitoes in Ahar County, East Azarbaijan Province, 2011
| Insecticides | species | *A | **B±SE | ***LT50 95%C.I (Minute) | ****LT90 95%C.I (Minute) | X2 (df) | Y= A+BX | P-value |
|---|---|---|---|---|---|---|---|---|
| DDT 4% | Cs. longiaerolata | −7.6975 | 1.9745±0.381 | 131.94 | 588.13 | 4.119(2) | Y= −7.6975+1.9745 X | <0.05 |
| Cx. theileri | −6.7965 | 1.6702±0.363 | 195.48 | 1144.01 | 2.624(2) | Y= −6.7965+1.6702 X | <0.05 | |
| Cx. pipiens | −6.6372 | 1.6985±0.305 | 134.75 | 765.75 | 1.778(2) | Y= −6.6372+1.6985X | <0.05 | |
| Deltamethrin 0.05% | Cs. longiaerolata | −4.2737 | 1.7124±0.148 | 5.21 | 29.24 | 9.996(2) | Y = −4.2737+1.7124 X | <0.05 |
| Cx. theileri | −4.691 | 1.6581±0.141 | 10.179 | 60.350 | 7.516(2) | Y = −4.6191+1.6581 X | <0.05 | |
| Cx. pipiens | −4.9528 | 1.7710±0.152 | 10.432 | 55.211 | 5.921(2) | Y = −4.9528+1.7710 X | <0.05 | |
| Lambda-cyhalothrin 0.05% | Cs. longiaerolata | −3.4762 | 1.1496±0.114 | 17.60 | 229.26 | 3.329(2) | Y= −3.4762+1.1496 X | <0.05 |
| Cx. theileri | −3.7667 | 1.1630±0.123 | 28.87 | 365.2 | 2.621(2) | Y= −3.7667+1.1630 X | <0.05 | |
| Cx. pipiens | −3.7260 | 1.1772±0.121 | 24.37 | 298.77 | 4.048(2) | Y= −3.7260+1.1772 X | <0.05 | |
| Malathion 5% | Cs. longiaerolata | −4.4964 | 1.8031±0.157 | 5.19 | 26.69 | 9.939(2) | Y= −4.4964+1.8031 X | <0.05 |
| Cx. theileri | −4.5115 | 1.6842±0.142 | 7.95 | 45.87 | 4.676(2) | Y= −4.5115+1.6842 X | <0.05 | |
| Cx. pipiens | −4.6712 | 1.7413±0.148 | 8.025 | 43.70 | 5.028(2) | Y= −4.6712+1.7413 X | <0.05 | |
| Propoxur 0.1% | Cs. longiaerolata | −3.7575 | 1.1588±0.123 | 29.12 | 371.76 | 8.809(2) | Y= −3.7575+1.1588 X | <0.05 |
| Cx. theileri | −4.1764 | 1.2443±0.137 | 37.86 | 405.64 | 9.727(2) | Y= −4.1764+1.2443 X | <0.05 | |
| Cx. pipiens | −4.2249 | 1.2666±0.137 | 36.105 | 371.07 | 5.825(2) | Y= −4.2249+1.2666 X | <0.05 | |
A= intercept.
B±SE= slope and its standard error.
LT50, 95 % CI= lethal time causing 50 % mortality and its 95 % confidence interval.
LT90, 95 % CI= lethal time causing 90 % mortality and its 95 % confidence interval.
Fig. 2.
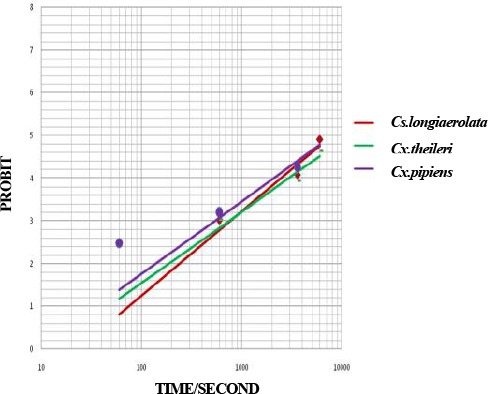
Probit regression line of Culicinae mosquitoes to DDT 4 % in Ahar County, East Azarbaijan Province, 2011
Fig. 6.
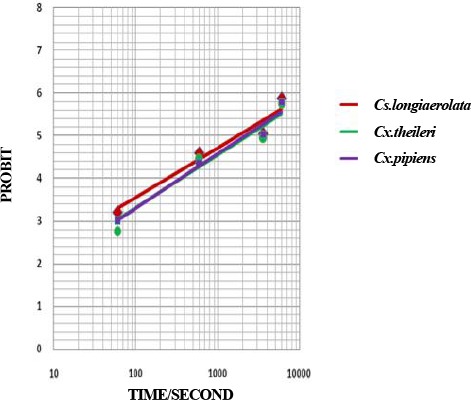
Probit regression line of Culicinae mosquitoes to Propoxur 0.1 % in Ahar County, East Azarbaijan Province, 2011
Fig. 3.
