Abstract
Background:
Zoonotic Cutaneous Leishmaniasis (ZCL) is endemic in many parts of Iran. Recently its incidence is considerable in different parts of Jahrom district, in Fars Province, southern Iran. The aims of our study were to investigate the prevalence of leishmania infection, and identify and characterize the Leishmania species present, among the rodents by molecular methods in a new endemic focus of ZCL, in an urban and rural area of the Jahrom district, Fars Province, southern Iran.
Methods:
From May to November 2010), 55 rodents in four regions of Jahrom focus were caught and checked for leishmania infection by the microscopical examination of liver, spleen, ears, and footpads’ smears.
Results:
Overall 18 Meriones persicus, 15 Tatera indica, 14 Mus musculus, and 8 Rattus rattus were caught. Totally, four (16.5%) and two (13.3%) of the Me. persicus and Ta. indica, but only one of Mu. musculus and Ra. rattus were found smear-positive for leishmania amastigotes, respectively. In the nested-PCR assay 8 (14.6%) smears were found positive for Leishmania major, none was found positive for any other Leishmania species. Sequencing based detection of Leishmania confirmed the microscopic and PCR findings. All positive specimens were shown 95–96% similarity with L. major Friedlin.
Conclusion:
Tatera indica and Me. persicus are incriminated as the main ‘reservoir’ hosts of L. major in the rural area of Jahrom, moreover, Mu. musculus and Ra. rattus have the minor but remarkable role in the maintenance of the disease in the urban regions of Jahrom focus.
Keywords: Rodent, Leishmania, PCR, Sequencing, Iran
Introduction
The leishmaniases are parasitic diseases with an extensive variety of clinical symptoms that have an effect on over 12 million people in 88 countries, mainly in the tropical and subtropical situated regions, as well as Iran (Javadian et al. 1976, Mohebali et al. 2004, Guedes et al. 2008). Zoonotic Cutaneous Leishmaniasis(ZCL) takes place in many rural endemic foci in different parts of Iran (Yaghoobi-Ershadi et al. 2005). Sandflies (Diptera: Phlebotominae) have been naturally found infected with Leishmania major and considered as the sole vectors of leishmaniasis. Moreover, gerbil rodents (Muridae: Gerbillinae) are the reservoir hosts of ZCL in Iran (Azizi et al. 2011 a). Rodents, as the ‘reservoir’ hosts, are the main bases in the epidemiology and control strategy of the ZCL (Pourmohammadi et al. 2008). Due to high similarity of different species of the parasite, various sources of Leishmania DNA such as kinetoplast DNA (kDNA), ssu rRNA, ITS1 and repetitive sequences have been used for molecular characterizations (Van Eys et al. 1992, Noyes et al. 1998, Parvizi and Ready 2008, Davami et al. 2011, Ghasemian et al. 2011).
Recently, PCR-based assays are characteristically used to detect Leishmania species in patients, vectors and reservoir hosts (Rodgers et al. 1990, Bulat et al. 1992, Parhizkari et al. 2011).
Materials and Methods
Study Area
Fars Province placed in southern Iran and covers an area of about 122,400 km2. Jahrom district is located in the south-east of province and situated at 30° 4′ 45″ North, 51° 43′ 29″ East, and 1050m above the sea level (Fars Budget and Planning Organization 2000). Due to a favorable ecological conditions for vectors and reservoirs of leishmaniasis, Jahrom has always been considered as one of the foci of cutaneous and visceral leishmaniasis in south of Iran (Fig. 1) (Davami et al. 2010, Davami et al. 2011).
Fig. 1.
Map of Iran, showing the locations of Fars Province and the city of Jahrom
Collection and Examination of Rodents
Between May to November 2010, rodents were caught alive in wire traps on agricultural plantations, surrounding and near the houses in four regions including Jahrom City and three villages in the area including Mousavieh, Ghotb-Abad and Fath-Abad. Each trap was set in the evening and checked in the next early morning. There were set at least 50 ‘trap-nights’ per months. Each rodent was identified to species (Eisenberg and Redford 1999), killed by over-anesthesia and dissected, so that four impressions smears (two of the liver, spleen, ear and footpad) could be prepared. Each smear was fixed in methanol, Giemsa-stained and checked for amastigotes under a light microscope (Mohebali et al. 2004).
