Abstract
Background:
Anopheles stephensi is a sub-tropical species and has been considered as one of the most important vector of human malaria throughout the Middle East and South Asian region including the malarious areas of southern Iran. Current reports confirmed An. stephensi resistance to temephos in Oman and India. However, there is no comprehensive research on mechanisms of temephos resistance in An. stephensi in the literature. This study was designed in order to clarify the enzymatic and molecular mechanisms of temephos resistance in this species.
Methods:
Profile activities of α- and ß-esterases, mixed function oxidase (MFO), glutathione-S-transferase (GST), insensitive acetylcholinesterase, and para-nitrophenyl acetate (PNPA)-esterase enzymes were tested for An. stephensi strain with resistance ratio of 15.82 to temephos in comparison with susceptible strain.
Results:
Results showed that the mean activity of α-EST, GST and AChE enzymes were classified as altered indicating metabolic mechanisms have considerable role in resistance of An. stephensi to temephos. Molecular study using PCR-RFLP method to trace the G119S mutation in ACE-1 gene showed lack of the mutation responsible for organophosphate insecticide resistance in the temephos-selected strain of An. stephensi.
Conclusion:
This study showed that the altered enzymes but not targets site insensitivity of ACE-1 are responsible for temephos resistance in An. stephensi in south of Iran.
Keywords: Anopheles stephensi, temephos, mechanisms of resistance, Acetylcholinesterase gene, malaria
Introduction
Malaria still remains as a public health problem in the world. Southern parts of Iran are involved with this problem (Vatandoost et al. 2010).
Anopheles stephensi is a sub-tropical species and also an important vector of human malaria throughout the Middle East and South Asian region, including the Indo-Pakistan subcontinent, with a westward extension through Iran and Iraq into the Middle East and Arabian Peninsula. This species is considered to be the main malaria vector in the Persian Gulf area (Oshaghi et al. 2006a and 2006b). Previous studies have shown An. stephensi to be the most prevalent anopheline species in the malarious areas of southern Iran (Vatandoost et al. 2004, Hanafi-Bojd et al. 2012).
Temephos, a most widely used organophosphorus insecticide, has been included in the list of World Health Organization (WHO) as a suitable and safe mosquito larvicide that can be used even in drinking water for controlling of the most mosquito vectors. The toxicity of this insecticide is low and unlikely to present acute hazard for human (WHO 2006).
Temephos (EC 50%) has been used for some years for larval control program of malaria in Southern Iran (Vatandoost et al. 2006). Many studies on the susceptibility level of An. stephensi to various pesticides have been done in Iran and other countries. Resistance of An. stephensi to different insecticide was reported from around the world (Vatandoost et al. 1996). Different levels of resistance to larvicides were reported in anopheline malaria vectors worldwide. Anopheles stephensi has an extensive resistance comparing to other species and is resistant or tolerant to fenitrothion, temephos and fenthion in India, fenitrothion and pirimiphos-methyl in Iraq, fenitrothion, pirimiphos-methyl, chlorfoxim and foxim in Iran and fenitrothion in Pakistan (Vatandoost and Hanafi-Bojd 2005a). Resistance of other anopheline mosquito such as An. dthali to temephos also was reported (Hanafi-Bojd et al. 2006).
In 2006 for the first time in the Middle East, resistance to temephos was confirmed in An. stephensi breeding in water storage tanks in the Al-Dhahira region of Oman (Anderasen 2006). The level of resistance was 2.5 times higher than that of the WHO diagnostic dose (0. 25 mg/l). However, there was no confirmed report of resistance of An. stephensi to temephos in Iran. Previous studies in Iran showed that this species was completely susceptible to temephos at the WHO diagnostic dose (Vatandoost et al. 2004, Vatandoost and Hanafi-Bojd 2005a, Vatandoost et al. 2005b, Vatandoost et al. 2006).
One of the most important molecular mechanisms of resistance to organophosphate insecticide in mosquitoes is structural mutations that occur in acetylcholinesterase gene. In mosquitoes two cholinesterase genes are existed (ACE-2 and ACE-1). ACE genes have been cloned from the mosquitoes Aedes aegypti and An. stephensi, both of these genes are also sex linked (Hemingway and Ranson 2000). The existence of both ACE genes in An. stephensi is approved by other researchers (Malcolm and Hall 1990, Weill et al. 2002,). But as yet, there is no recorded ACE-based resistance mechanism in An. stephensi (Hemingway and Ranson 2000). It is known that insensitive acetylcholinesterase (AChE) due to a G119S mutation is associated with tolerance to carbamate and organophosphate insecticides in Anopheles gambiae and the mutation can be detected using a PCR-RFLP assay (Weill et al. 2004a).
As yet there is no comprehensive research about mechanisms of temephos resistance in An. stephensi in the literature. The current study was designed in order to clarify the enzymatic and molecular mechanisms of temephos resistance in this species.