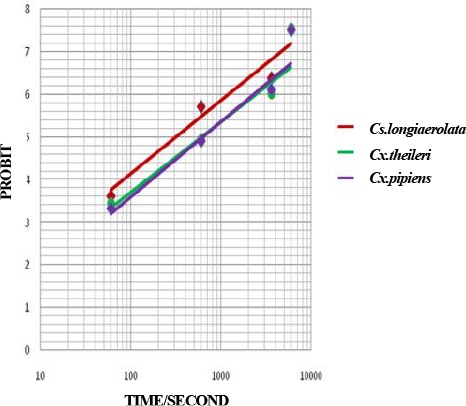
Probit regression line of Culicinae mosquitoes to Deltamethrin 0.05 % in Ahar County, East Azarbaijan Province, 2011
Fig. 4.
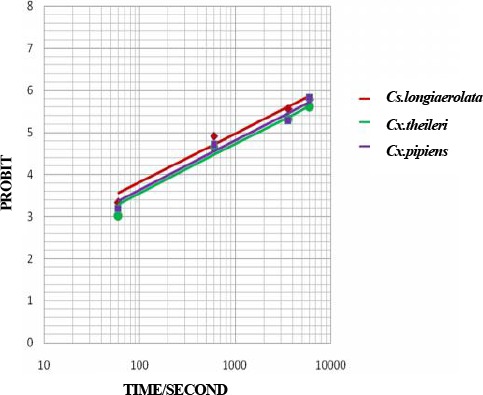
Probit regression line of Culicinae mosquitoes to Lambda-cyhalothrin 0.05 % in Ahar County, East Azarbaijan Province, 2011
Fig. 5.
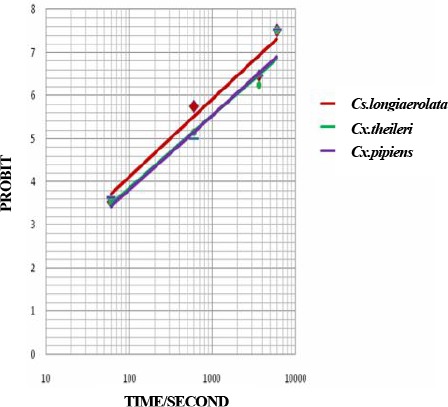
Probit regression line of Culicinae mosquitoes to Malathion 5 % in Ahar County, East Azarbaijan Province, 2011
Considering the criteria of resistance of the species described by WHO (Table 2), it should be mentioned that the species of Cx. pipiens, Cx. theileri and Cs. longiaerolata were resistant to DDT 4 %, Lambda-cyhalothrin 0.05 %, and propoxur 0.1 %. These mosquitoes were found tolerant to deltamethrin 0.05 % and malathion 5 %. Our results based on probit regression line showed that Cs. longiaerolata was more susceptible than Cx. theileri and Cx. pipiens when exposed to deltamethrin 0.05 % and malathion 5 %. In addition, Cs. longiaerolata was found more susceptible to Cx. pipiens and Cx. theileri after exposed to lambda-cyhalotherin 0.05 %, propoxur 0.1 % and DDT 4 %.
Table 2.
Susceptibility status of culicinae species to insecticides in Ahar County, East Azarbaijan Province, 2011
| Species | Insecticide | MR±EB* | Resistance status** | |
|---|---|---|---|---|
| Cs. longiaerolata | DDT 4 % | 18±4 | R | |
| Deltamethrine 0.05 % | 87±4 | T | ||
| Lambda-cyhalothrine 0.05 | 73±5 | R | ||
| Malathion 5 % | 90±3 | T | ||
| Propoxur 0.1 % | 55±5 | R | ||
| Cx. theileri | DDT 4 % | 15±4 | R | |
| Deltamethrine 0.05 % | 88±4 | T | ||
| Lambda-cyhalothrine 0.05 | 65±5 | R | ||
| Malathion 5 % | 93±3 | T | ||
| Propoxur 0.1 % | 50±6 | R | ||
| Cx. pipiens | DDT 4 % | 23±5 | R | |
| Deltamethrine 0.05 % | 91±3 | T | ||
| Lambda-cyhalothrine 0.05 | 63±5 | R | ||
| Malathion 5 % | 97.5±2 | T | ||
| Propoxur 0.1 % | 53±6 | R | ||
Mortality Rate±Error Bar
R: Resistance, T tolerance
Discussion
In this study, a total of 3 genera and 7 species of mosquito larvae and adults were collected in Ahar County. Culicidae species belonged to the genus of Anopheles, Culex and Culiseta.
The most predominant species was Cx. theileri as 42.8% of adult and 33.6% larvae collection. This species is reported as one of the most predominant species in Ardebil (Azari-Hamidian et al. 2009), Isfahan (Moosa-Kazemi et al. 2000) and Guilan Province (Lotfi 1970, Azari-Hamidian et al. 2000, Azari-Hamidian et al. 2001, Azari-Hamidian 2007). This species cited as predominant in Zanjan (Ghavami and Ladoni 2005), East Azarbaijan (Abai et al. 2007), and Southeastern Iran (Moosa-Kazemi et al. 2009). Simsek (2004) noted the dominant species in Ankara, Turkey (Simsek 2004). Zoophilic index on goat, dog, cattle, and human reported as 58.3%, 50%, 44.4% and 13.6%, respectively. Culex theileri found 1.49% of the total collection in Neka County (Nikookar et al. 2010). The seasonal activity of larvae started at the end of May reach to peak in the end of June in Isfahan Province. Seasonal activity of adults began in the end of May reach to peak in mid of July (Mousakazemi 1995). The maximum blood feeding activity reported as 22.30 PM–00.30 AM, 23% of feeding observed in the second third of the night while 19% found in the first third of the night (Moosa Kazemi e al. 2010).