DNA Extraction
All specimens were checked in a nested-PCR assay for Leishmania kDNA. Therefore, each dry smear was scraped from the slides and mixed with 200μl of lysis buffer [1 mM EDTA, 50 mM Tris-HCl (pH 7.6, 1% (v/v) Tween 20] containing 5μl of a proteinase K solution which already had 23mg/ml of enzyme. The specimen was incubated for 2 h at 56 ºC or 12h at 37 ºC before 75μl of a phenol: chloroform: isoamyl-alcohol solution (25:24: 1, by vol.) was added (Motazedian et al. 2002). After being shaken vigorously, specimen was centrifuged at 6000× g for 10min. DNA in the supernatant solution was precipitated with 300μl of cold ethanol, resuspended in 100μl of double distilled water, and stored at 4 ºC before using in the PCR- assay (Noyes et al. 1998).
Nested-PCR assay
The specific and sensitive nested-PCR was used to amplify the variable area of the minicircle kDNA of Leishmania spp. in the rodent liver, spleen, ear and footpad as previously described by Noyes et al. (1998) with slight modification (Moemenbellah-Fard et al. 2003, Davami et al. 2011, Ghasemian et al. 2011). The first-round (external) primers were CSB1XR (ATTTTTCGCGATTTTCGCAGA ACG) and CSB2XF (CGAGTAGCAGAAA CTCCCGTTCA). A reaction mixture containing of 1.5 mM of MgCl2, 2 mM of dntp, 2.5μL of 10 X PCR buffer (Boehringer Mannheim, Mannheim, Germany), 1unit of Taq DNA polymerase (Cinagene, Tehran), and 10pmol of each primer were used in a total reaction volume of 25μL including 5μL of DNA sample. The second-round (internal) primers were 13Z (ACTGGGGGTTGGTGTAAAA TAG) and LIR (TCGCAGAACGCCCCT). The reaction mixture was used in a total volume of 30μL including 2μL of DNA product of the first round. These mixtures were amplified in a programmable thermocycler (Eppendorf AG (Mastercycler gradient), Germany) for 5min at 94 ºC (1 cycle) followed by 30 cycles at 94 ºC for 30 seconds, 55 ºC for 60 seconds and 72 ºC for 1.5min followed by a final elongation step at 72 ºC for 5 minutes. The WHO reference strains of L. major (MHOM/IL/67/LV561) was used as standard DNA. A band of 560bp indicated that L. major kDNA is present in the sample (Davami et al. 2011).
Electrophoresis
A 6μl sample of each second-round product of PCR was subjected to electrophoresis in 1.5% agarose gel, stained with ethidium bromide and visualized by ultraviolet trans-illumination (Davami et al. 2011).
Sequencing
The PCR products of all positive samples were purified by Gel Purification Kit (Accu Prep®, Cat. No. k-3035-1, Bioneer, USA). The strands of amplified DNA were sequenced (Both forward and reverse sequencing) with the PCR primers on an automated sequencer (Applied Biosystems 377XL). The nucleotide homologies of the sequenced products were investigated with the TritrypDB blast programme. The characterization of consensus sequences was performed by using FASTA formatted sequences aligned with the Chromas programme.
Results
The 55 rodents trapped over the study belonged to four species; totally, 18 (32.7%) Me. persicus, 15 (27.3%) Ta. indica, 14 (25.5%) Mu. musculus, and 8 (14.5%) Ra. rattus were caught from Jahrom district (Table 1).
Table 1.
The species, numbers and geographical distributions of the rodents caught in Jahrom district
| Study areas |
No. and (%) of rodents caught |
||||
|---|---|---|---|---|---|
| Meriones persicus | Tatera indica | Mus musculus | Rattus rattus | All species | |
| *Jahrom | 14 (77.8) | 13 (86.7) | 6 (42.9) | 8 (100) | 41 (74.5) |
| Fath-Abad | 3 (16.7) | 0 (0) | 3 (21.4) | 0 (0) | 6 (10.9) |
| Mousavieh | 0 (0) | 2 (13.3) | 4 (28.6) | 0 (0) | 6 (10.9) |
| Ghotb-Abad | 1 (5.5) | 0 (0) | 1 (7.1) | 0 (0) | 2 (3.6) |
| All | 18 (100) | 15 (100) | 14 (100) | 8 (100) | 55 (100) |
Urban and suburban areas of Jahrom
Amastigotes were seen in the liver, spleen, ear and/or footpad smears of seven (12.7%) of the rodents- three (16.7%) of the Me. persicus, two (13.3%) of the T. indica, one (7.1%) of the Mu. Musculus, and one (12.5%) of the Ra. Rattus- caught in Jahrom district (Table 2).