Materials and Methods
Study area
Eight different areas in two most important malarious provinces of Iran were considered to collect live wild specimens of An. stephensi including: Bandar Abbas Port, Minab County and Hormoodar Village in Hormozgan Province, and Chabahar Port, villages of Bampoor and Abtar from Iranshahr County, villages of Angoori and Machkor from Sarbaz County in Sistan and Baluchistan Province (Fig. 1).
Fig. 1.
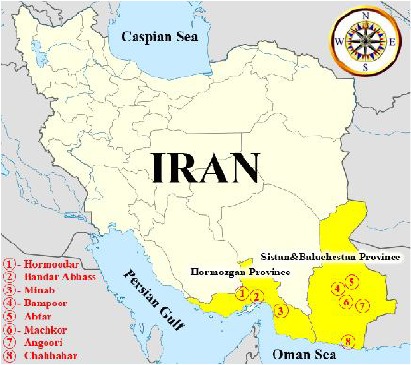
Location of Anopheles stephensi collection sites from malarious areas of Iran, 2011
Mosquito strains
The field collected strains of An. stephensi were reared in the insectarum for further tests.
A susceptible laboratory strain of An. stephensi (Beech-Lab from insectarium of department of Medical Entomology and Vector Control group, School of Public Health, Tehran University of Medical Sciences) was used to compare the susceptibility status of the field strains. This strain has been maintained in the laboratory without exposure to insecticides for 28 years.
Insecticide
Technical grade insecticide used in the present study was Temephos 90 % (Batch No: TEM/136-229) which was obtained from Levant Overseas Development Ltd., Argenteuil, France.
Based on pre-tests, five concentrations of the larvicide (0.25, 0.0625, 0.0156, 0.0039 and 0.00195 mg/l) were considered for susceptibility assays. Bioassay consisted of five concentrations resulting 10–90% mortality. Butanone 2% in absolute ethanol was used as a control.
Larval bioassays
Susceptibility assays was carried out according to the method described by World Health Organization (WHO 2012). The toxicity of temephos to An. stephensi, from field-collected population was determined and compared with laboratory reared susceptible Beech-Lab strain.
Abbott’s formula was used to correct the observed mortality of larvae. All the data were corrected if the control mortality is between 5 and 20 % (Abbott 1965). Data were analyzed using probit analysis to determine the 50% lethal concentration values (LC50) and 90% lethal concentration values (LC90) of the field and Beech-Lab strains (Finney 1971).
Selection process
The strain which showed the highest resistance ratio (RR) to temephos was preceded for selection pressure. This strain was selected for 5 generations by exposing late third or early fourth instars to the concentrations which produced 50–70 % mortality (Paeporn et al. 2004). Selection was continued as long as a homogenous resistant population with resistance ratio more than 10-fold was achieved.
Biochemical assays
Thirty mosquito larvae from each susceptible and resistant strain were assayed for α-and ß-esterases, mixed function oxidase (MFO) and glutathione-S-transferase (GST), insensitive acetylcholinesterase and PNPA-esterase enzymes. Each larva was homogenized in 100 μL of potassium phosphate (KPO4) buffer (6.6 g dibasic potassium phosphate/1.7g mono basic potassium phosphate/1000mL distilled water (dH2O), pH 7.2) and then diluted to 2 mL with the same buffer. Each mosquito was analyzed in duplicate with 100 μL of mosquito homogenate transferred to two wells on a 96 well flatbottomed microtitration plate. Absorbance levels were measured spectrophotometrically with a microplate reader (ELX808 Ultra Microplate Reader BIO-TEK ®), at wave lengths indicated for each enzyme, and the mean absorbance calculated based on data for the two replicate wells per mosquito.
Procedures were followed based on slight modifications of a protocol from the Centers of Disease Control (Polson et al. 2011). The activities of all enzymes were evaluated according to this protocol. The details of procedures were described completely in this research (Polson et al. 2011). Reagents and substrates for biochemical assays were provided by Sigma.
Data analyses of Biochemical assays
Absorbance values which were obtained for mosquito replicates were corrected in relation to the volume of mosquito homogenates, the enzyme activity unit and the total protein content of each mosquito (Polson et al. 2011). The means of enzyme activities for each An. stephensi larval strain were compared with the susceptible (Beech-Lab strain) by Unpaired t-test, Mann-whitney test (P<0.05).
The Beech-Lab 99th percentile was calculated for each enzyme and the percentage of specimens with enzymatic activity above that of the Beech-Lab 99th percentile was calculated. Enzyme activities were then classified as “altered”, “incipiently altered” or “unaltered” if the rate was >50 %, between 15 % and 50 % and <15 %, respectively (Montella et al. 2007).