For the first time Di. imimitis was isolated from Cx. theileri with 10% infection in Meshkin Shahr (Azari-Hamidian et al. 2009). Nazari and Janbakhsh (2000) noted the resistance of this species to DDT, tolerant to propoxur and dieldrin in southern Tehran.
In our study, LT50 value of Cx. theileri for DDT, deltamethrin, lambda-cyhalothrin, malathion and propoxur was calculated as 195.48, 10.179, 28.87, 7.95 and 37.86 minutes respectively. LT90 value found as 1144.01, 60.35, 365.2, 45.87 and 405.64 minutes for DDT, deltamethrin, lambda-cyhalothrin, malathion and propoxur respectively. In contrast, LT50 value of this species for propoxur and malathion reported as 31, and 22 minutes respectively (Nazari and Janbakhsh 2000). At present, seems to, this species was resistance to DDT, lambda-cyhalothrin and propoxur whereas tolerant to deltamethrin and malathion.
In our study, Cx. pipiens was dominant species comprised as 29.9% of adult and 25.3% of larvae. Minimum and maximum temperature in the larval habitat found as 22 °C, 29 °C respectively. The larvae collected in the larval habitat as a range of altitude 834–1382 meters. Based on morphological characteristic Cx. pipiens with Cx. vagans and Cx. torentium has mentioned in one group by Zaim and Cranston (1986). Lotfi (1970) reported the Cx. torentium from Fars Province based on the siphon index and size of siphonal setae (Lotfi 1970). Culex pipiens complex have a great abundance in north and north west of the country (Lotfi 1970, Azari-Hamidian et al. 2000, Azari-Hamidian et al. 2001) East Azarbaijan (Abai et al. 2007), Zanjan (Ghavami and Ladoni 2005). This species reported the dominant species in Isfahan Province. Larvae were collected from rice fields (Mousakazemi 1995). The larval activity in Isfahan began in late May reached peak in late June and then declined. The seasonal activity of adult started in the end of June and reached peak in mid-July and declined in end of August. The attractiveness of this species to light trap had been reported more than to human bait collection (Mousakazemi 1995).
Azari-Hamidian et al. (2001) reported the blood feeding of this species on cattle in north of Iran. In contrast Mousakazemi (1995) reported the tendency of blood feeding on human in central Iran (Mousakazemi 1995). It seems that the limitation of animal host in the urban area of Lenjan County, leads to attractive of this species to light trap. This species was reported one of the most predominant species in Mazandaran Province in North of Iran (Nikookar et al. 2010). The adaptation of Cx. pipiens to variety of breeding places with different degrees of pollution near the human residence reported by Ghavami and Ladoni (2005) and Dehghan et al. (2010).
Culex pipiens and Cx. quinquefaciatus larvae were detectable based on Siphon/Saddle index and seta 1 on abdominal segment III-IV (Harbach 1985, Azari-Hamidian and Harbach 2009, Dehghan et al. 2010). Seta 1 on abdominal segment III-IV in Cx. pipiens is usually single while this character in Cx. quinquefaciatus is usually double. Culex pipiens form molestus can be separated from Cx. pipiens pipiens mainly by ecological and physiological characters. Culex pipiens form molestus this found in open spaces and the accumulated water. The adults are able to laying the first batch of eggs without a blood-sucking (Autogenes). They are mating in small spaces and they hibernate in the winter (Stenogamus). The blood feeding of Cx. pipiens form molestus reported from mammals more than other animals.
The main different characteristic between the adult of Cx. quinquefasciatus and Cx. pipiens pipiens was reported as the ratio of DV/D in male genitalia (Sundaramans 1949). The distance of tip of ventral arm to dorsal arm of phalosoma considered as DV and D mentioned the distance of tip of dorsal arms of phalosoma (Barr and Kabtman 1951, Mattingly et al. 1951). In addition some characters are important including: the number of setae on the side in the ninth tergit hairs on maxillary pulp of the adult male, and DV/D (Vinogradova 2000). The most reliable character for identifying larval stages of Cx. torentium and Cx. pipiens reported as siphon/saddle index and Seta 1 on abdominal segment III-IV. In Cx. torentium reported with four branches (Harbach 1985, Azari-Hamidian and Harbach 2009, Dehghan et al. 2010).
Culex pipiens includs pipiens and molestus forms. Culex pipiens pipiens is considered as unatogenous laying eggs on open breeding places, and pass the winter with diapose while Cx. pipiens form molestus is considered as autogenous, stenogamus, and pass the winter without diapose. Culex pipiens pipiens blood feeds on birds than human whereas Cx. pipiens form molestus feed on human. In addition blood feeding of Cx. pipiens form molestus is reported from birds, rodents and guinea pigs. Culex pipiens pipiens, Cx. quinquefasciatus and Cx. pipiens form molestus as the most common and most widespread in the world. Culex pipiens form molestus habitat found in temperate climates, whereas Cx. quinquefasciatus has been distributed in most parts of the world and considered as cosmotropical (Vinogradova 2000).