Table 2.
The prevalences of leishmania infection in the rodents, as revealed by microscopy and, nested-PCR based detection of Leishmania major kDNA in Jahrom district
| Meriones persicus |
No. and (%) of rodents found positive |
Rattus rattus | All species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tatera indica | Mus musculus | ||||||||||||||
| Study areas | *Ch. | ** Mic. | PCR | Ch. | Mic. | PCR | Ch. | Mic. | PCR | Ch. | Mic. | PCR | Ch. | Mic. | PCR |
| ***Jahrom | 14 | 1(7.1) | 2(14.3) | 13 | 0(0) | 0(0) | 6 | 0(0) | 0(0) | 8 | 1(12.5) | 1(12.5) | 41 | 2(4.9) | 3 (7.3) |
| Fath-Abad | 3 | 2(2.67) | 2(66.7) | 0 | 0 (0) | 0(0) | 3 | 0 (0) | 0 (0) | 0 | 0 (0) | 0 (0) | 6 | 2(33.3) | 2 (33.3) |
| Mousavieh | 0 | 0(0) | 0(0) | 2 | 2(100) | 2(100) | 4 | 1 (25) | 1(25) | 0 | 0 (0) | 0 (0) | 6 | 3 (50) | 3 (50) |
| Ghotb-Abad | 1 | 0(0) | 0(0) | 0 | 0 (0) | 0 (0) | 1 | 0 (0) | 0(0) | 0 | 0 (0) | 0 (0) | 2 | 0 (0) | 0 (0) |
| All | 18 | 3(16.7) | 4 (22.2) | 15 | 2(13.3) | 2(13.3) | 14 | 1(7.1) | 1(7.1) | 8 | 1(12.5) | 1(12.5) | 55 | 7 (12.7) | 8 (14.6) |
Checked,
Microscopy,
Urban and suburban areas of Jahrom district
The nested-PCR results showed that 8 (14.6%) of rodents-four (22.2%) of the Me. persicus, two (13.3%) of the Ta. indica, one (7.1%) of the Mu. Musculus, and one (12.5%) of the Ra. Rattus-were positive for leishmania kDNA, Also 10 out of the 220 smears belonged to different parts of rodents organs (liver, spleen, ear and/or footpad) were found positive, separately (Table 2,3).
Table 3.
The prevalence of leishmania infection in the studied organs of rodents, as revealed by the nested-PCR in Jahrom district
| Footpad |
Studied organs of rodents (No. and %) |
All | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ear | Liver | Spleen | ||||||||||||
| Species | *Ch. ** | Pos. (%) | Ch. | Pos. | (%) | Ch. | Pos. | (%) | Ch. | Pos. | (%) | Ch. | Pos. | (%) |
| Meriones persicus | 18 | 2 (11.1) | 18 | 1 | (5.6) | 18 | 2 | (11.1) | 18 | 0 | (0) | 72 | 5 | (6.9) |
| Tatera indica | 15 | 2 (13.3) | 15 | 1 | (6.7) | 15 | 0 | (0) | 15 | 0 | (0) | 60 | 3 | (5) |
| Mus musculus | 14 | 1 (7.1) | 14 | 0 | (0) | 14 | 0 | (0) | 14 | 0 | (0) | 56 | 1 | (1.8) |
| Rattus rattus | 8 | 0 (0) | 8 | 0 | (0) | 8 | 1 | (12.5) | 8 | 0 | (0) | 32 | 1 | (3.1) |
| All | 55 | 5 (9.1) | 55 | 2 | (3.6) | 55 | 3 | (5.5) | 55 | 0 | (0) | 220 | 10 | (4.6) |
Checked,
Positive
All the kDNA detected appeared to come from L. major (Fig. 2). Using TritrypDB sequence analysis against Trypanosomatidae species, the target sequence of PCR products showed 95–96% similarity with L. major strain Iran J Wmaj (GenBank accession no. AB67 8349.1) (Fig. 3).