Molecular study of resistance
Mosquito genomic DNA was extracted from triplex homogenate of mosquitoes by QIAamp DNA Mini Kit. DNA was then PCR amplified with the degenerated primers Moustdir1 5′CCGGGNGCSACYATGTGGAA3′ and Moustrev1 5′ACGATMACGTTCTCYTCCGA3′ according to the conditions and thermal cycles already introduced by Weill et al. (2004). The PCR products were digested with AluI restriction enzyme according to the manufacturer’s instructions and fractionated on a 2% agarose ethidium bromide gel (Weill et al. 2004a).
The primers created a 194 bp amplicon in both temephos resistance and susceptible strains, however after restriction enzyme digestion, homozygous resistant individuals cut to 120 bp and 74 bp fragments if the G119S mutation was existed.
Representative PCR products of both temephos resistance and susceptible strains of An. stephensi were sent for sequencing in order to confirm the PCR-RFLP assays as well as to find other possible mutation on ACE-1 gene except for the G119S mutation.
Bioinformatic softwares such as Clustal W2, Blast, and Mega 5 were used for sequence alignment, homology, and phylogenetic analysis. We also used TranSeq software for translation nucleic acids to amino acids.
Results
Larval bioassays
Considerable variation in temephos resistance ratio of filed strains in comparison with susceptible strain was noticed from all the locations studied. A low level of resistance ratio was observed in the populations of An. stephensi except in Chabahar strain. (RR= 4.27 folds) compared to Beech-Lab strain (P< 0.05) (Fig. 2). According to our findings, Chabahar strain of An. stephensi with resistance ratio more than 4-folds was chosen for selection process as the most tolerant strain.
Fig. 2.
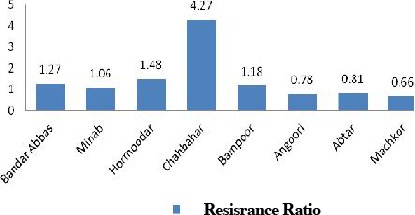
Temephos resistance ratio pattern in Anopheles stephensi field strains from malarious area of southern Iran
Selection process
After larval bioassays, Chabahar strain was established into the insectary for selection process. Selection process was continued for 15 months. After 5th selection a resistant population of An. stephensi was achieved with 15.82 and 35.34-folds resistance ratio at LC50 and LC90 level, respectively.
Biochemical assays
Analyses were conducted through comparing the median value for Beech-Lab strain (S) with those of the temephos selected strain (R), for each enzyme. By Unpaired t-test and Mann-Whitney test, the median activity for all enzymes differed significantly (P< 0.05). According to the classification scheme detailed in method, for each respective enzyme, activities were classified as “unaltered”, “incipiently altered” or “altered” if the values were <15%, between15 and 50% and >50%, respectively. Tables 1 and 2 show the number of mosquitoes assessed in each assay, along with the median values and percentage of strains with enzymatic activities in relation to Beech-Lab strain (S).
Table 1.
Quantification of enzymatic activity of esterases in two strains (resistant and susceptible) of Anopheles stephensi
| Strains | α-EST (nmol/mg ptn/min) | ß-EST (nmol/mg ptn/min) | PNPA-EST(Δabs/mg ptn/min) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N a | Median b | p99 c | N | Median | p99 | N | Median | p99 | |
| Beech-Lab | 30 | 0.00006881 | 0.00010411 | 30 | 0.00014011 | 0.00020971 | 30 | 0.04560881 | 0.07969278 |
| N | Median | %>p99d | N | Median | %>p99 | N | Median | %>p99 | |
| Chabahar (Selected with Temephos) | 30 | 0.00013654 | 95 | 30 | 0.00017725 | 8.33 | 30 | 0.05132526 | 6.67 |
Number of mosquitoes tested.
Median value for each enzymatic activity.
99th percentile for Beech-Lab reference strain.
Percentage of mosquito specimen with activity above 99th percentile for Beech-Lab reference strain.
Table 2.
Quantification of enzymatic activity of MFO, GST and iAChE in two strains (resistant and susceptible) of Anopheles stephensi
| Strains | MFO (nmol cyt/mg ptn) | GST (nmol/mg ptn/min) | AChE (% activity) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N a | Median b | p99 c | N | Median | p99 | N | Median | p99 | |
| Beech-Lab | 30 | 0.00003264 | 0.00008108 | 30 | −.00085944 | 0.00028138 | 30 | 1.40618583 | 5.36517282 |
| N | Median | %>p99 d | N | Median | %>p99 | N | Median | %>p99 | |
| Chabahar (Selected with Temephos) | 30 | 0.00002279 | 1.67 | 30 | 0.00033844 | 86.67 | 30 | 14.16817118 | 90 |
Number of mosquitoes tested.
Median value for each enzymatic activity.
99th percentile for Beech-Lab reference strain.
Percentage of mosquito specimen with activity above 99th percentile for Beech-Lab reference strain.