Gjullin and Peters (1952) reported the tolerant of Cx. pipiens complex to DDT and organophosphoros insecticidae. The first reports related to resistance of the Cx. quinquefasciatus to organophosphoros insecticidae was reported by Isaak (Isaak 1961). In addition, the increase of the resistance to organophosphoros insecticides reported by Toma et al. (2011). Many studies indicated the tolerance to organophosphoros from Tunisia (Ben Cheikh et al. 1998), Cuba (Bisset et al. 1991, Rodriguez et al. 1993), Burkina Faso (Chandre et al. 1998), Saudi Arabia (Amin and Hemingway 1998), China (Jinfu 1999) and North America (McAbee et al. 2003).
In Iran, indoor residual spraying was carried out during 1950–1968 against malaria vector. Following the resistance of An. stephensi, the main malaria vector to DDT, other insecticides such as dieldrin, malathion, propoxur, lambda-cyhalothrin and recently deltamethrin used. The resistance of Cx. pipiens complex to DDT reported in southern Tehran. LT50 for propoxur and malathion calculated as 51, and 31 minutes respectively. The species was quit susceptible to propoxur and malathion (Nazari and Janbakhsh 2000). Khyami-Horani et al. (1995) reported two standard strains, Bacillus thuringiensis, and Ba. sphaericus had killing effects as 0.0006 to 0.006 ppm and 0.003 to 0.06 ppm respectively against Cx. pipiens molestus larvae.
In our study, LT50 value of Cx. pipiens for DDT, deltamethrin, lambda-cyhalothrin, malathion and propoxur calculated as 134.75, 10.43, 24.37, 8.025 and 36.105 minutes respectively. LT90 value found as 765.75, 55.211, 298.77, 43.70 and 371.07 minutes for DDT, deltamethrin, lambda-cyhalothrin, malathion and propoxur respectively. LT50 value of this species in our study was lower than the previous study cited by Nazari and Janbakhsh (2000).
In our study, Cs. longiaerolata observed resistance to DDT, propoxur, lambda-cyhalotrin and tolerant to malathion and deltamethrin. LT50 value found as 131.94, 5.21, 17.60, 5.19 and 29.12 minutes for DDT, deltamethrin, lambda-cyhalothrin, malathion and propoxur respectively. LT90 value of this species for DDT, deltamethrin, lambda-cyhalothrin, malathion and propoxur calculated as 588.13, 29.24, 229.26, 26.69 and 371.76 minutes respectively.
Some reports emphasizing to susceptibility of Cs. longiareolata larvae to Ba. sphaericus and Ba. thuringiensis (Katbeh Bader et al. 1999). The range of mortality found as a 0.003–0.004 ppm. The mortality of larvae in temperature of 28±1 °C found more than 20±1 °C (Katbeh Bader et al. 1999). Bouaziz et al. (2011) reported the LC50 values of Novaluron (IGR) against Cs. longiareolata as 0.51 and 0.91 μg/l respectively. The LC90 values were reported as 2.32–4.30 μg/l (species was collected to 41.1 % in larval collection (Bouaziz et al. 2011).
Conclusion
Cross-resistance of Cs. longiareolata, Cx. pipiens and Cx. theileri found after exposed to DDT and lambda-cyhalothrin. Also, we recommend the same procedure in field trial in different parts of Iran such as West Azerbaijan, Ardebil, Tehran, Fars, Khuzestan, Mazandaran and Golestan Provinces to obtain the unique conclusion about criteria for susceptibility status. The results could be useful for controlling of vector-borne diseases and Dirofolaria immitis in the future.
Acknowledgements
The authors are grateful to M Mohebali and Eng Zare for cooperation during the study. We express our appreciation to people of the villages that helped us during the study. The authors declare that there is no conflict of interests.
References
- 1. Abai MR. ( 1990) Fauna and seasonal activity of the sand flies and their animal reservoirs of visceral leishmaniasis in Meshgin Shahr County. [MSPH dissertation], School of Public Health, Tehran University of Medical Sciences, Iran. [Google Scholar]
- 2. Abai MR, Azari-Hamidian S, Ladonni H, Hakimi M, Mashhadi-Esmail K, Sheikhzadeh K, Kousha A, Vatandoost H. ( 2007) Fauna and checklist of mosquitoes (Diptera: Culicidae) of East Azerbaijan, northwestern Iran. Iran J Artropod-Borne Dis. 1: 27– 33. [Google Scholar]
- 3. Abbott WS. ( 1925) A method for computing the effect of an insecticide. J Econ Entomol. 18: 265– 267. [Google Scholar]
- 4. Almeida APG, Galao RP, Sousa CA, Novo MT, Parreira R, Pinto J, Piedade J, Esteves A. ( 2008) Potential mosquito vectors of arboviruses in Portugal: species, distribution, abundance and West Nile infection. Trans R Soc Trop Med Hyg. 102: 823– 832. [DOI] [PubMed] [Google Scholar]