Fig. 2.
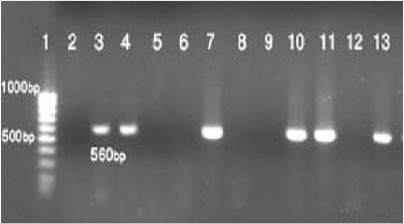
The results of the nested PCR-based amplification of kDNA recovered either from a negative control (lane 2), and the reference samples of Leishmania major (lane 3), or positive and negative smears of liver, spleen, ear, or footpad of Mus musculus (lane 4, 5 and 6), Rattus rattus (lanes 7, 8), Meriones persicus (lanes 9, 10 and 11), and Tatera indica (lanes 12 and 13). Molecular-weight markers were run in lanes 1
Fig. 3.

Alignment analysis of the kDNA of Leishmania major isolated from Mus musculus foot-pad, L. major strain Iran J Wmaj (GenBank accession no. AB678349.1) and L. major isolate MHOM/IL/67/LV561 (accession no. AF308685.1). Target sequence of PCR product of Mu. musculus showed 95% similarity with L. major strains, of Iran J Wmaj. Stars indicate the different regions between the isolates
Discussion
Detection of the mammalian ‘reservoir’ hosts is one of the main problems in front of researchers who make an effort to evaluate the epidemiology of ZCL to control the disease (Pourmohammadi et al. 2008). In the present study using a specific and sensitive nested-PCR on scrapings from Giemsa-stained smears of rodent tissues, the overall prevalence of rodents with leishmania infection was outcome as 14.6% that showed the higher infection than microscopical observations (12.7%). Different biochemical, immunological, and molecular assays such as iso-enzyme electrophoresis, monoclonal antibodies, and PCR have been used to characterize the causative agents of leishmaniasis (Fakhar et al. 2008, Parvizi et al. 2008, Fakhar et al. 2010, Pourmohammadi et al. 2010, Azizi et al. 2011b). Usuallym, in the leshmanial detections molecular assays are more sensitive than the other diagnostic methods such as micriscopical examinations or culture (Fakhar et al. 2011). As it is doable to use the molecular techniques to detect and identify the leishmania parasites in fixed and stained smears utilized for microscopy, recently, those methods chiefly based on nested-PCR and sequencing have been remarkably raised to detect the leishmania infections (Noyes et al. 1998).
In Iran, Rhombomys opimus, Ta. indica, Me. lybicus, and Me. hurrianae have been reported as the major ‘reservoir’ hosts of ZCL in endemic foci of the central and north-east, west and south-west, south, and south-eastern parts of Iran, respectively (Yaghoobi-Ershadi et al. 1996, Moemenbellah-Fard et al. 2003, Parvizi et al. 2005, Asgari et al. 2007).
Meriones lybicus, Me. persicus, Me. Hurrianae and Ta. indica have been found positive for Leishmania kDNA in Fars Province (Rassi et al. 2001, Moemenbellah-Fard et al. 2003, Rassi et al. 2006, Mehrabani et al. 2007, Mehrabani et al. 2011, Parhizkari et al. 2011, Azizi et al. 2012). In our study, the results of rodents trapped in Jahrom district of Fars Province, indicated that Ta. indica and Me. persicus were the most common rodents in that part of the province, and the results of the nested-PCR showed that 22.2% and 13.3% of mentioned rodents were found to be positive for L. major. Me. persicus and Ta. indica are the most important mammalian hosts of L. major in the rural areas of Jahrom district. No R. opimus and Me. hurrianae were caught in the present study in Jahrom.
Nesokia indica, Gerbillus nanus have been described as an accidental, main or a probable ‘reservoir’ hosts in different parts of Iran (Pourmohammadi et al. 2008, Azizi et al. 2011a, Azizi et al. 2011c, Azizi et al. 2012). In our investigation any Nesokia or Gerbilus species were not caught.
Mu. Musculus has been found naturally infected with L. major in Fars Province (Parhizkari et al. 2011). In the present study, the footpad of one out of 14 (7.1%) Mu. Musculus were found to be both microscpical and molecular positive.