α-Esterase
In relation to Beech-Lab strain (S), temephos resistant strain (R) was significantly different in α-EST activity levels (P<0.0001). R strain showed altered activity with >50 % (95%) of individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain (Table 1).
ß-Esterase
The median activity levels of ß-EST seen in the resistant strain were significantly different from the Beech-Lab strain (P<0.0001). Based on the classification of activity profiles, R strain showed unaltered activity with <15 % (8.33%) of individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain (Table 1).
PNPA-Esterase
There were significant differences observed between the median PNPA-EST activities of the Beech-Lab strain and temephos resistant strain (P= 0.0096). An unaltered profile of PNPA-EST (6.67%) was found in R strain of An. stephensi (Table 1).
Mixed function oxidase (MFO)
In relation to Beech-Lab strain (S), temephos resistant strain (R) was significantly different in MFO activity levels (P= 0.0002). R strain showed unaltered activity with <15 % (1.67%) of individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain (Table 2).
Glutathione-S-transferase (GST)
The GST activity in the Beech-Lab strain was significantly different from that of the temephos resistant strain (P< 0.0001). Based on the classification of activity profiles, R strain showed altered activity with >50 % (86.67%) of individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain (Table 2).
Insensitive acetylcholinestersase (iAChE)
The rate of activity of AChE in the presence of propoxur for Beech-Lab strain was significantly different from that observed in the temephos resistant strain (P< 0.0001). An altered profile of AChE (90%) was found in the R strain of An. stephensi (Table 2).
The activity levels of the enzymes in both strains (R and S) are graphically displayed in scatter plots (Figs. 3–8).
Fig. 3.
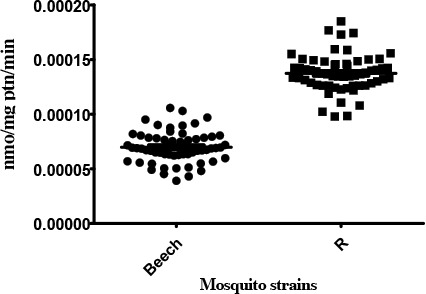
Activity profile of α-esterase enzymes
Fig. 8.
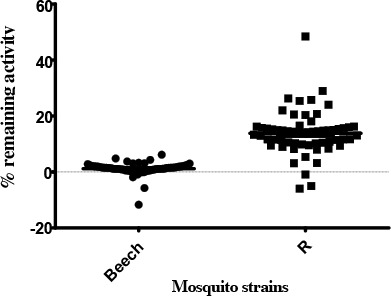
Percent remaining activity acetylcholinesterase (AChE)
Fig. 4.
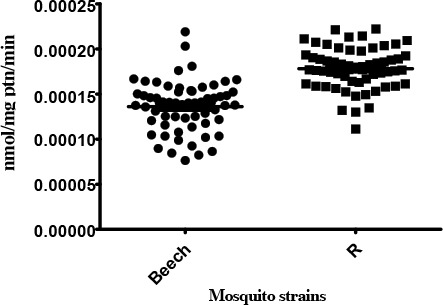
Activity profile of ß-esterase enzymes
Fig. 5.
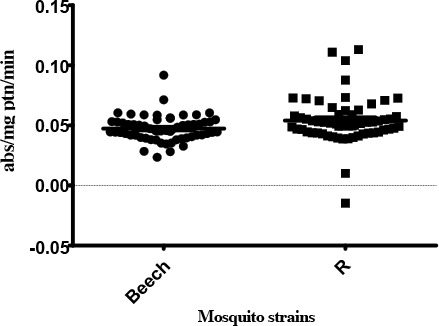
Activity profile of PNPA-esterase enzymes
Fig. 6.
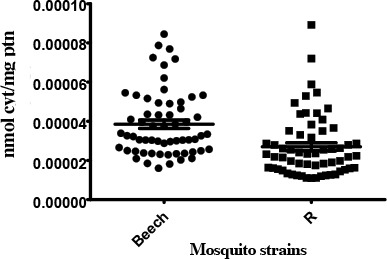
Activity profile of MFO enzymes
Fig. 7.
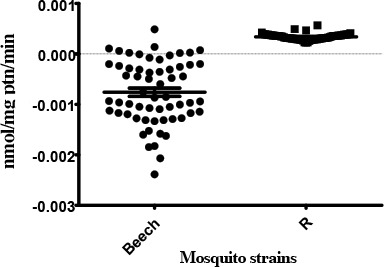
Activity profile of GST enzymes
Molecular study on ACE-1 Resistance in Anopheles stephensi
With Moustdir1 and Moustrev1 primers, a 194 bp amplicon was amplified by PCR. The result of PCR-RFLP with AluI showed that PCR products of both temephos resistance and susceptible strains of An. stephensi were remain intact (Fig. 9) indicating lack of the G119S mutation in ACE-1 of resistance strain.