- 5. Amin AM, Hemingway J. ( 1998) Preliminary investigation of the mechanisms of DDT and pyrethroid resistance in Culex quinquefasciatus Say (Diptera: Culicidae) from Saudi Arabia. Bull Entomol Res. 79: 361– 366. [Google Scholar]
- 6. Anderson RC. ( 2000) Nematod Parasites of Vertebrates. Their Development and Transmission. Vol. 2 Wallingford, CABI Press, Oxon, UK. [Google Scholar]
- 7. Aranda C, Panyello O, Eritja R, Costella J. ( 1998) Canine filariasis importance and transmission in the BaixLlobregat area, Barcelona (Spain). Vet Parasitol. 77: 267– 275. [DOI] [PubMed] [Google Scholar]
- 8. Ariamanesh MR. ( 2000) Infection rate of Dirofilaria immitis on stray dogs in Urmia County. Proceedings of the Forth National Congress of Zoonotic diseases, Mashhad, pp. 189– 190. [Google Scholar]
- 9. Ashrafi J, Eslami A, Shirani D, Meshgi B, Mostofi S. ( 2001) A study on the prevalence, clinical findings and treatment of feline heartworm disease in Iran. University of Tehran, Iran. J Vet Fac Univ Tehran. 56( 4): 21– 23. [Google Scholar]
- 10. Atas AD, Ozcelik S, Saygi G. ( 1997) The occurrence of helminth species in stray dogs, their prevalence and significance to public health in Sivas. Acta Parasitol Turcica. 21( 3): 305– 309. [Google Scholar]
- 11. Athari A. ( 2003) Zoonotic subcutaneous dirofilariasis in Iran. Arch. Iran Med. 6: 63– 65. [Google Scholar]
- 12. Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E. ( 2000) The mosquito fauna (Diptera: Culicidae) in Rasht City. Modares J Med Sci. 3( 2): 65– 70 (in Persian with English abstract). [Google Scholar]
- 13. Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E. ( 2001) The distribiution and larval habitat characteristics of mosquitoes (Diptera: Culicidae) in Rasht City. Modares J Med Sci. 4( 2): 96– 87 (in Persian with English abstract). [Google Scholar]
- 14. Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Mobedi I, Abai MR. ( 2007) Review of dirofilariasis in Iran. J Med Fac Guilan Univ Med Sci. 15( 60): 102– 113 (In Persian). [Google Scholar]
- 15. Azari-Hamidian S. ( 2007) Checklist of Iranian mosquitoes (Diptera: Culicidae). J Vect Ecol. 32( 2): 235– 242. [DOI] [PubMed] [Google Scholar]
- 16. Azari-Hamidian S, Harbach RE. ( 2009) Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2078: 1– 33. [Google Scholar]
- 17. Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE. ( 2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 23( 2): 111– 121. [DOI] [PubMed] [Google Scholar]
- 18. Barr AR, Kabtman L. ( 1951) Biometrical notes on the hybridization of Culex pipiens L. and C. quinquejasciatus Say. J Parasit. 37: 419– 420. [PubMed] [Google Scholar]
- 19. Ben Cheikh H, Ben Ali-Haouas Z, Marquine Mand Pasteur N. ( 1998) Resistance to organophosphates and pyrethroid insecticides Culex pipiens (Diptera: Culicidae) from Tunisia. J Med Entomol. 35: 251– 260. [DOI] [PubMed] [Google Scholar]
- 20. Bisset JA, Rodriguez MM, Hemingway J, Diaz C, Small GJ, Ortiz E. ( 1991) Malathion and pyrethroid resistance in Culex quinquefasciatus from Cuba: efficacy of Pirimiphos-methyl in the presence of at least three resistance mechanisms. Med Vet Entomol. 5: 223– 228. [DOI] [PubMed] [Google Scholar]
- 21. Bokai S, Moobedi A, Mohebali M, Hoseini H, Nadim A. ( 1998) Study on prevalence of dirofilariosis in Meshkinshahr-Northwest of Iran. J Fac Vet Med Tehran Univ. 53( 1,2): 23 (in Persian with English abstract). [Google Scholar]
- 22. Bouaziz A, Boudjelida H, Soltani N. ( 2011) Toxicity and perturbation of the metabolite contents by a chitin synthesis inhibitor in the mosquito larvae of Culiseta longiareolata. Ann Biol Res. 2( 3): 134– 143. [Google Scholar]
- 23. Chandre F, Darriet F, Darder M, Cuany A, Doannio JMC, Pasteur N, Guillet P. ( 1998) Pyrethroid resistance in Culex quinquefasciatus from west Africa. Med Vet Entomol. 12: 359– 366. [DOI] [PubMed] [Google Scholar]
- 24. Chen KY, Liang S. ( 1993) Blood chemistry of dogs with heart Worm in the Taipei area. J Chinese Soc Vet Sci. 19( 2): 130– 136. [Google Scholar]
- 25. Bazzocchi C, Ceciliani F, Mc Call JW, Ricci I, Genchi C, Bandi C. ( 2000) Antigenic role of the endosymbionts of filarial nematodes: IgG response against the Wolbachia surface protein in cats infected with Dirofilaria immitis. Proc R Soc Lond B. 267: 2511– 2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dehghan H, Sadraei J, Moosa-Kazemi SH. ( 2010) The morphological variations of Culex pipiens larvae (Diptera: Culicidae) in Yazd Province, central Iran. Iran J Arthropod-Borne Dis. 4( 2): 42– 49. [PMC free article] [PubMed] [Google Scholar]