Recently Motazedian et al. has isolated the causative agents of ZCL (L. major) from Ra. norvegicus in Fars Province (Motazedian et al. 2010). In this investigation one liver of Ra. rattus caught in urban area of Jahrom district was found to be infected with Leishmania kDNA, the present report appears to be the first to describe natural infection of Ra. rattus with L. major in Iran. Mu. Musculus and Ra. rattus infection with L. major may explain why, in recent years, ZCL causative has been remarkably increased in some urban areas of Iran (Razmjou et al. 2009, Davami et al. 2010).
The importance of the ‘reservoir’ hosts in the epidemiology of ZCL appreciated with the local sandflies feeding on that species and the infectivity of the leishmania infections in each host species (Motazedian et al. 2006). Even though Mu. musculus and Ra. rattus were found infected with L. major, but some reports show that the prevalence of leishmania infection have been observed from those which caught from other regions of Iran (Parhizkari et al. 2011).
There was no evidence of L. infantum or L. tropica in the tested smears. This study includes the first isolation and characterisation of L. major from Iranian Me. persicus and Ta. indica, caught in an area where zoonotic CL has recently occurred.
In Jahrom, whatever the time of the year, more than half of all rodents attracted to walnut-baited traps are infected with L. major. If local sandflies take their bloodmeals from both rodents and humans, there is clearly much scope for transmission of these parasites to humans.
Conclusion
Based on observing of amastigotes in livers, spleens, ears, and footpads smears, using a high sensitive and specific nested-PCR designed for kDNA of Leishmania, and comparing the kDNA of sequenced products with GenBank that confirmed the highest homology of 95–96% with L. major, concluded that the species isolated form rodents is L. major.
Acknowledgements
The authors are grateful to the office of the Vice-Chancellor for Research at Jahrom and Shiraz University of Medical Sciences for financial support, and the Department of Parasitology and Mycology in the Medical School of Shiraz University of Medical Sciences for facilitating the PCR assays. They also thank to Dr R Zarei for her assistance in the identification of the rodents caught, and to Dr B Sarkari, for his critical review of the manuscript. The authors declare that there is no conflict of interests.
Reference
- 1. Asgari Q, Motazedian MH, Mehrabani D, Oryan A, Hatam GR, Owji SM, Paykari H. ( 2007) Zoonotic cutaneous leishmaniasis in Shiraz Iran: a molecular, isoenzyme and morphologic approach. J Res Med Sci. 12: 7– 15. [Google Scholar]
- 2. Azizi K, Davari B, Kalantari M, Fekri S. ( 2011a) Gerbillid rodents fauna (Muridae: Gerbillinae) and detection of reservoir hosts(s) of zoonotic cutaneous leishmaniasis using a nested-PCR technique in Jask city in Hormozgan Province in 2008. Sci J Kurdistan Univ Med Sci. 16: 66– 76. [Google Scholar]
- 3. Azizi K, Kalantari M, Fekri S. ( 2011b) The Nested-PCR based detection of cutaneous leishmaniasis vectors in Jask County, Hormozgan, Iran. Iran J Epidemiol. 7: 27– 33. [Google Scholar]
- 4. Azizi K, Moemenbellah-Fard MD, Fakoorziba MR, Fekri S. ( 2011c) Gerbillus nanus a new reservoir host of Leishmania major. Ann Trop Med Parasitol. 105: 431– 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azizi K, Moemenbellah-Fard MD, Fakoorziba MR, Kalantari M. ( 2012) Molecular detection of Leishmania major kDNA from rodents in a new focus of zoonotic cutaneous leishmaniasis in oriental region of Iran. Vector Borne Zoonotic Dis. (in press). [DOI] [PubMed] [Google Scholar]