Fig. 9.
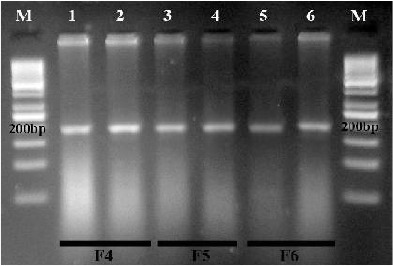
Diagnostic PCR-RFLP to identify G119S mutation in amplified region of ACE-1 (194 bp) in individuals of Anopheles stephensi. M: 50 bp ladder (Fermentas), Lane 1–6: temephos-resistant strain (Lane 1–2: 4th generation of selected strain with temephos, Lane 3–4: 5th generation of selected strain with temephos, Lane 5–6: 6th generation of selected strain with temephos) CLUSTAL 2.1 multiple sequence alignment
Three specimens from each resistant and susceptible strain of An. stephensi to temephos, were sent for sequencing. The sequences were deposited in the European Nucleotide Archive (ENA) with accession numbers (HG380320-24).
The results of 5 sequencing that were trustable, analyzed with Blast, and Clustal W2 softwares. The Blast analysis revealed that there was no counterpart sequence data of the ACE-1 gene of An. stephensi in the genbank database. The most similar sequence data available in genbank database were Anopheles albimanus S (Accession number: AJ566402), An. albimanus R (AN: AJ566403) and Anopheles funestus R (AN: DQ534435) (Fig. 10).
Fig. 10.
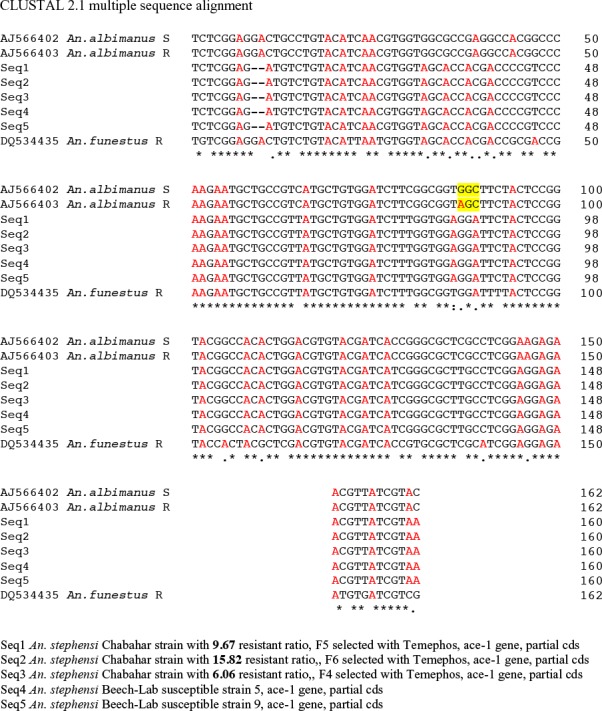
Comparison of sequencing results of this study (Seq1, Seq2, Seq3, Seq4, Seq5*) with other three registered genes in gene bank
The results showed sequences of ACE-1 for both resistant and susceptible strains were identical and no G119S mutation was observed in resistance strainthat equenced. The Blast analysis of this region of ACE-1 for An. stephensi, showed sequence of ACE-1 was more similar to An. albimanus than An. funestus (Table 3). In comparison with available data in genbank, 2 indels, and 34 substitutions were observed (Fig. 10). Construction of Phylogram was done using Mega 5 for ACE-1 sequences of this study (An. stephensi R/S) and other available data in genbank (An. albimanus S, An. albimanus R and An. funestus R) (Fig. 11).
Table 3.
Blast analyze of ACE-1 region sequence for temephos-resistant Anopheles stephensi R/S (this study) and other species of mosquitoes (Anopheles albimanus S, Anopheles albimanus R and Anopheles funestus R)
| SeqA | Name | Length | SeqB | Name | Length | Score |
|---|---|---|---|---|---|---|
| 1 | An. stephensi R/S | 160 | 2 | AJ566402 An.albimanus S | 162 | 88.75 |
| 1 | An. stephensi R/S | 160 | 3 | AJ566403 An.albimanus R | 162 | 88.12 |
| 1 | An. stephensi R/S | 160 | 4 | DQ534435 An.funestus R | 162 | 83.12 |
| 2 | AJ566402 An.albimanus S | 162 | 3 | AJ566403 An.albimanus R | 162 | 99.38 |
| 2 | AJ566402 An.albimanus S | 162 | 4 | DQ534435 An.funestus R | 162 | 82.72 |
| 3 | AJ566403 An.albimanus R | 162 | 4 | DQ534435 An.funestus R | 162 | 82.1 |
Fig. 11.