- 27. Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. ( 2003) Molecular evidence for a Kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 17: 138– 144. [DOI] [PubMed] [Google Scholar]
- 28. Farahnak A, Mobedi I, Mohamadi F. ( 1998) Study of zoonotic helminthes of carnivores in Khuzestan, Iran. Iran J Publ Health. 27( 3–4): 15– 20. [Google Scholar]
- 29. Fallah A, Onishi A, Mehdipour Zare N. ( 2005) the prevalence of Dirofilaria immitis in Meshgin Shahr County. Proceedings of the Fifth National Congress of parasitic disease in Iran, pp. 14– 15. [Google Scholar]
- 30. Finney DJ. ( 1971) Probit Analysis, 3rd Ed. Cambridge University Press, Cambridge, UK. [Google Scholar]
- 31. Genchi C, Rinaldi L, Cascone C, Mortarino M, Cringoli G. ( 2005) Is heartworm disease really spreading in Europe? Vet Parasitol. 133: 137– 148. [DOI] [PubMed] [Google Scholar]
- 32. Gjullin CM, Peters RF. ( 1952) Abstract of recent studies of mosquito resistance to insecticides in California. Calif. Mosquito Control Assoc. Ann Confr Proc and Papers -20: 44– 45. [Google Scholar]
- 33. Ghavami MB, Ladoni H. ( 2005) Fauna and density of (Diptera: Culicidae) in Zanjan. J Zanjan Univ Med Sci Health Serv. 13( 53): 46– 54. [Google Scholar]
- 34. Grieve RB, Lok JB, Glickman LT. ( 1983) Epidemiology of canine heartworm infection. Epidemiol Rev. 5: 220– 246. [DOI] [PubMed] [Google Scholar]
- 35. Harbach RE. ( 1985) Pictorial keys to the genera of mosquitoes, subgenera of Culex and the species of Culex ( Culex) occurring in southwestern. Asia and Egypt, with a note on the subgeneric placement of Culex deserticola (Diptera: Culicidae): Mosq Syst. 17( 2): 83– 107. [Google Scholar]
- 36. Hatsushika R, Okino T, Shimizu M, Ohyama F. ( 1997) The prevalence of dog heart worm ( Dirofilariaimmitis) infection in stray dogs in Okayama, Kawasaki. Japan Med J. 3( 4): 75– 83. [Google Scholar]
- 37. Isaak LW. ( 1961) Review of insecticide resistance in Kern Mosquito Abatement District. In: Proceedings and Papers of the Twenty-nine Annual Conference of the California Mosquito Control Association, Vol. 1 Visalia press, Cleveland, Ohio, USA, pp. 105– 106. [Google Scholar]
- 38. Jafari S, Gaur NS, Khaksar Z. ( 1996) Prevalence of Dirofilari aimmitis on dog of Fars province of Iran. J Appl Anim Res. 9( 1): 27– 31. [Google Scholar]
- 39. Jamali R, HashemzadeFarhang H. ( 1996) Infection study to Dirofilaria immitis on stray dogs in the city of Tabriz. Proceedings of the Third National Congress of zoonotic diseases, Mashhad, p. 179. [Google Scholar]
- 40. Jinfu W. ( 1999) Resistance to deltamethrin in Culex pipiens pallens (Diptera: Culicidae) from Zhejiang, China. J Med Entomol. 36: 389– 393. [DOI] [PubMed] [Google Scholar]
- 41. Choi JS, Choi SY, Kim MK, Kim CH, Yeom BW, Park SH. ( 2002) The first human case of hepatic dirofilariasis. J Korean Med Sci. 17: 686– 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katbeh Bader A, Khyami Horani H, Mohsen ZH. ( 1999) Effect of temperature on the susceptibility of Culiseta longiareolata (Macquart) (Diptera: Culicidae) to two standard strains of biocontrol bacteria. J Appl Entomol. 123: 629– 631 [Google Scholar]
- 43. Khanmohammadi M, Fallah E, Reyhani rad S. ( 2011) Epidemiological studies on fauna and prevalence of parasite helminthes on Red Fox ( Vulpes vulpes) in Sarab district, East Azerbaijan Province, Iran. Ann Biol Res. 2( 5): 246– 251. [Google Scholar]
- 44. Lotfi MD. ( 1970) Iranian species of genus Culex (Culicinae: Diptera). Bull Sot. Pathol Exotique. 63: 400– 403. [PubMed] [Google Scholar]
- 45. Maraghi S, Rahdar M, Akbari H, Radmanesh M, Saberi AA. ( 2006) Human dirofilariasis due to Dirofilaria repens in Ahvaz-Iran: a report of three cases. Pak J Med Sci. 22: 211– 213. [Google Scholar]
- 46. Mattingly PF, Rozeboom LE, Knight KL, Laven H, Drummond FH, Christophers R, Shute PG. ( 1951) The Culex pipiens complex, Trans. Roy Ent Sot Lond. 102: 331– 382. [Google Scholar]
- 47. McAbee RD, Kang KD, Stanich MA, Christiansen JA, Wheelock CE, Inman AD, Hammock BD, Cornel AJ. ( 2003) Pyrethroid tolerance in Culex pipiens pipiens var molestus from Marin County, California. Pest Manag Sci. 60: 359– 368. [DOI] [PubMed] [Google Scholar]