- 6. Bulat SA, Strelkova MV, Sysoev VV. ( 1992) The identification of marker strains Leishmania major, L. turanica and L. gerbili by the polymer chain reaction with a universal primer. Med Parasitol (Mosk). 1: 21– 222. [PubMed] [Google Scholar]
- 7. Davami MH, Motazedian MH, Kalantari M, Badzohre A, Mohammadpour I. ( 2011) First microscopical and molecular-based characterization of Leishmania major within naturally infected Phlebotomus salehi (Diptera: Psychodidae) in Fars Province, southern Iran. Ann Trop Med Parasitol. 105: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davami MH, Motazedian MH, Sarkari B. ( 2010) The changing profile of cutaneous leishmaniasis in a focus of the disease in Jahrom district, southern Iran. Ann Trop Med Parasitol. 104: 377– 382. [DOI] [PubMed] [Google Scholar]
- 9. Eisenberg JF, Redford KF. ( 1999) Mammals of the Neotropics: the Central Neotropics. University of Chicago Press, Chicago, USA. [Google Scholar]
- 10. Fakhar M, Motazedian MH, Asgari Q, Kalantari M. ( 2011) Asymptomatic domestic dogs are carriers of Leishmania infantum: possible reservoirs host for human visceral leishmaniasis in southern Iran. Comp Clin Path. 21( 5): 801. [DOI] [PubMed] [Google Scholar]
- 11. Fakhar M, Motazedian MH, Asgari Q, Kalantari M, Hatam GR, Akbarpoor MA, Gharachahi MA. ( 2010) The efficacy of PCR for early diagnosis and detection of asymptomatic cases of visceral leishmaniasis in human and dog. J Jahrom Univ Med Sci. 8: 1– 6. [Google Scholar]
- 12. Fakhar M, Motazedian MH, Hatam GR, Asgari Q, Kalantari M, Mohebali M. ( 2008) Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann Trop Med Parasitol. 102: 577– 583. [DOI] [PubMed] [Google Scholar]
- 13. Fars Budget and Planning Organization ( 2000) Position of Fars province: annual report. Management and Planning Press; pp. 20– 30 (In Persian). [Google Scholar]
- 14. Ghasemian M, Maraghi S, Samarbafzadeh AR, Jelowdar A, Kalantari M. ( 2011) The PCR-based detection and identification of the parasites causing human cutaneous leishmaniasis in the Iranian city of Ahvaz. Ann Trop Med Parasitol. 105: 209– 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guedes H, Carvalho R, Gomes D, Rossi-Bergmann B, De-Simone S. ( 2008) Oligopeptidase B-2 from Leishmania amazonensis with an unusual C-terminal extension. Acta Parasitol. 53: 197– 204. [Google Scholar]
- 16. Javadian E, Nadim A, Tahvildare-Bidruni G, Assefi V. ( 1976) Epidemiology of cutaneous leishmaniasis in Iran: Khorassan. Bull Soc Pathol Exot. 69: 140– 143. [PubMed] [Google Scholar]
- 17. Mehrabani D, Motazedian MH, Hatam GR, Asgari Q, Owji SM, Oryan A. ( 2011) Leishmania major in Ttera iindica in Fasa, southern Iran: Microscopy, Culture, Isoenzyme, PCR and Morphologic study. Asian J Anim Vet Adv. 6: 255– 264. [Google Scholar]
- 18. Mehrabani D, Motazedian MH, Oryan A, Asgari Q, Hatam GR, Karamian M. ( 2007) A search for the rodents hosts of Leishmania major in the Larestan region of southern Iran: demonstration of the parasite in Tatera indica and Gerbilus sp., by microscopy, culture and PCR. Ann Trop Med Parasitol. 101: 315– 322. [DOI] [PubMed] [Google Scholar]
- 19. Moemenbellah-Fard MD, Kalantari M, Rassi Y, Javadian E. ( 2003) The PCR-based detection of Leishmania major infections in Meriones libycus (Rodentia: Muridae) from southern Iran. Ann Trop Med Parasitol. 97: 811– 816. [DOI] [PubMed] [Google Scholar]
- 20. Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjran H, Abaei MR. ( 2004) Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 10: 415. [PubMed] [Google Scholar]
- 21. Motazedian H, Karamian M, Noyes HA, Ardehali S. ( 2002) DNA extraction and amplification of Leishmania from archived, Giemsa-stained slides, for the diagnosis of cutaneous leishmaniasis by PCR. Ann Trop Med Parasitol. 96: 31– 34. [DOI] [PubMed] [Google Scholar]