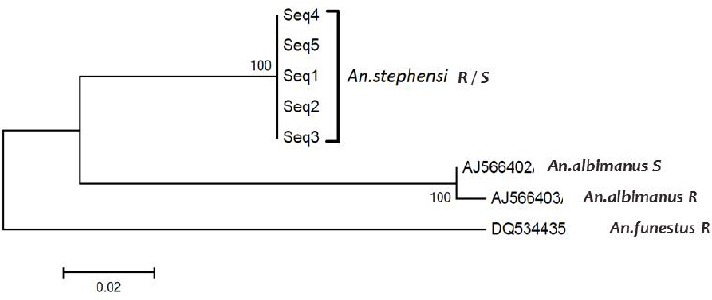
Phylogram a part of ACE-1 for sequences of this study (Anopheles stephensi R/S) and other similar registered genes in gene bank (Anopheles albimanus S, Anopheles albimanus R and Anopheles funestus R)
Amino acids sequences of An. stephensi ACE-1 gene were compared with other similar amino acid sequences of mosquitoes were available in the genbank (Fig. 12). The results showed lack of Glycine to Serine substitution at position 119 in the ACE-1 gene that confers high levels of resistance to organ-ophosphate in the resistant (R) strain of An. stephensi of this study. This substitution only observed in R strain of An. albimanus. Two species specific amino acid sequences in ACE-1 gene of An. stephensi were observed in analogy with other sequences. In this species two Arginine have been substituted with Glutamic acid and Aspartic acid. These differences seem to be structural and not related to insecticide resistance property of this species (Fig. 12).
Fig. 12.
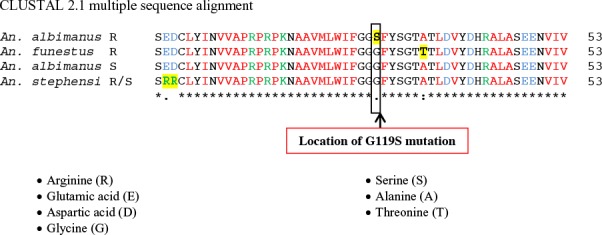
Comparison of translated ACE-1 region amino acids sequence of Anopheles stephensi R/S (this study) with other similar registered genes in gene bank (Anopheles albimanus S, Anopheles albimanus R and Anopheles funestus R)
Discussion
In this study, it was found that An. stephensi in southern part of Iran would normally be susceptible to insecticides but become resistant to temephos under insecticide pressure in laboratory condition. On the other hand, in field condition, temephos resistance of this species has been reported from otherneighboring malarious countries such as India and Oman (Vatandoost and Hanafi-Bojd 2005a, Anderasen 2006). This warrants precautions and insecticide vector management (IVM) before the resistance becomes widespread in Iran and neighboring countries. Biochemical assays were carried out by several researchers in order to characterizing the mechanisms of temephos resistance in different vector species. Some of them are mentioned in Table 4.
Table 4.
Summary of some biochemical studies were done in order to characterizing the mechanisms of temephos resistance in different vector species
| Species | Year | Country | insecticide | Main mechanism of resistance | reference |
|---|---|---|---|---|---|
| Culex quinquefasciatus | 1990 | Srilanka | Temephos | General esterase (α and ß) | Peiris and Hemingway 1990 |
| Anopheles albimanus | 1998 | Mexico | Organophosphate | PNPA-estrase and MFO | Penilla et al. 1998 |
| Aedes aegypti | 2003 | Thailand | Temephos | General esterase (α and ß | Paeporn et al. 2003 |
| Aedes aegypti | 2005 | Thailand | Temephos | General esterase (α and ß) | Saelim et al. 2005 |
| Aedes aegypti | 2007 | Brazil | Temephos | General esterase (α and ß), PNPA estrase and GST (only in north-east strain of Brazil) | Montella et al. 2007 |
| Aedes aegypti | 2010 | Brazil | Temephos | General esterase (α and ß) and GST | Melo-Santosa et al. 2010 |
| Aedes aegypti | 2011 | Trinidad | Temephos | General esterase (α and ß), GST, MFO and AChE | Polson 2011 |
| Anopheles stephensi | 2013 | Iran | Temephos | α-EST, GST and AChE | This study |
Biochemical assays were done in temephos selected strain of An. stephensi for the first time in the literature. Profile of enzyme activity in temephos-resistant An. stephensi showed that the mean enzymatic activity of α-EST, GST and AChE were classified as altered. These results clarified that metabolic mechanisms have considerable role in resistance of An. stephensi to temephos. And the most important mechanisms of resistance are α-EST, GST and AChE. It seems ß-EST, MFO and PNPA-esterase are not important in resistance of An. stephensi to this insecticide.