- 48. Meshgi B, Eslami A. ( 2001) Study on filariosis of sheepdogs around of Tehran. J Fac Vet Med Tehran Univ. 55( 4): 53– 57 (In Persian). [Google Scholar]
- 49. Meshgi B, Eslami A, Ashrafi Helan J. ( 2002) Epidemiological survey of blood filariae in rural and urban dogs of Tabriz. J Fac Vet Med Tehran Univ. 57( 4): 59– 63 (In Persian). [Google Scholar]
- 50. Montoya JA, Morales M, Ferrer O, Molina JM, Corbera JA. ( 1998) the prevalence of Dirofilaria immitisin Gran Canaria, Canary Islands, Spain (1994–1996). Vet Parasitol. 75: 221– 226. [DOI] [PubMed] [Google Scholar]
- 51. Moosa Kazemi SH, Karimiana F, Davari B. ( 2010) Culicinae mosquitoes in Sanandaj County, Kurdistan Province, western Iran. J Vector Borne Dis, 47: 103– 107. [PubMed] [Google Scholar]
- 52. Moosa-Kazemi SH, Vatandoost H, Nikookar H, Fathian M. ( 2009) Culicinae (Diptera: Culicidae) mosquitoes in Chabahar County, Sistan and Baluchistan Province, southeastern Iran. Iran J Arthropod-Borne Dis. 3( 1): 29– 35. [PMC free article] [PubMed] [Google Scholar]
- 53. Moosa-Kazemi SH, Vatandoost H, Raeisi A, Akbarzadeh K. ( 2007) Deltamethrin impregnated bed nets in a malaria control program in Chabahar, south-east Baluchistan, Iran. Iran J Arthropod-Borne Dis. 1( 1): 43– 51. [Google Scholar]
- 54. Moosa-Kazemi SH, Zaim M, Zahra'i Ramezani A. ( 2000) Funa and ecological study of culicidae mosquito in Lenjan area, Isfahan Province. J Yasuj Uni Med Sci. 5( 17–18): 46– 53. [Google Scholar]
- 55. Mousakazemi SH. ( 1995) Fauna and ecology of culicidae mosquitoes (Diptera) in the region of Mobarake and ZarinShahr, Isfahan Province. [MSPH dissertation]. School of Public Health. Tehran University of Medical Sciences, Iran. [Google Scholar]
- 56. Muller R. ( 2002) Worms and Human disease. 2nd Ed. CABI publication, Wallingford; Oxon, UK. [Google Scholar]
- 57. Mullen GR, Durden L. ( 2009) Medical and Veterinary Entomology, Mosquitoes (Culicidae) Woodbridge A. Foster and Edward D Walter. Vol. 2 Elsevier, Burlington, pp. 207– 260. [Google Scholar]
- 58. Nazari M, Janbakhsh B. ( 2000) a survey of the susceptibility level of Culex theileri and Cx. pipiens to DDT, Dieldrin, Propoxur and Malathion in the southern area of Tehran. J Uromia Univ Med Sci. 11( 1): 13– 19. [Google Scholar]
- 59. Nikookar SH, Moosa-Kazemi SH, Oshaghi MA, Yaghoobi-Ershadi MR, Vatandoost H, Kianinasab A. ( 2010) Species composition and diversity of mosquitoes in Neka County, Mazandaran Province, northern Iran. Iran J Arthropod-Borne Dis. 4( 2): 26– 34. [PMC free article] [PubMed] [Google Scholar]
- 60. Peribanez MA, Lucientes J, Arce S, Morales M, Castillo JA, Gracia MJ. ( 2001) Histochemical differentiation of Dirofilaria immitis, Dirofilaria repens and Acanthocheilonema dracunculoides microfilariae by staining with a commercial kit, Leucognost-SP. Vet Parasitol. 102: 173– 175. [DOI] [PubMed] [Google Scholar]
- 61. Radmanesh M, Saberi A, Maraghi S, Emad-Mostowfi N. ( 2006) Dirofilaria repens: a dog parasite presenting as a paranasal subcutaneous nodule. Int J Dermatol. 45: 477– 478. [DOI] [PubMed] [Google Scholar]
- 62. Ranjbar-Bahadori S, Eslami A. ( 2005) Camparison of modified Knott and ELISA methods for diagnosis of dirofilariosis in dogs in Golestan Province and determining of its periodicity. The 20th International Conference of the World Association for the advancement of Veterinary Parasitology, Christchurch, New Zealand, p. 249. [Google Scholar]
- 63. Ranjbar-Bahadori Sh, Eslami A, Meshgi B, Mohammad Mohtasham R. ( 2005) Study on blood filaria of dogs in Tonekabon. J Fac Vet Med Tehran Univ. 60( 4): 353– 356 (In Persian). [Google Scholar]
- 64. Razm Arayi N, Ebrahimi M, Ameghi A. ( 2000) Report of Dirofilaria immitis on wild carnivores in Northern of East Azerbaijan Province. Proceedings of the Fourth National Congress of Zoonotic diseases, Mashhad, pp. 220– 221. [Google Scholar]
- 65. Razmi Gh. ( 1999) Study on situation of infection to dogs of Mashhad to types of filaria. J Fac Vet Med Tehran Univ. 54( 1): 5– 7 (In Persian). [Google Scholar]
- 66. Rosa A, Ribicich M, Betti A, Kistermann N, Cardillo N, Basso N, Hallu R. ( 2002) Prevalence of canine dirofilariosis in the city of Buenos Aires and its outskirts (Argentina). Vet Parasitol. 109: 261– 264. [DOI] [PubMed] [Google Scholar]