- 22. Motazedian MH, Mehrabani D, Oryan A, Asgari Q, Karamian M, Kalantari M. ( 2006) Life cycle of cutaneous leishmaniasis in Larestan, southern Iran. Iran J Clin Infect Dis. 1: 137– 143. [Google Scholar]
- 23. Motazedian MH, Parhizkari M, Mehrabani D, Hatam GR, Asgari Q. ( 2010) First Detection of Leishmania major in Rattus norvegicus from Fars Province, southern Iran. Vector Borne Zoonotic Dis. 10: 969– 975. [DOI] [PubMed] [Google Scholar]
- 24. Noyes HA, Reyburn H, Baiely JW, Smith D. ( 1998) A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 36: 2877– 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parhizkari M, Motazedian MH, Asqari Q, Mehrabani D. ( 2011) The PCR-based detection of Leishmania major in Mus musculus and other rodents caught in southern Iran: a guide to sample selection. Ann Trop Med Parasitol. 105: 319– 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parvizi P, Mauricio I, Aransay AM, Miles MA, Ready PD. ( 2005) First detection of Leishmania major in peridomestic Phlebotomus papatasi from Isfahan Province, Iran: comparison of nested PCR of nuclear ITS ribosomal DNA and semi-nested PCR of minicircle kinetoplast DNA. Acta Trop. 93: 75– 83. [DOI] [PubMed] [Google Scholar]
- 27. Parvizi P, Moradi G, Akbari G, Farahmand M, Ready PD, Piazak N, Assmar M, Amirkhani A. ( 2008) PCR detection and sequencing of parasite ITS-rDNA gene from reservoirs host of zoonotic cutaneous leishmaniasis in central Iran. Parasitol Res. 103: 1273– 1278. [DOI] [PubMed] [Google Scholar]
- 28. Parvizi P, Ready PD. ( 2008) Nested PCRs and sequencing of nuclear ITS-rDNA fragments detect three Leishmania species of gerbils in sand flies from Iranian foci of zoonotic cutaneous leishmaniasis. Trop Med Int Health. 13: 1159– 1171. [DOI] [PubMed] [Google Scholar]
- 29. Pourmohammadi B, Motazedian MH, Hatam GR, Kalantari M, Habibi P, Sarkari B. ( 2010) Comparison of Three Methods for Diagnosis of Cutaneous Leishmaniasis. Iran J Parasitol. 5: 1– 8. [PMC free article] [PubMed] [Google Scholar]
- 30. Pourmohammadi B, Motazedian MH, Kalantari M. ( 2008) Rodent infection with Leishmania in a new focus of human cutaneous leishmaniasis, in northern Iran. Ann Trop Med Parasitol. 102: 127– 133. [DOI] [PubMed] [Google Scholar]
- 31. Rassi Y, Jalali M, Javadian E, Motazedian MH. ( 2001) Confirmation of Meriones libycus (Rodentia: Gerbillidae) as the main reservoir host of zoonotic cutaneous leishmaniasis in Arsanjun, Fars Province, south of Iran. Iranian J Public Health. 30: 143– 144. [Google Scholar]
- 32. Rassi Y, Javadian E, Amin M, Rafizadeh S, Vatandoost H, Motazedian H. ( 2006) Meriones libycus is the main reservoir of zoonotic cutaneous leishmaniasis in south Islamic Republic of Iran. East Mediterr Health J. 12: 474– 477. [PubMed] [Google Scholar]
- 33. Razmjou S, Hejazy H, Motazedian MH, Baghaei M, Emamy M, Kalantary M. ( 2009) A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Trans R Soc Trop Med Hyg. 103: 727– 730. [DOI] [PubMed] [Google Scholar]
- 34. Rodgers MR, Popper SJ, Wirth DF. ( 1990) Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. EXP Parasitol. 71: 267– 275. [DOI] [PubMed] [Google Scholar]
- 35. Van Eys GJ, Schoone GJ, Kroon NCM, Ebeling SB. ( 1992) Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 51: 133– 142. [DOI] [PubMed] [Google Scholar]
- 36. Yaghoobi-Ershadi MR, Akhavan AA, Mohebali M. ( 1996) Meriones libycus and Rhombomys opimus (Rodentia: Gerbillidae) are the main reservoir hosts in a new focus of zoonotic cutaneous leishmaniasis in Iran. Trans R Soc Trop Med Hyg. 90: 503– 504. [DOI] [PubMed] [Google Scholar]
- 37. Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, Jalali-Zand AR, Piazak N. ( 2005) Bionomics of Phlebotomus papatasi (Diptera: Psychodidae) in an endemic focus of zoonotic cutaneous leishmaniasis in central Iran. J Vector Ecol. 30: 115– 118. [PubMed] [Google Scholar]