Vector control programs have, for a long time, utilized bioassays to monitor insecticide resistance in field mosquito populations. These assays only inform the susceptibility level of a certain population to a specific insecticide (Polson et al. 2011). Biochemical assays also should be included in routine activities of surveillance programs in order to finding incipiently altered enzyme activity of field populations. These are more informative than the bioassays in that they provide some information on the resistance mechanisms involved. With this information we can prevent the development of insecticide resistance in whole population by proper and timely interventions.
Biochemical assays should be simultaneously carried out with routine bioassays in order to improve the surveillance of resistance and monitoring of the efficacy of insecticides in malarious area.
Considering that the mechanisms and molecular basis of resistance are very diverse, these mechanisms (metabolic and molecular) should be identified as well for each insecticide which will be used. In this case, the efficient monitoring strategies shall be applicable and finally management of insecticide resistance in vectors can be obtained.
High insecticide resistance resulting from insensitive acetylcholinesterase has emerged in mosquitoes. A single mutation (G119S of the ACE-1 gene) explains this high resistance in Culex pipiens and in An. gambiae (Weill et al. 2004 a,b). It has been recently shown that the high insensitivity of acetylcholinesterase displayed by Cx. pipiens and An. gambiae is due to the same glycine to serine substitution (G119S mutation), resulting from a single point mutation GGC to AGC in the gene ACE-1 (Weill et al. 2002).
The results of Weill et al. study (2004) showed that, the Gly 119 codon was found serine immutable in 31 vector species including An. stephensi.
Molecular and biochemical assays were carried out to identify ACE-1 mutation in An. gambiae and Culex quinquefasciatus. In this study less than 1 % of mosquitoes showed the presence of the ACE-1 mutation (Corbel et al. 2007).
Another study was surveyed acetyl cholinesterase sequencing in Ae. aegypti. In all individuals, a PCR product of 507 bp was amplified. Sequences were aligned and no mutations were observed within this region of ACE. Resistant and susceptible individuals presented the same nucleotide and amino acid sequence, with 100 % homology to the sequence (Melo-Santosa et al. 2010).
These studies clarified that the frequency of ACE-1 mutation into the field population of mosquitoes are very low.
The mechanisms of temephos resistance based on the existence of G119S mutation on ACE-1 gene for one of the most important malaria vector An. stephensi were studied. In this study PCR-RFLP showed no G119S mutation was existed in this part of gene of the An. stephensi strains. Resistant and susceptible individuals presented the same nucleotide and amino acid sequence, with 100 % homology to the sequence.
These results are completely similar to other researchers' results and approved the immutable characteristic of this region of ACE-1 gene in An. stephensi. Finally we can conclude based on molecular studies of temephos resistance, there is no mechanisms of temephos resistance in relation to studied region of Acetylcholinesterase 1 gene in An. stephensi. Probably mechanisms of temephos resistance in An. stephensi are more enzymatic or are belong to other parts of the mosquito genome that we didn't studied in this research.
Conclusion
The results of this study will provide information about mechanisms of temephos resistance in the main malaria vector in Iran. This finding is very crucial for management of malaria vector control.
Acknowledgements
This article is a part of the first author’s dissertation for fulfillment of a PhD degree in Medical Entomology and Vector Control from Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. The authors are very grateful to Mr M Yarian from Hormozgan University of Medical Sciences and Mr A Pakari and Mr Shahbakhsh technicians of the National Institute of Health Research, Bandar Abbas and Iranshahr Research Stations, for their kind collaboration during this study. This study was financially supported by the Deputy for Research, Tehran University of Medical Sciences. The authors declare that there is no conflict of interest.