- 67. Reifur L, Thomaz-Soccol V, Montiani-Ferreira F. ( 2004) Epidemiological aspects of filariosis in dogs on the coast of Parana state, Brazil: with emphasis on Dirofilaria immitis. Vet Parasitol. 122: 273– 86. [DOI] [PubMed] [Google Scholar]
- 68. Rodriguez M, Ortiz E, Bisset JA, Hemingway J, Saledo E. ( 1993) Changes in malathion and pyrethroid resistance after cypermethrin selection of Culex quinquefasciatus field populations of Cuba. Med Vet Entomol. 7: 117– 121. [DOI] [PubMed] [Google Scholar]
- 69. Siavashi MR, Masoud J. ( 1995) Human cutaneous dirofilariasis in Iran: a report of two cases. Iran J Med Sci. 20: 85– 86. [Google Scholar]
- 70. Sadjjadi S, Mehrabani D, Oryan A. ( 2004) Dirofilariosis of stray dogs in Shiraz Iran. J Vet Parasitol. 18( 2): 181– 182. [DOI] [PubMed] [Google Scholar]
- 71. Sadighian A. ( 1969) Helminth parasites of stray dogs and jackals in Shahsavar area, Caspian region, Iran. J Helminth. 2: 372– 374. [PubMed] [Google Scholar]
- 72. Shahgudian ER. ( 1960) A key to the anophelines of Iran. Acta Med Iran. 3: 38– 48. [PubMed] [Google Scholar]
- 73. Service MW. ( 2003) Medical Entomology for Students. Vol. 3 United Kingdom: Cambridge: University Press, Cambridge. [Google Scholar]
- 74. Silver BJ. ( 2008) Mosquito Ecology: Field Sampling Methods. Vol. 3 Springer-Verlag Press, New York. [Google Scholar]
- 75. Simsek FM. ( 2004) Seasonal larval and adult population dynamics and breeding habitat diversity of Culex theileri Theobald, 1903 (Diptera: Culicidae) in the Golbasi District, Ankara, Turkey. Turk J Zool. 28: 337– 44. [Google Scholar]
- 76. Slocombe JOD, Villeneure A. ( 1993) Heart worm in dogs in Canada in 1991. Canadian Vet J. 4( 10): 630– 633. [PMC free article] [PubMed] [Google Scholar]
- 77. Sundaramans Y. ( 1949) Biometrical studies on intergradations in the genitalia of certain populations of Culex pipiens and Culex quinquefasciatus in the United States, Amer. J Hyg. 60: 307– 314. [DOI] [PubMed] [Google Scholar]
- 78. Tiawsirisup S, Thanapaisarnkit T, Varatorn E, Apichonpongsa T, Bumpen KN, Rattanapuchpong S, Chungpiwat S, Sanprasert V, Nuchprayoon S. ( 2010) Canine Heartworm ( Dirofilaria immitis (Leidy)) Infection and Immunoglobulin G Antibodies against Wolbachia (Rickettsiales: Rickettsiaceae) in Stray Dogsin Bangkok, Thailand. Thai J Vet Med. 40( 2): 165– 170. [Google Scholar]
- 79. Toma L, Menegon M, Romi R, De Matthaeis E, Montanari M, Severini C. ( 2011) Status of insecticide resistance in Culex pipiens field populations from northeastern areas of Italy before the withdrawal of OP compounds. Pest Manag Sci. 67( 1): 6– 100. [DOI] [PubMed] [Google Scholar]
- 80. Venco L, McCall JW, Guerrero J, Genchi C. ( 2004) Efficacy of long term monthly administration of Ivermectin on the progress of naturally acquired heartworm infections in dogs. Vet Parasitol. 124: 259– 268. [DOI] [PubMed] [Google Scholar]
- 81. Vinogradova EB. ( 2000) Mosquitoes Culex pipiens pipiens: taxonomy, distribution, ecology, physiology, genetics and control. PenSoft, Sofia. [Google Scholar]
- 82. WHO/FAO/OIE ( 1984) Animal health Yearbook 1983. Animal health Service, Animal production and Health Division, World Health Organization, Food and Agriculture Organization, International des Epizooties Rome. [Google Scholar]
- 83. WHO ( 2002) Entomology and vector control. Training module on malaria control. Guide for participants. World Health Organization, Global Malaria Programme WHO Press, World Health Organization, Avenue Appia, Geneva Switzerland. [Google Scholar]
- 84. WHO ( 1998) Test procedures for insecticide resistance monitoring in malaria Vectors, bio-efficacy and persistence of insecticides on treated surfaces. Report of WHO Informal Consultation, WHO/CDS/CPC/MAL/98.12. Geneva, Switzerland. [Google Scholar]
- 85. Yildirim A, Ica A, Atalay O, Duzlu O, Inci A. ( 2006) Prevalence and epidemiological aspects of Dirofilariaimmitis (Leidy) (Leidy) in dogs from Kayseri Province, Turkey. Res Vet Sci. 82: 358– 363. [DOI] [PubMed] [Google Scholar]
- 86. Zaim M. ( 1987) Malaria Control in Iran. J Am Mosq Cont Assoc. 3: 392– 396. [PubMed] [Google Scholar]
- 87. Zaim M, Cranston PS. ( 1986) Checklist and keys to the Culicinae of Iran (Diptera: Culicidae). Mosq Syst. 18: 233– 45. [Google Scholar]
- 88. Zarriffard MR. ( 1993) A study on helminthic parasites of wild carnivorous of east Azerbaijan with emphasis on Echinococcus multilocularis. [PhD dissertation]. School of Public Health, Tehran University of Medical Sciences, Iran. [Google Scholar]