References
- 1. Abbott WS. (1965) A method of comparing the effectiveness of an insecticide. J Econ Entomol. 18: 265– 267. [Google Scholar]
- 2. Anderasen MH. (2006) Emerging resistance to temephos in Anopheles stephensi in the Al-Dhahira region of oman. Mission report of World Health Organization, EM/MAL/328/E/R/6.06. [Google Scholar]
- 3. Corbel V, N'Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Akogbéto M, Hougard JM, Rowland M. (2007) Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 101: 207– 216. [DOI] [PubMed] [Google Scholar]
- 4. Finney DJ. (1971) Probit analysis, III ed. Cambridge University Press, Cambridge. [Google Scholar]
- 5. Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Sedaghat MM, Yeryan M, Pakari A. (2012) Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 121: 85– 92. [DOI] [PubMed] [Google Scholar]
- 6. Hanafi-Bojd AA, Vatandoost H, Jafari R. (2006) Susceptibility status of Anopheles dthali and Anopheles fluviatilis to commonly used larvicides in an endemic focus of malaria, southern Iran. J Vector Borne Dis. 43(1): 34– 38. [PubMed] [Google Scholar]
- 7. Hemingway J, Ranson H. (2000) Insecticide resistance in insect vectors of human disease. Ann Rev Entomol. 45: 371– 391. [DOI] [PubMed] [Google Scholar]
- 8. Malcolm CA, Hall LMC. (1990) Cloning and characterization of a mosquito acetylcholinesterase gene, In Molecular insect science, ed. by Hagedorn HH, Hildebrand JG, Kindwell MG, Lawet JH , New York: Plenum, pp. 57– 65. [Google Scholar]
- 9. Melo-Santosa MAV, Varjal-Meloa JJM, Arajoa AP, Gomesa TCS, Paivaa MHS, Regisa LN, Furtadoa AF, Magalhaesa T, Macorisd MLG, Andrighettid MTM, Ayresa CFJ. (2010) Resistance to the organophosphate temephos: Mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. ActaTrop. 113: 180– 189. [DOI] [PubMed] [Google Scholar]
- 10. Montella IR, Martins AJ, Viana-Medeiros PF, Lima JB, Braga IA, Valle D. (2007) Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. Am J Trop Med Hyg. 77: 467– 477. [PubMed] [Google Scholar]
- 11. Oshaghi MA, Yaghoobi F, Vatandoost H, Abai MR, Akbarzadeh K. (2006a) Anopheles stephensi biological forms, geographical distribution, and malaria transmission in malarious regions in Iran. Pak J Biol Sci. 9: 294– 298. [Google Scholar]
- 12. Oshaghi MA, Yaaghoobi F, Abaie MR. (2006b) Pattern of mitochondrial DNA variation between and within Anopheles stephensi (Diptera: Culicidae) biological forms suggests extensive gene flow. Acta Trop. 99: 226– 233. [DOI] [PubMed] [Google Scholar]
- 13. Paeporn P, Ya-umphan P, Supaphathom K, Savanpanyalert P, Wattanachai P, Patimaprakorn R. (2004) Insecticide susceptibility and selection for resistance in a population of Aedes aegypti from Ratchaburi Province, Thailand. Trop Biomed. 21(2): 1– 6. [PubMed] [Google Scholar]
- 14. Polson KA, Brogdon WG, Rawlins SC, Chadee DD. (2011) Characterization of insecticide resistance in Trinidadian strains of Aedes aegypti mosquitoes. Acta Trop. 117(1): 31– 38. [DOI] [PubMed] [Google Scholar]
- 15. Vatandoost H. (1996) The functional Basis of pyrethroids resistant in the malaria vector Anopheles stephensi [PhD dissertation]. University of Liverpool, UK. [Google Scholar]
- 16. Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. (2004) Larval habitats of main malaria vectors in Hormozgan Province and their susceptibility to different larvicides. Southeast Asian J Trop Med and Public Hlth. 35(2): 22– 25. [PubMed] [Google Scholar]
- 17. Vatandoost H, Hanafi-Bojd AA. (2005a) Current Resistant Status of Anopheles stephensi Liston to Different Larvicides in Hormozgan Province, Southeastern Iran, 2004. Pak J Biol Sci. 8: 1568– 1570. [Google Scholar]
- 18. Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I. (2005b) Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district, Kerman Province, southeastern Iran. J Vector Borne Dis. 42(3): 100– 108. [PubMed] [Google Scholar]
- 19. Vatandoost H, Oshaghi M, Abaie MR, Shahi M, Yaghoobi F, Baghai M, Hanafi-Bojd AA, Zamani G, Townson H. (2006) Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, southern Iran. ActaTrop. 97: 196– 205. [DOI] [PubMed] [Google Scholar]
- 20. Vatandoost H, Akbarzadeh K, Hanafi-Bojd AA, Mashayekhi M, Saffari M, Elfatih MM, Kenyi L, Abakar John B, Busaq A, Esmailpour M, Hassen A, Oshaghi MA. (2010) Malaria stratification in a malarious area, a field exercise. Asian Pac J Trop Med. 3: 807– 811. [Google Scholar]
- 21. Weill M, Fort P, Berthomieu A, Dubois MP, Pasteur N, Raymond M. (2002) A novel acetylcholinesterase gene in mosquitoes codes for the insecticide target and is non-homologous to the ace gene in Drosophila. Proc Biol Sci. 269 (1504): 2007– 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, Raymond M. (2004a) The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 13: 1– 7. [DOI] [PubMed] [Google Scholar]
- 23. Weill M, Berthomieu A, Berticat C, Lutfalla G, Negre V, Pasteur N, Philips A, Leonetti JP, Fort P, Raymond M. (2004b) Insecticide resistance: a silent base prediction. Curr Biol. 14: 552– 553. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization (2006) Pesticides and their application for the control of vectors and pests of public health importance ( Sixth edition). WHO, Geneva, Switzerland, WHO/CDS/NTD/WHOPES/GCDPP/2006.1. [Google Scholar]
- 25. World Health Organization (2012) Malaria Entomology and Vector Control (Learner's Guide). WHO, Geneva, Switzerland. [Google Scholar]


